
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rui Figueiredo | -- | 1470 | 2024-02-02 13:32:18 | | | |
| 2 | Catherine Yang | Meta information modification | 1470 | 2024-02-04 02:34:03 | | |
Video Upload Options
Customized subperiosteal implants (CSIs) are a promising treatment option for rehabilitating edentulous patients with atrophic jaws; they seem to have an excellent short-term survival rate, a low incidence of major complications, and less morbidity in comparison with complex bone grafting procedures.
1. Introduction
2. Indications and Contraindications of CSIs
CSIs indications:
-
Patients who present insufficient bone to place standard dental implants;
-
When complex regenerative techniques cannot be performed or are not accepted by the patients because of the associated morbidity;
-
Patients who do not tolerate removable prostheses or when these cannot be made;CSIs might be considered as an alternative to zygomatic implants when a fixed prosthesis is required;
-
CSIs should be used with caution in cases of partial edentulism since the available clinical data are limited in these situations (EO).
CSI contraindications:
-
Patients with systemic pathologies that contraindicate the surgical procedure;
-
Patients under treatment with therapies or drugs that contraindicate the surgical procedure.
3. Planning and Designing CSIs
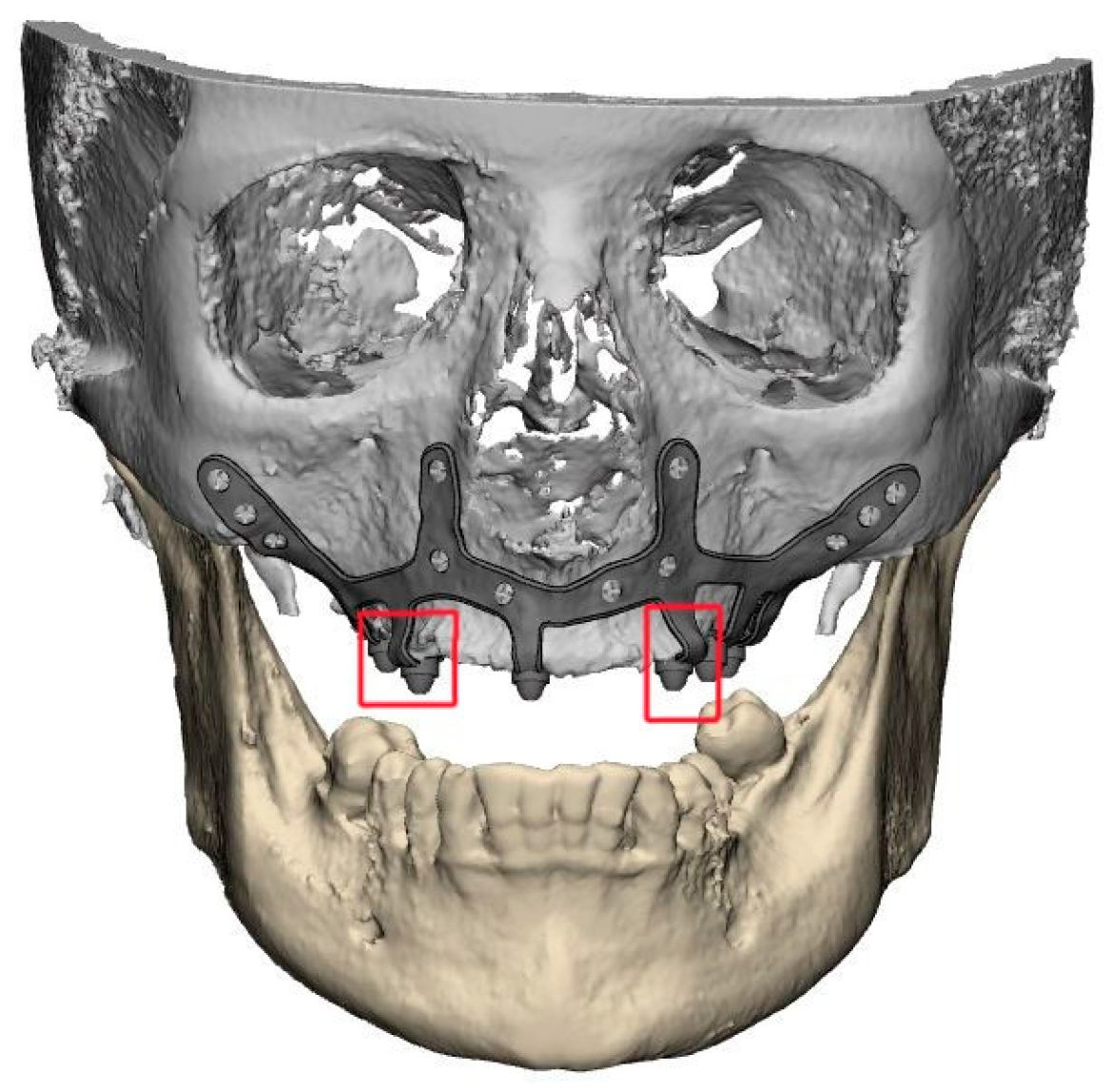
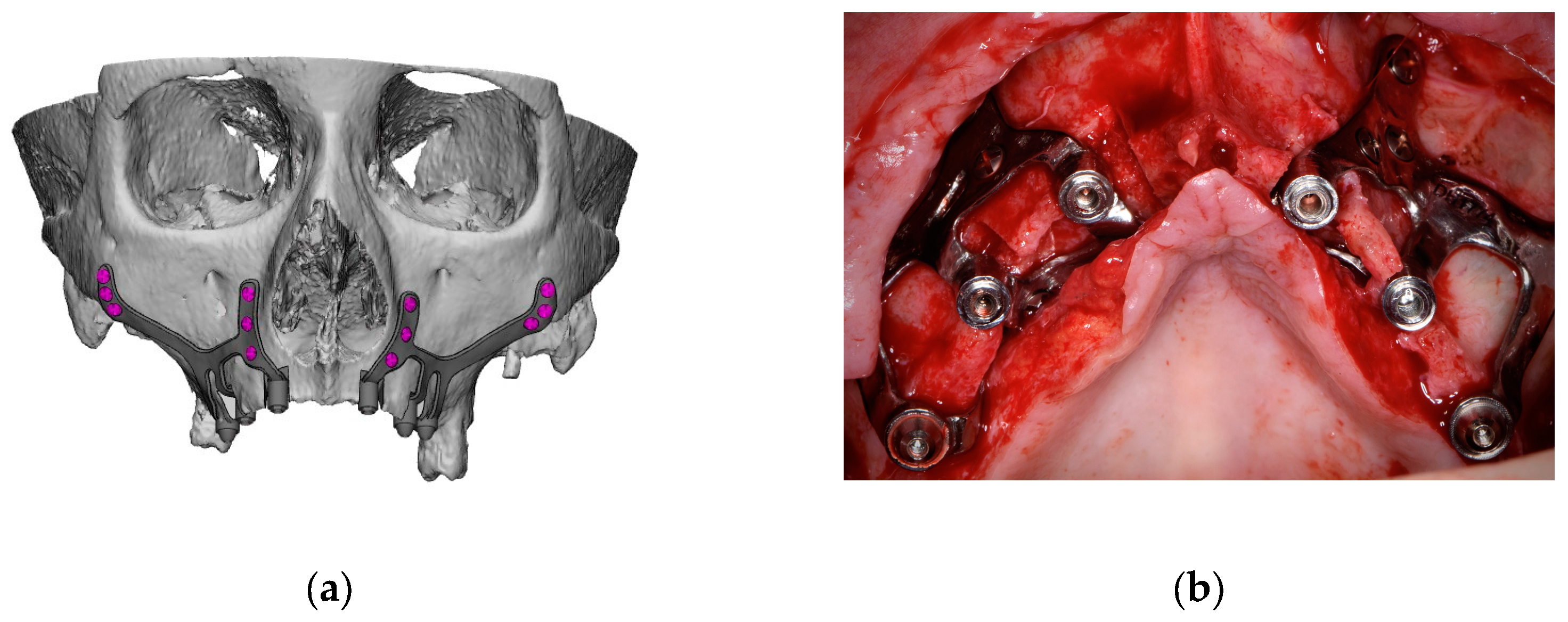
A thorough diagnosis is paramount for adequate treatment planning. High-resolution computer tomography (CT) following the instructions provided by the CSI manufacturer is mandatory. Cone-beam computer tomography (CBCT) is not suitable for designing CSIs (EO). A proper diagnosis should include the occlusal position, a standard tessellation language (STL) file with the intraoral anatomy, and a CT scan. Passive fit of the CSI to the surrounding bone is critical since this is a custom-made device. Since the most frequent complication is CSI exposure, a polished titanium surface is recommended (EO). It is essential to avoid abrupt transitions and sharp angles in the areas between the CSI frame and the prosthetic connections (Figure 1). A thorough diagnosis is paramount for adequate treatment planning. High-resolution computer tomography (CT) following the instructions provided by the CSI manufacturer is mandatory. Cone-beam computer tomography (CBCT) is not suitable for designing CSIs (EO). Fixation of the CSI is a key factor for achieving a successful treatment outcome. The fixation elements should be placed in high anatomic buttress areas (nasal and zygomatic) and the palatal region. The use of self-drilling screws is recommended. In cases with totally edentulous arches, clinicians should consider designing two independent frames to facilitate the implant insertion path during the procedure (Figure 2). This issue is particularly important when high fixation zones are selected (EO). Specific surgical templates are recommended to guide the removal of the residual alveolar ridge (Figure 3). This will improve the adaptation of the CSI, facilitate its design, and reduce the risk of postoperative soft tissue dehiscence (EO). From a biomechanical perspective, there is no contraindication for connecting CSIs with previously placed conventional dental implants (EO). It is advisable to print a 3D model of the patient before surgery (EO).
4. Surgical protocol and associated complications
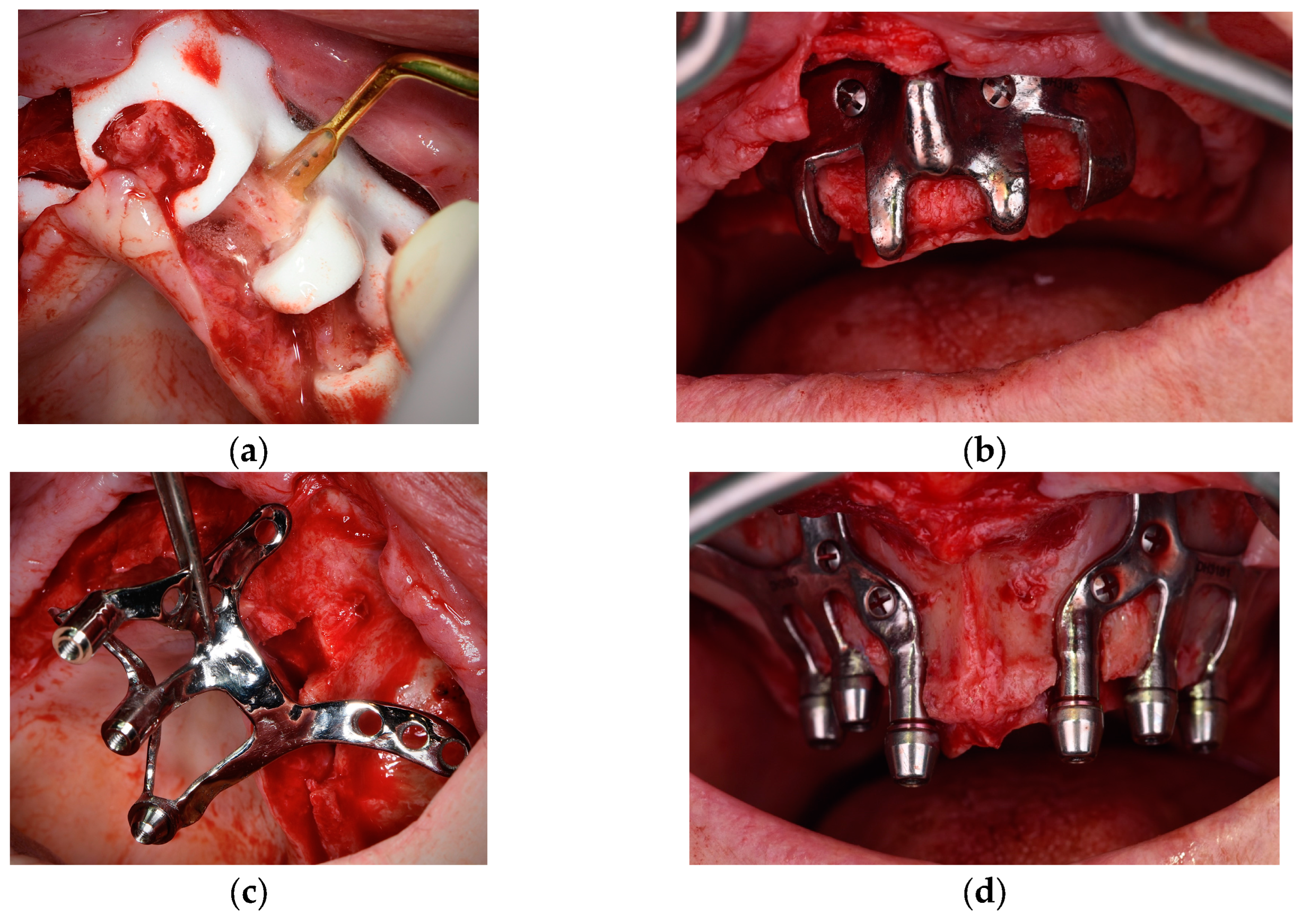
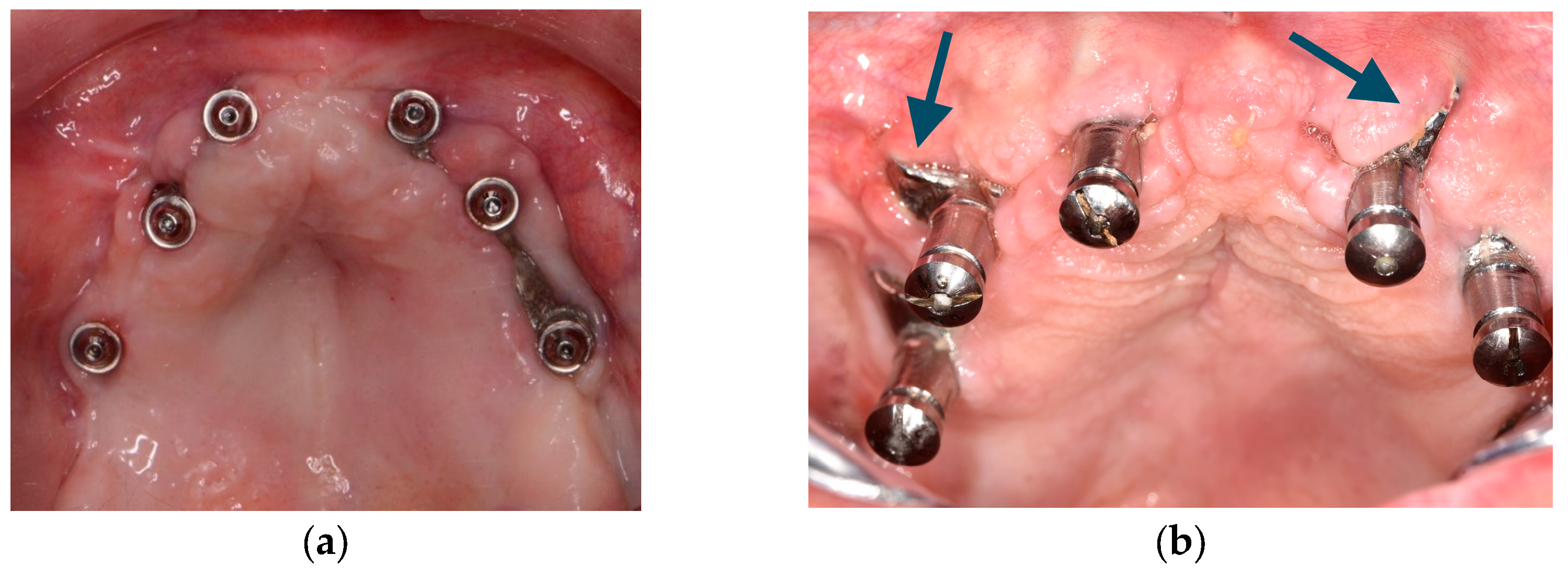
Although it is possible to place CSIs under local anesthesia, it is advisable to combine them with conscious sedation techniques or general anesthesia. Surgical asepsis guidelines must be followed during the procedure. The incision should be performed considering the final position of the keratinized mucosa since this tissue is essential to prevent long-term complications (EO). If the keratinized mucosa width is insufficient, it is advisable to perform soft tissue augmentation procedures. Soft tissue dehiscence leading to exposure of the CSI is the most common postoperative complication (Figure 4). This complication does not seem to affect CSI survival in the short term. Removal of the CSI is indicated when the implant has lost its stability or when recurrent infections occur. It is advisable to have a sterile 3D model of the patient present during the surgical procedure (Figure 5) (EO). After testing the insertion of the implant in the model, the CSI should be securely fixed with screws to the maxilla or mandible. The flap should be repositioned, leaving the abutments exposed.

5. Prosthetic Protocol and Associated Complications
A thorough and complete preoperative prosthetic diagnosis is mandatory. This prosthetic planning is essential for designing the CSI correctly. The prosthodontic treatment principles and steps used in rehabilitation with conventional dental implants should be followed when using CSI. It is essential to create a prosthesis with ovoid pontics that allows correct assessment for oral hygiene. The clinical results of this group of experts support the use of fixed screw-retained restorations over CSIs. The literature also reports on the use of other types of rehabilitation (EO). The CSI can be immediately loaded. The provisional and definitive prostheses should not apply pressure on the soft tissues (EO). A minimum of 4 prosthetic connections are required to rehabilitate an entire arch. Whenever possible, the use of transepithelial abutments should be considered (EO). The materials employed in conventional implant-supported prostheses are also suitable for rehabilitation with CSIs. It is advisable to use an occlusal splint after the prosthetic rehabilitation to prevent the occurrence of mechanical complications, especially when the patient has natural dentition or a fixed implant-supported rehabilitation in the opposing arch (EO).
6. Peri-Implant Supportive Therapy
There is no specific evidence reporting the maintenance protocol for CSI restorations. Control visits are recommended every 6 months to avoid or diagnose biological (e.g., bone loss under CSIs) or mechanical complications (e.g., prosthetic fracture) (EO). The main goal of peri-implant supportive therapy is to remove plaque accumulation and biofilm around implant abutments and prostheses. In the case of screw-retained restorations, these can be removed to thoroughly clean the surfaces (EO). Patients should be informed of the importance of these visits for the long-term maintenance of their rehabilitation and of the most common pathologies or complications. Patients should also be advised to seek clinical attention in cases of CSI mobility or soft tissue dehiscences (CSI exposure) (EO). These visits should include professional advice in case of risk factors/indicators. Patients should be informed that redness, bleeding, or inflammation of the peri-implant mucosa are important signs that, if left untreated, might result in significant long-term complications (EO).
7. General Recommendations and Future Perspectives (EO)
The available data on the use of CSIs are very scarce, precluding the establishment of clinical recommendations based on scientific evidence. It is essential to perform randomized clinical trials to compare the use of CSIs with other therapeutic alternatives. Additionally, cohort studies with a long-term follow-up could help determine the incidence, repercussions, and prognosis of complications associated with CSIs. Finite analysis studies evaluating different CSI designs would be desirable. Professionals are encouraged to undergo specific training in the use of CSIs. Professionals could benefit from developing additional tools or guides to reduce the margin of error. The creation of specifically designed custom guides for all steps of the treatment would be desirable. The development of specific prosthetic connections for CSIs might be interesting.
References
- Tofé-Povedano, A.; Parras-Hernández, J.; Herce-López, J.; Matute-García, D.; González-Moguena, V.A.; Rollón-Mayordomo, A. Design modifications in subperiosteal implants to avoid complications. Presentation of a case series study and literature re-view. Rev. Esp. Cir. Oral Maxilofac. 2023, 45, 57–63.
- Branemark, P.-I. Surgery and fixture installation. In Zygomaticus Fixture Clinical Procedures, 1st ed.; Nobel Biocare AB: Goteborg, Sweden, 1998.
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Survival and complications of zygomatic implants: An updated systematic review. J. Oral Maxillofac. Surg. 2016, 74, 1949–1964.
- Pons, M.C.; Gallo, J.A.; Perez, L.M.; Martinez, G.D.; Vicario, A.C. Subperiosteal personalised implants for the rehabilitation of the severely deficient edentulous maxilla. Revision of a clinical series of 8 cases. Rev. Esp. Cir. Oral Maxilofac. 2021, 43, 140–148.
- Borre, C.V.D.; Rinaldi, M.; De Neef, B.; Loomans, N.A.J.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Radiographic evaluation of bone re-modeling after additively manufactured subperiosteal jaw implantation (Amsji) in the maxilla: A one-year follow-up study. J. Clin. Med. 2021, 10, 3542.
- Borre, C.V.D.; Rinaldi, M.; De Neef, B.; Loomans, N.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Patient- and clinician-reported outcomes for the additively manufactured sub-periosteal jaw implant (AMSJI) in the maxilla: A prospective multicentre one-year follow-up study. Int. J. Oral Maxillofac. Surg. 2022, 51, 243–250.
- Korn, P.; Gellrich, N.C.; Jehn, P.; Spalthoff, S.; Rahlf, B. A new strategy for patient-specific implant-borne dental rehabilitation in patients with extended maxillary defects. Front. Oncol. 2021, 11, 718872.
- Rahlf, B.; Korn, P.; Zeller, A.-N.; Spalthoff, S.; Jehn, P.; Lentge, F.; Gellrich, N.-C. Novel approach for treating challenging implant-borne maxillary dental rehabilitation cases of cleft lip and palate: A retrospective study. Int. J. Implant. Dent. 2022, 8, 6.
- Mommaerts, M.Y. Additively manufactured sub-periosteal jaw implants. Int. J. Oral Maxillofac. Surg. 2017, 46, 938–940.
- Mommaerts, M.Y. Evolutionary steps in the design and biofunctionalization of the additively manufactured sub-periosteal jaw implant ‘AMSJI’ for the maxilla. Int. J. Oral Maxillofac. Surg. 2019, 48, 108–114.
- Gellrich, N.-C.; Zimmerer, R.M.; Spalthoff, S.; Jehn, P.; Pott, P.-C.; Rana, M.; Rahlf, B. A customised digitally engineered solution for fixed dental rehabilitation in severe bone deficiency: A new innovative line extension in implant dentistry. J. Craniomaxillofac. Surg. 2017, 45, 1632–1638.
- Cerea, M.; Dolcini, G.A. Custom-made direct metal laser sintering titanium subperiosteal implants: A retrospective clinical study on 70 patients. Biomed. Res. Int. 2018, 2018, 5420391.
- Claffey, N.; Bashara, H.; O’Reilly, P.; Polyzois, I. Evaluation of new bone formation and osseointegration around subperiosteal titanium implants with histometry and nanoindentation. Int. J. Oral Maxillofac. Implant. 2015, 30, 1004–1010.
- Peev, S.; Sabeva, E. Subperiosteal implants in treatment of total and partial edentulism—A long term follow up. Int. J. Sci. Res. 2016, 5, 98–99.





