
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hizkia Manuel Vieri | -- | 3025 | 2024-02-02 01:18:01 | | | |
| 2 | Mona Zou | Meta information modification | 3025 | 2024-02-05 08:37:54 | | | | |
| 3 | Hizkia Manuel Vieri | -401 word(s) | 2624 | 2024-02-05 08:47:37 | | | | |
| 4 | Mona Zou | Meta information modification | 2624 | 2024-02-05 08:54:11 | | | | |
| 5 | Mona Zou | + 1 word(s) | 2625 | 2024-03-07 10:13:32 | | | | |
| 6 | Mona Zou | -1 word(s) | 2624 | 2024-03-07 10:15:27 | | |
Video Upload Options
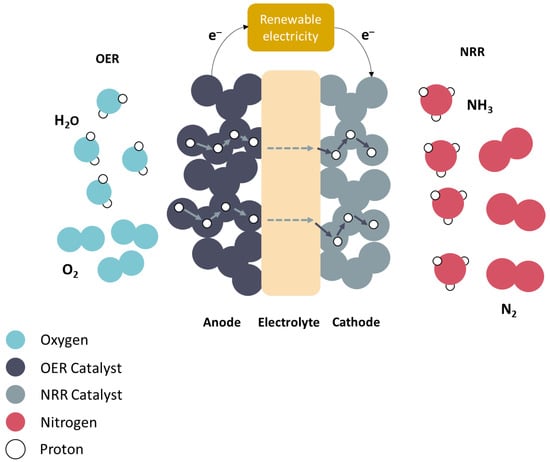
The application of protonic ceramic electrolysis cells (PCECs) for ammonia (NH3) synthesis has been evaluated over the past 14 years. While nitrogen (N2) is the conventional fuel on the cathode side, various fuels such as methane (CH4), hydrogen (H2), and steam (H2O) have been investigated for the oxygen evolution reaction (OER) on the anode side. Because H2 is predominantly produced through CO2-emitting methane reforming, H2O has been the conventional carbon-free option thus far. Although the potential of utilizing H2O and N2 as fuels is considerable, studies exploring this specific combination remain limited.
1. Introduction
- ●
-
At the anode, an electric current passes through water, thereby splitting water molecules into hydrogen (protons) and oxygen ions.
- ●
-
Reaction: 2H2O → O2 + 4H+ + 4e−
- ●
-
Electrons generated at the anode during water splitting are transported through an external electric circuit.
- ●
-
Protons generated at the anode during water splitting are transported through the electrolyte [18].
- ●
-
At the cathode, protons (H+) from the anode and nitrogen atoms react to produce NH3
- ●
-
Reaction: N2 + 3H+ + 3e− → NH3
2. PCEC Design Strategies
2.1. Electrolyte Design Strategies
| Electrolyte | Method | Conductivity (S cm−1) | Thickness (mm) | Reference |
|---|---|---|---|---|
| SrCe0.95Yb0.05O3−δ | sol–gel | Unknown | 1.5 | [28] |
| BaZr0.8−x−yCexNdyY0.1Yb0.1O3−δ | Pechini method | 500 °C: 3.77 × 10−4 | 0.8–1.5 | [29] |
| BaZr0.85Y0.15O3−δ | hydrothermal process | 600 °C: 2.5 × 10−3 | 1.6 | [30] |
| Electrolyte | Method | Conductivity (S cm−1) | Thickness (µm) | Reference |
|---|---|---|---|---|
| BaCe0.7Zr0.1Y0.2 | co-precipitation solid-state reaction dip-coating |
650 °C: 2.8 × 10−2 | ~20 | [32] |
| BaCe0.8Y0.2−xNdxO3−δ | citrate–nitrate combustion | 350 °C: 8.5 × 10−3 | ~20 | [33] |
| BaCe1−xInxO3−δ | auto-combustion reaction | 700 °C: 5 × 10−3 | 20–25 | [34] |
| BaZr0.1Ce0.7Y0.1Yb0.1 | solid-state reaction | 500 °C: 1.2 × 10−2 | 10 | [35] |
| BaHf0.8Yb0.2O3−δ | pulsed laser deposition (PLD) | 500 °C: 2.5 × 10−3 | 110 | [35] |
| BaZr0.1Ce0.7Y0.1Yb0.1 | solid-state reaction | 500 °C: 1.3 × 10−2 | ~10 | [21] |
| BaZr0.4Ce0.4Y0.1Yb0.1 | solid-state reaction | 500 °C: 5.6 × 10−3 | ~15 | [20] |
| BaZr0.2Ce0.6Y0.1Yb0.1O3−δ | Pechini method inkjet printing |
600 °C: 24.39 | 1 | [36] |
| BaCe0.5Zr0.35Y0.15O3−δ | citric nitrate method PLD |
Unknown | 2–4 | [37] |
| BaZr1−x−yCexYyO3 | ultrafast microwave-assisted sintering tape casting |
Unknown | ~12 | [38] |
| BaZr0.2Ce0.6Y0.2O3 | solid-state reaction spin coating |
800 °C: 1 × 10−2 | ~7 | [39] |
| BaCe0.55Zr0.3Y0.15O3−δ | screen printing | Unknown | ~2.5 | [40] |
2.2. Electrode Design Strategies
| Cathode | Deposition Method | Thickness (µm) |
Reference |
|---|---|---|---|
| La0.6Sr0.4Co0.2Fe0.8O3−δ | Unknown | 44 | [50] |
| Ag | - | 4 | |
| Pt | - | 8 | |
| Fe | doctor blade | 15–25 | [51] |
| 10-Fe-BCY | doctor blade | 15–25 | |
| 0.5W-10Fe-BCY | doctor blade | 15–25 | |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | - | 10–20 | [23] |
| Ru–Ag/MgO | Unknown | - | [28] |
| Ni-BCYR | - | - | [52] |
| NdBa0.5Sr0.5Co1.5Fe0.5O5+δ (NBSCF)-BZCYYb | drop coating | 15 | [53] |
| Pr2NiO4-BZCY | screen printing | 13 | [54] |
| PrCo0.05Ni0.5O3−δ | tape casting | 29 | [55] |
| Ba0.9Co0.7Fe0.2Nb0.1O3−δ | screen printing | 15 | [56] |
| Pr0.2Ba0.2Sr0.2La0.2Ca0.2CoO3−δ | spray coating | 20 | [57] |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | PLD | 20 | [58] |
| Gd0.3Ca2.7Co3.82Cu0.18O9−δ | screen printing | 30 | [59] |
4. Current Progress
The NRR and OER have been studied extensively; the catalysts used for these reactions are summarized in Tables 5 and 6, respectively.
Table 5. Notable NRR catalysts in PCECs for NH3 synthesis.
|
Cathode * |
Electrolyte |
NH3 Production Rate [mol cm−2 s−1] × 10−9 |
Thickness (µm) |
Reference |
|
La0.6Sr0.4Co0.2Fe0.8O3−δ |
BaZr0.8Y0.2O3−δ |
0.0850 |
44 |
[24] |
|
Ag |
BaZr0.8Y0.2O3−δ |
0.0490 |
4 |
|
|
Pt |
BaZr0.8Y0.2O3−δ |
<0.0010 |
8 |
|
|
Fe |
BaCe0.9Y0.1O3−δ |
14.000 |
15–25 |
[26] |
|
10-Fe-BCY |
BaCe0.9Y0.1O3−δ |
0.4200 |
15–25 |
|
|
0.5W-10Fe-BCY |
BaCe0.9Y0.1O3−δ |
0.5700 |
15–25 |
|
|
Ru–Ag/MgO |
SrCe0.95Yb0.05O3−δ |
0.0003 |
- |
[41] |
|
Ni-BCYR |
BaCe0.9Y0.1O3−δ |
0.0110 |
- |
[63] |
Based on Table 5, metallic catalysts evidently yield higher reaction rates. This could be attributed to two main factors. First, perovskite-based electrocatalysts may possibly be affected by degradation at the interface and thermal mismatch with the electrolyte, thus decreasing the performance [24]. Second, the electrochemical promotion of catalysis is more pronounced in pure metal catalysts with a higher effective double layer (S*eff) on their surfaces than in supported electrocatalysts [26]. However, pure metallic catalysts are typically expensive.
Ru is regarded as a suitable catalyst for thermochemical NH3 synthesis because of its peak position on Skulason’s volcano diagram, which shows that it requires a minimum potential for electrochemical NH3 synthesis. It has also been reported to be an ultra-efficient electrocatalyst for the NRR, with a lower reduction potential than that of Fe [71–75]. However, Ag is a more cost-effective option because of its natural abundance. Although noble-metal-based electrocatalysts exhibit favorable activity, efficiency, and selectivity, their practical application is inhibited by their high cost and scarcity [27]. Consequently, extensive research has been conducted on transition-metal-based electrocatalysts for the NRR. The NH3 synthesis rate of Pt catalysts can be primarily attributed to their strong HER activity [76,77]. At negative potentials, the surface of Pt nanoparticles tends to adsorb hydrogen atoms rather than nitrogen atoms, thus affecting the overall performance [78].
Table 6. Notable OER catalysts in PCECs for NH3 synthesis.
|
Anode |
Electrolyte |
Current Density @1.3 V and 550 °C [A cm−2] |
Thickness (µm) |
Reference |
|
Pr0.2Ba0.2Sr0.2La0.2Ca0.2CoO3−δ |
BaZr0.1Ce0.7Y0.1Yb0.1O3−δ |
−0.800 |
20 |
[68] |
|
PrBa0.5Sr0.5Co1.5Fe0.5O5+δ |
BaZr0.4Ce0.4Y0.1Yb0.1O3−δ |
−1.059 |
20 |
[69] |
|
Gd0.3Ca2.7Co3.82Cu0.18O9−δ |
BaZr0.1Ce0.7Y0.1Yb0.1O3−δ |
−1.241 |
30 |
[70] |
Considering NH3-producing PCECs, most studies have only focused on the NRR, whereas the OER has been overlooked. The NH3 synthesis reaction is typically performed at 475–600 °C [24]. Pei et al. briefly summarized the OER performance at a cell voltage of 1.3 V and operating temperature of 550 °C [67].
Currently, transition metals, particularly compounds based on Fe, Co, and Ni, have demonstrated remarkable catalytic activity for the OER [79]. A successful method to enhance the OER activity involves altering the surface electronic structure through the addition of supporting materials to the active metal (Fe, Co, or Ni). This strategy has attracted attention, particularly with reference to multi-metal materials such as high-entropy perovskites, because they provide numerous possibilities for modifying the characteristics and improving the catalytic performance [80].
Among multi-metal materials, Co-based double perovskite oxides are notable for their rapid ion diffusion and enhanced surface catalysis, resulting in high electrochemical performance in single cells [35,69,81]. Various studies have explored the application of OER catalysts in PCECs (Table 6). For example, Gd0.3Ca2.7Co3.82Cu0.18O9−δ exhibits the highest current density owing to various factors, including abundant oxygen vacancies in the central Co–O layer of the Ca3Co2O3 rock–salt subsystem, which alters the electronic charge carrier concentration. The needlelike grain morphology aids in the complex flow of the reaction components via triple conduction and open diffusion paths [70].
Despite the promising characteristics of Co-based double perovskite oxides, these high-entropy perovskite oxides have disadvantages such as instability, thermal mismatch, and high cost, which limit their widespread implementation [35,69,81,82]. Thermal mismatch occurs when the OER catalyst and electrolyte materials have different coefficients of thermal expansion (CTE). The CTE indicates the extent to which a material expands when exposed to changes in temperature. If the OER catalyst and electrolyte have significantly different CTE, they may expand at different rates as the temperature changes [83]. Therefore, catalysts with compatible mechanical properties need to be used.
References
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy Storage in Residential and Commercial Buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054.
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber–Bosch Process Revisited: On the Real Structure and Stability of “Ammonia Iron” under Working Conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726.
- Haber, F.; Rossignol, R. Le Über Die Technische Darstellung von Ammoniak Aus Den Elementen. Zeitschrift für Elektrochemie und Angew. Phys. Chemie 1913, 19, 53–72.
- Aika, K.i.; Hori, H.; Ozaki, A. Activation of Nitrogen by Alkali Metal Promoted Transition Metal I. Ammonia Synthesis over Ruthenium Promoted by Alkali Metal. J. Catal. 1972, 27, 424–431.
- IEA Ammonia Technology Roadmap. Available online: https://www.iea.org/reports/ammonia-technology-roadmap (accessed on 4 October 2023).
- Brown, T. Feeding Life 2030: The Vision of Fertilizers Europe. Available online: https://www.ammoniaenergy.org/articles/feeding-life-2030-the-vision-of-fertilizers-europe/ (accessed on 4 October 2023).
- Shipman, M.A.; Symes, M.D. Recent Progress towards the Electrosynthesis of Ammonia from Sustainable Resources. Catal. Today 2017, 286, 57–68.
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for Nitrogen Reduction to Ammonia. Nat. Catal. 2018, 1, 490–500.
- Jeerh, G.; Zhang, M.; Tao, S. Recent Progress in Ammonia Fuel Cells and Their Potential Applications. J. Mater. Chem. A 2021, 9, 727–752.
- Gunduz, S.; Deka, D.J.; Ozkan, U.S. A Review of the Current Trends in High-Temperature Electrocatalytic Ammonia Production Using Solid Electrolytes. J. Catal. 2020, 387, 207–216.
- Liu, F.; Ding, D.; Duan, C. Protonic Ceramic Electrochemical Cells for Synthesizing Sustainable Chemicals and Fuels. Adv. Sci. 2023, 10, e2206478.
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of Electrochemical Ammonia Production Technologies and Materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594.
- Garagounis, I.; Kyriakou, V.; Skodra, A.; Vasileiou, E.; Stoukides, M. Electrochemical Synthesis of Ammonia in Solid Electrolyte Cells. Front. Energy Res. 2014, 2, 1.
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a Green Energy Carrier: Electrochemical Synthesis and Direct Ammonia Fuel Cell—A Comprehensive Review. Fuel Process. Technol. 2022, 235, 107380.
- Juangsa, F.B.; Irhamna, A.R.; Aziz, M. Production of Ammonia as Potential Hydrogen Carrier: Review on Thermochemical and Electrochemical Processes. Int. J. Hydrogen Energy 2021, 46, 14455–14477.
- Medvedev, D. Trends in Research and Development of Protonic Ceramic Electrolysis Cells. Int. J. Hydrogen Energy 2019, 44, 26711–26740.
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical Ammonia Synthesis: Mechanistic Understanding and Catalyst Design. Chem 2021, 7, 1708–1754.
- Trivinho-Strixino, F.; Santos, J.S.; Souza Sikora, M. 3—Electrochemical Synthesis of Nanostructured Materials. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N.B.T.-N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 53–103. ISBN 978-0-323-49782-4.
- Iwahara, H. Oxide-Ionic and Protonic Conductors Based on Perovskite-Type Oxides and Their Possible Applications. Solid State Ionics 1992, 52, 99–104.
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional Power Density and Stability at Intermediate Temperatures in Protonic Ceramic Fuel Cells. Nat. Energy 2018, 3, 202–210.
- Liu, M.; Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7y0.2−XYbXO3−δ. Science 2009, 326, 126–129.
- Zhu, H.; Kee, R.J. Membrane Polarization in Mixed-Conducting Ceramic Fuel Cells and Electrolyzers. Int. J. Hydrogen Energy 2016, 41, 2931–2943.
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly Efficient Reversible Protonic Ceramic Electrochemical Cells for Power Generation and Fuel Production. Nat. Energy 2019, 4, 230–240.
- Han, D.; Liu, X.; Bjørheim, T.S.; Uda, T. Yttrium-Doped Barium Zirconate-Cerate Solid Solution as Proton Conducting Electrolyte: Why Higher Cerium Concentration Leads to Better Performance for Fuel Cells and Electrolysis Cells. Adv. Energy Mater. 2021, 11, 2003149.
- Zhu, J.; Li, X.L.; Wu, C.; Gao, J.; Xu, H.; Li, Y.; Guo, X.; Li, H.; Zhou, W. A Multilayer Ceramic Electrolyte for All-Solid-State Li Batteries. Angew. Chemie Int. Ed. 2021, 60, 3781–3790.
- Brett, D.J.L.; Aguiar, P.; Clague, R.; Marquis, A.J.; Schöttl, S.; Simpson, R.; Brandon, N.P. Application of Infrared Thermal Imaging to the Study of Pellet Solid Oxide Fuel Cells. J. Power Sources 2007, 166, 112–119.
- Feng, W.; Wu, W.; Jin, C.; Zhou, M.; Bian, W.; Tang, W.; Gomez, J.Y.; Boardman, R.; Ding, D. Exploring the Structural Uniformity and Integrity of Protonic Ceramic Thin Film Electrolyte Using Wet Powder Spraying. J. Power Sources Adv. 2021, 11, 100067.
- Skodra, A.; Stoukides, M. Electrocatalytic Synthesis of Ammonia from Steam and Nitrogen at Atmospheric Pressure. Solid State Ionics 2009, 180, 1332–1336.
- Yilmaz, S.; Kavici, B.; Ramakrishnan, P.; Celen, C.; Horri, B.A. Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells. Energies 2023, 16, 4318.
- François, M.; Lescure, V.; Heintz, O.; Combemale, L.; Demoisson, F.; Caboche, G. Synthesis of Y-Doped BaZrO3 Proton Conducting Electrolyte Material by a Continuous Hydrothermal Process in Supercritical Conditions: Investigation of the Formation Mechanism and Electrochemical Performance. Ceram. Int. 2023, 49, 25344–25352.
- Konwar, D.; Yoon, H.H. A Methane-Fueled SOFC Based on a Thin BaZr0.1Ce0.7Y0.1Yb0.1O3−δ Electrolyte Film and a LaNi0.6Co0.4O3 Anode Functional Layer. J. Mater. Chem. A 2016, 4, 5102–5106.
- Fan, Z.; Cao, D.; Zhou, M.; Zhu, Z.; Chen, M.; Liu, J. Barium Cerate-Zirconate Electrolyte Powder Prepared by Carbonate Coprecipitation for High Performance Protonic Ceramic Fuel Cells. Ceram. Int. 2023, 49, 8524–8532.
- Wang, S.; Shen, J.; Zhu, Z.; Wang, Z.; Cao, Y.; Guan, X.; Wang, Y.; Wei, Z.; Chen, M. Further Optimization of Barium Cerate Properties via Co-Doping Strategy for Potential Application as Proton-Conducting Solid Oxide Fuel Cell Electrolyte. J. Power Sources 2018, 387, 24–32.
- Malešević, A.; Radojković, A.; Žunić, M.; Dapčević, A.; Perać, S.; Branković, Z.; Branković, G. Evaluation of Stability and Functionality of BaCe1−xInxO3−δ Electrolyte in a Wider Range of Indium Concentration. J. Adv. Ceram. 2022, 11, 443–453.
- Kane, N.; Luo, Z.; Zhou, Y.; Ding, Y.; Weidenbach, A.; Zhang, W.; Liu, M. Durable and High-Performance Thin-Film BHYb-Coated BZCYYb Bilayer Electrolytes for Proton-Conducting Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2023, 15, 32395–32403.
- Kim, Y.S.; Chang, W.; Jeong, H.J.; Kim, K.H.; Park, H.S.; Shim, J.H. High Performance of Protonic Ceramic Fuel Cells with 1-Μm-Thick Electrolytes Fabricated by Inkjet Printing. Addit. Manuf. 2023, 71, 103590.
- Bae, K.; Lee, S.; Jang, D.Y.; Kim, H.J.; Lee, H.; Shin, D.; Son, J.W.; Shim, J.H. High-Performance Protonic Ceramic Fuel Cells with Thin-Film Yttrium-Doped Barium Cerate-Zirconate Electrolytes on Compositionally Gradient Anodes. ACS Appl. Mater. Interfaces 2016, 8, 9097–9103.
- Kim, D.; Bae, K.T.; Kim, K.J.; Im, H.N.; Jang, S.; Oh, S.; Lee, S.W.; Shin, T.H.; Lee, K.T. High-Performance Protonic Ceramic Electrochemical Cells. ACS Energy Lett. 2022, 7, 2393–2400.
- Lee, K.R.; Tseng, C.J.; Jang, S.C.; Lin, J.C.; Wang, K.W.; Chang, J.K.; Chen, T.C.; Lee, S.W. Fabrication of Anode-Supported Thin BCZY Electrolyte Protonic Fuel Cells Using NiO Sintering Aid. Int. J. Hydrogen Energy 2019, 44, 23784–23792.
- Choi, S.M.; An, H.; Yoon, K.J.; Kim, B.K.; Lee, H.W.; Son, J.W.; Kim, H.; Shin, D.; Ji, H.I.; Lee, J.H. Electrochemical Analysis of High-Performance Protonic Ceramic Fuel Cells Based on a Columnar-Structured Thin Electrolyte. Appl. Energy 2019, 233–234, 29–36.
- Zhong, Z.; Li, Z.; Li, J.; Guo, X.; Hu, Q.; Feng, Y.; Sun, H. A Facile Method to Synthesize BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) Nanopowders for the Application on Highly Conductive Proton-Conducting Electrolytes. Int. J. Hydrogen Energy 2022, 47, 40054–40066.
- Wang, M.; Wu, W.; Lin, Y.; Tang, W.; Gao, G.; Li, H.; Stewart, F.F.; Wang, L.; Yang, Y.; Ding, D. Improved Solid-State Reaction Method for Scaled-Up Synthesis of Ceramic Proton-Conducting Electrolyte Materials. ACS Appl. Energy Mater. 2023, 6, 8316–8326.
- Sari, S.N.; Nieroda, P.; Pasierb, P. The BaCeO3-Based Composite Protonic Conductors Prepared by Spark Plasma Sintering (SPS) and Free-Sintering Methods. Int. J. Hydrogen Energy 2023, 48, 29748–29758.
- Timurkutluk, C.; Timurkutluk, B.; Kaplan, Y. Experimental Optimization of the Fabrication Parameters for Anode-Supported Micro-Tubular Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 23294–23309.
- Ren, C.; Xu, P.; Zhang, Y.; Liu, T. Understanding the Polymer Binder Effect on the Microstructure and Performance of Micro-Tubular Solid Oxide Fuel Cells with Continuously Graded Pores Fabricated by the Phase Inversion Method. Appl. Surf. Sci. 2023, 612, 155928.
- Sun, H.; Zhang, S.; Li, C.; Rainwater, B.; Liu, Y.; Zhang, L.; Zhang, Y.; Li, C.; Liu, M. Atmospheric Plasma-Sprayed BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) Electrolyte Membranes for Intermediate-Temperature Solid Oxide Fuel Cells. Ceram. Int. 2016, 42, 19231–19236.
- Xiao, Y.; Wang, M.; Bao, D.; Wang, Z.; Jin, F.; Wang, Y.; He, T. Performance of Fuel Electrode-Supported Tubular Protonic Ceramic Cells Prepared through Slip Casting and Dip-Coating Methods. Catalysts 2023, 13, 182.
- Hou, M.; Zhu, F.; Liu, Y.; Chen, Y. A High-Performance Fuel Electrode-Supported Tubular Protonic Ceramic Electrochemical Cell. J. Eur. Ceram. Soc. 2023, 43, 6200–6207.
- Oneill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825.
- Yun, D.S.; Joo, J.H.; Yu, J.H.; Yoon, H.C.; Kim, J.N.; Yoo, C.Y. Electrochemical Ammonia Synthesis from Steam and Nitrogen Using Proton Conducting Yttrium Doped Barium Zirconate Electrolyte with Silver, Platinum, and Lanthanum Strontium Cobalt Ferrite Electrocatalyst. J. Power Sources 2015, 284, 245–251.
- Li, C.I.; Matsuo, H.; Otomo, J. Effective Electrode Design and the Reaction Mechanism for Electrochemical Promotion of Ammonia Synthesis Using Fe-Based Electrode Catalysts. Sustain. Energy Fuels 2021, 5, 188–198.
- Kosaka, F.; Nakamura, T.; Otomo, J. Electrochemical Ammonia Synthesis Using Mixed Protonic-Electronic Conducting Cathodes with Exsolved Ru-Nanoparticles in Proton Conducting Electrolysis Cells. J. Electrochem. Soc. 2017, 164, F1323–F1330.
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.H.; Kim, G. Hybrid-Solid Oxide Electrolysis Cell: A New Strategy for Efficient Hydrogen Production. Nano Energy 2018, 44, 121–126.
- Li, W.; Guan, B.; Ma, L.; Hu, S.; Zhang, N.; Liu, X. High Performing Triple-Conductive Pr2NiO4+δ Anode for Proton-Conducting Steam Solid Oxide Electrolysis Cell. J. Mater. Chem. A 2018, 6, 18057–18066.
- Ding, H.; Wu, W.; Jiang, C.; Ding, Y.; Bian, W.; Hu, B.; Singh, P.; Orme, C.J.; Wang, L.; Zhang, Y.; et al. Self-Sustainable Protonic Ceramic Electrochemical Cells Using a Triple Conducting Electrode for Hydrogen and Power Production. Nat. Commun. 2020, 11, 1907.
- Pei, K.; Zhou, Y.; Xu, K.; Zhang, H.; Ding, Y.; Zhao, B.; Yuan, W.; Sasaki, K.; Choi, Y.M.; Chen, Y.; et al. Surface Restructuring of a Perovskite-Type Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nat. Commun. 2022, 13, 2207.
- He, F.; Zhou, Y.; Hu, T.; Xu, Y.; Hou, M.; Zhu, F.; Liu, D.; Zhang, H.; Xu, K.; Liu, M.; et al. An Efficient High-Entropy Perovskite-Type Air Electrode for Reversible Oxygen Reduction and Water Splitting in Protonic Ceramic Cells. Adv. Mater. 2023, 35, e2209469.
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic Ceramic Electrochemical Cells for Hydrogen Production and Electricity Generation: Exceptional Reversibility, Stability, and Demonstrated Faradaic Efficiency. Energy Environ. Sci. 2019, 12, 206–215.
- Saqib, M.; Choi, I.G.; Bae, H.; Park, K.; Shin, J.S.; Kim, Y.D.; Lee, J.I.; Jo, M.; Kim, Y.C.; Lee, K.S.; et al. Transition from Perovskite to Misfit-Layered Structure Materials: A Highly Oxygen Deficient and Stable Oxygen Electrode Catalyst. Energy Environ. Sci. 2021, 14, 2472–2484.




