
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naiying Du | -- | 1931 | 2024-02-01 16:13:24 | | | |
| 2 | Fanny Huang | Meta information modification | 1931 | 2024-02-05 03:28:52 | | |
Video Upload Options
Direct air capture (DAC) is an emerging negative CO2 emission technology that aims to introduce a feasible method for CO2 capture from the atmosphere. Unlike carbon capture from point sources, which deals with flue gas at high CO2 concentrations, carbon capture directly from the atmosphere has proved difficult due to the low CO2 concentration in ambient air. Current DAC technologies mainly consider sorbent-based systems; however, membrane technology can be considered a promising DAC approach since it provides several advantages, e.g., lower energy and operational costs, less environmental footprint, and more potential for small-scale ubiquitous installations. Several recent advancements in validating the feasibility of highly permeable gas separation membrane fabrication and system design show that membrane-based direct air capture (m-DAC) could be a complementary approach to sorbent-based DAC, e.g., as part of a hybrid system design that incorporates other DAC technologies (e.g., solvent or sorbent-based DAC).
1. Introduction
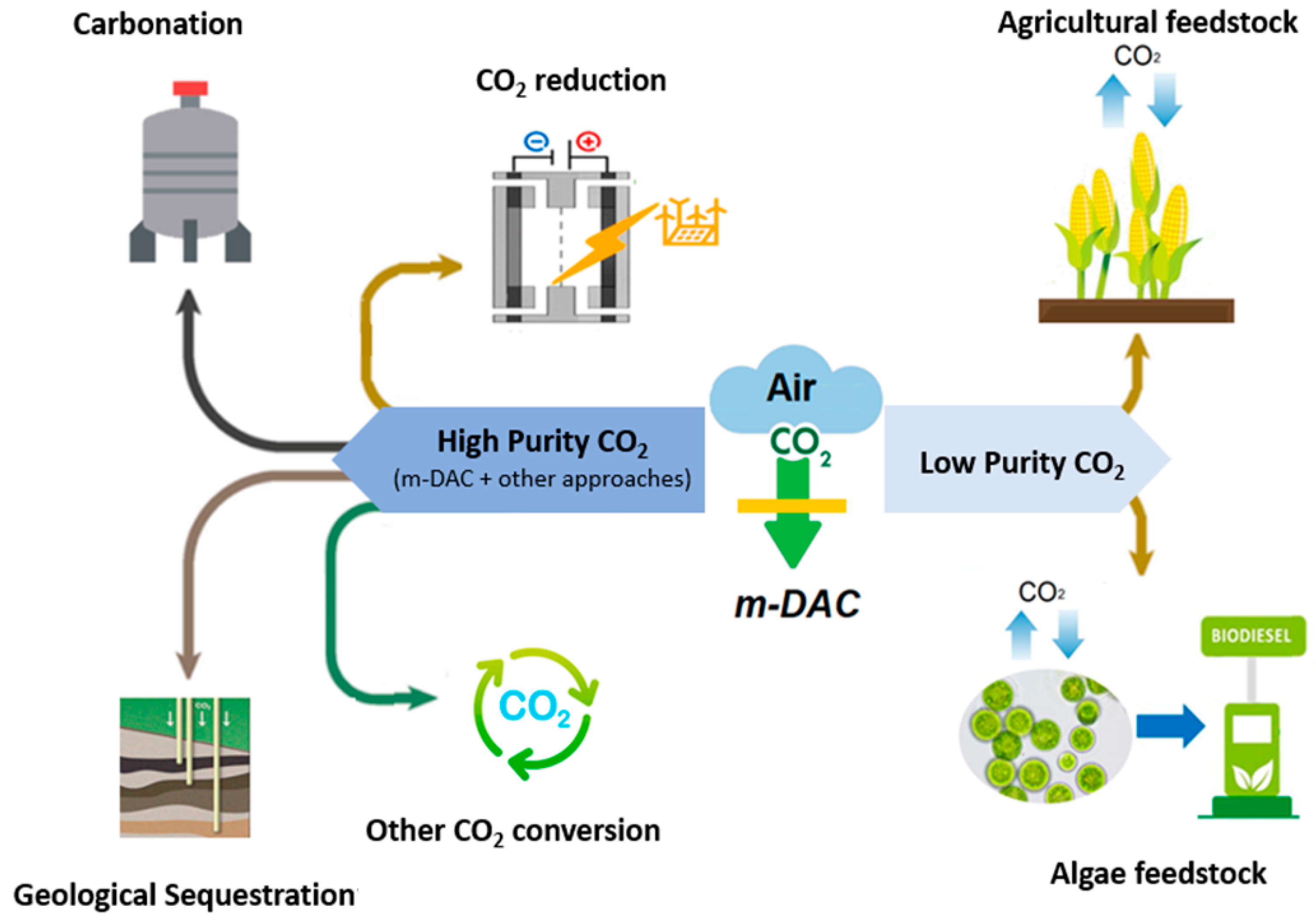
2. Membrane-Based Direct Air Capture Application
References
- Minx, J.C.; Lamb, W.F.; Callghan, M.W.; Fuss, S.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; De Oliveira Garcia, W.; Hartmann, J. Negative emissions-Part1: Research landscape and synthesis. Environ. Res. Lett. 2018, 13, 063001.
- IPCC. 2023: Summary for Policymakers. In Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; 36p, in press.
- National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019.
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331.
- Fujikawa, S.; Selyanchyn, R. Direct air capture by membranes. MRS Bull. 2022, 47, 416–423.
- Chiwaye, N.; Majozi, T.; Daramola, M.O. On optimisation of N2 and CO2-selective hybrid membrane process systems for post-combustion CO2 capture from coal-fired power plants. J. Membr. Sci. 2021, 638, 119691.
- Lackner, K.S.; Brennan, S.; Matter, J.M.; van der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162.
- Shayegh, S.; Bosetti, V.; Tavoni, M. Future Prospects of Direct Air Capture Technologies: Insights from an Expert Elicitation Survey. Front. Clim. 2021, 3, 630893.
- Ozkan, M. Direct air capture of CO2: A response to meet the global climate targets. MRS. Energy Sustain. 2021, 8, 51–56.
- Beuttler, C.; Charles, L.; Wurzbacher, J. The Role of Direct Air Capture in Mitigation of Anthropogenic Greenhouse Gas Emissions. Front. Clim. 2019, 1, 10.
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594.
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A new strategy for membrane-based direct air capture. Polym. J. 2021, 53, 111–119.
- Socolow, R.; Desmond, M.; Aines, R.; Blackstock, J.; Bolland, O.; Kaarsberg, T.; Lewis, N.; Mazzotti, M.; Pfeffer, A.; Sawyer, K.; et al. Direct Air Capture of CO2 with Chemicals: A Technology Assessment for the APS Panel on Public Affairs; American Physical Society: College Park, MD, USA, 2011.
- McQueen, N.; Gomes, K.V.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A review of direct air capture (DAC): Scaling up commercial technologies and innovating for the future. Prog. Energy 2021, 3, 032001.
- Kikkawa, S.; Anamoto, K.; Fujiki, Y.; Hirayama, J.; Kato, G.; Miura, H.; Shishido, T.; Yamazoe, S. Direct Air Capture of CO2 Using a Liquid Amine-Solid Carbamic Acid Phase-Separation System Using Diamine Bearing an Aminocyclohexyl Group. ACS Environ. Au 2022, 2, 354–362.
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct air capture: Process technology, Techno-economic and socio political challanges. Energy Environ. Sci. 2022, 15, 1360–1405.
- Wiegner, J.F.; Grimm, A.; Weimann, L.; Gazzani, M. Optimal Design and Operation of Solid Sorbent Direct Air Capture Processes at Varying Ambient Conditions. Ind. Eng. Res. 2022, 61, 12649–12667.
- Lackner, K.S. A Guide to CO2 Sequestration. Science 2003, 300, 1677–1678.
- Osterloh, F.E. The Low Concentration of CO2 in the Atmosphere Is an Obstacle to a Sustainable Artificial Photosynthesis Fuel Cycle Based on Carbon. ACS Energy Lett. 2016, 1, 1060–1061.
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139.
- IEAGHG. Effecs of Impurities on Geological Storage of CO2; IEAGHG: Cheltenham, UK, 2011.
- Anantharaman, R.; Berstad, D.; Roussanaly, S. Techno-economic Performance of a Hybrid Membrane—Liquefaction Process for Post-combustion CO2 Capture. Energy Procedia 2014, 61, 1244–1247.
- Sreenath, S.; Sam, A.A. Hybrid membrane-cryogenic CO2 capture technologies: A mini-review. Front. Energy Res. 2023, 11, 1167024.
- Belaissaoul, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 415–416, 424–434.
- Zhang, Z.; Pan, S.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 25, 109799.
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867.
- Kim, B.; Ma, S.; Jhong, H.M.; Kenis, P.J. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta 2015, 166, 271–276.
- Dittmeyer, R.; Klumpp, M.; Kant, P.; Ozin, G. Crowd oil not crude oil. Nat. Commun. 2019, 10, 1818.
- Seppänen, O.A.; Fisk, W.J.; Mendell, W.J. Association of Ventilation Rates and CO2 Concentrations with Health and Other Responses in Commercial and Institutional Buildings. Indoor Air 1999, 9, 226–252.





