Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosario Nicoletti | -- | 1943 | 2024-02-01 12:20:40 | | | |
| 2 | Wendy Huang | Meta information modification | 1943 | 2024-02-02 03:36:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nicoletti, R.; Russo, E.; Becchimanzi, A. Cladosporium Entomopathogenicity. Encyclopedia. Available online: https://encyclopedia.pub/entry/54643 (accessed on 13 January 2026).
Nicoletti R, Russo E, Becchimanzi A. Cladosporium Entomopathogenicity. Encyclopedia. Available at: https://encyclopedia.pub/entry/54643. Accessed January 13, 2026.
Nicoletti, Rosario, Elia Russo, Andrea Becchimanzi. "Cladosporium Entomopathogenicity" Encyclopedia, https://encyclopedia.pub/entry/54643 (accessed January 13, 2026).
Nicoletti, R., Russo, E., & Becchimanzi, A. (2024, February 01). Cladosporium Entomopathogenicity. In Encyclopedia. https://encyclopedia.pub/entry/54643
Nicoletti, Rosario, et al. "Cladosporium Entomopathogenicity." Encyclopedia. Web. 01 February, 2024.
Copy Citation
The range of interactions between Cladosporium, a ubiquitous fungal genus, and insects, a class including about 60% of the animal species, is extremely diverse. Conventionally, Cladosporium species are not considered full-right representatives of the guild of entomopathogens, which is generally restricted to specialized fungi such as Beauveria, Metarhizium and Lecanicillium/Akanthomyces. However, like other fungi that are widely associated with crops such as Trichoderma and Talaromyces, the evidence is increasing that Cladosporium may also infect insects and cause epizootics in pest populations or promote plant defense reactions.
interactions
biocontrol
Cladosporium
insects
fungi
plant
1. Introduction
Fungi in the genus Cladosporium (Dothideomycetes, Cladosporiaceae) are ubiquitous and reported from any terrestrial and marine substrate, including all kinds of living organisms [1]. This is linked to their profuse sporulation, which allows the spread of conidia through atmospheric agents over long distances. Thus, the mere isolation of these fungi from plants and animals does not necessarily imply a symbiotic association. However, generality is not a rule, and in many cases, this pervasiveness subtends either occasional or more systematic biotic relationships that influence the fitness of the associated organisms in diverse ways [2][3][4].
Direct observations of the parasitic aptitude of insects are limited and essentially concern the case of C. cladosporioides on the sugarcane white wooly aphid (Ceratovacuna lanigera: Hemiptera, Aphididae); both light and electron microscopy at the host–parasite interface showed that nymphs and adults of the aphid were completely overgrown by the fungal mycelium, which penetrated and disrupted their powdery waxy coating [5].
However, circumstantial evidence of entomopathogenic aptitude in Cladosporium derives from several studies reporting on mortality induced by conidial suspensions administered at various concentrations and exposure times. In this regard, the available data concerning strains that proved to be effective against various targeted pests in experimental trials are summarized in Table 1.
Table 1. Reported effectiveness of conidial suspensions of Cladosporium in inducing mortality on insect pests.
| Cladosporium Species | Source | Insect Targets | Country | Reference |
|---|---|---|---|---|
| C. cladosporioides | Bemisia sp. | Bemisia sp. | Egypt | [6] |
| Brevicoryne brassicae | B. brassicae | Egypt | [7] | |
| Culex quinquefasciatus | C. quinquefasciatus | Iraq | [8] | |
| endophytic | Duponchelia fovealis | Brazil | [9] | |
| Kermes sp. | Hemiberlesia pitysophila | China | [10] | |
| Lycorma delicatula | Tenebrio molitor | USA | [11] | |
| Myzus persicae | M. persicae | Iraq | [12] | |
| Nilaparvata lugens | Bemisia tabaci | Bangladesh | [13] | |
| Pulvinaria aurantii | Aphis fabae | Iran | [14] | |
| Sitophilus oryzae | Rhyzopertha dominica Sitophilus zeamais Trogoderma granarium |
Pakistan | [15] | |
| soil | Metopolophium dirhodum | Egypt | [16] | |
| C. oxysporum | endophytic | A. fabae | Algeria | [17] |
| endophytic | Chilo partellus | India | [18] | |
| Planococcus citri | Pseudococcus longispinus Pulvinaria aethiopica Toxoptera citricida Trioza erytreae |
South Africa | [19] | |
| unknown | Aphis craccivora | India | [20] | |
| C. sphaerospermum | endophytic | D. fovealis | Brazil | [9] |
| Cladosporium sp. | Helicoverpa armigera | Aphis gossypii B. tabaci H. armigera |
Australia | [21] |
| Spodoptera frugiperda | S. frugiperda | China | [22] | |
| Cladosporium spp. | several species of sap-sucking Hemiptera | A. craccivora A. gossypii B. tabaci |
Egypt | [23][24] |
| C. subuliforme | Diaphorina citri | D. citri | China | [25] |
| C. tenuissimum | M. persicae | M. persicae | Iraq | [12] |
| Trachymela sloanei | S. frugiperda | China | [26] | |
| C. uredinicola | A. gossypii B. tabaci |
A. gossypii B. tabaci |
Egypt | [27] |
| Bemisia sp. | Bemisia sp. | Egypt | [6] | |
| C. xanthocromaticum | M. persicae | M. persicae | Iraq | [12] |
Alternatively, the anti-insectan effect can be assessed through the addition of the fungi or their products to the laboratory diet. In this respect, when incorporated in the feed of larvae of the tobacco budworm (Chloridea virescens: Lepidoptera, Noctuidae), an isolate of C. cladosporioides was found to reduce larval and pupal weights by 56% and 7%, respectively; moreover, in preference tests, the caterpillars showed a marked tendency to avoid feed amended with the fungus [28]. Development of another noctuid moth, the tobacco cutworm (Spodoptera litura), was significantly prolonged when larvae were fed on a diet amended with ethyl acetate extract of C. uredinicola at concentrations of 1.25–2.00 μL g−1; moreover, at 2.00 μL g−1, a significantly higher number of adults emerged showing morphological deformities. At higher concentrations, significant reductions in adult emergence, longevity and reproductive potential were recorded. Finally, the toxicity of the ethyl acetate extract was further evidenced by a reduction in feed utilization by the larvae [29].
The ethyl acetate and methylene chloride extracts of a strain of C. cladosporioides were effective against nymphs and adults of the cotton aphid (Aphis gossypii: Hemiptera, Aphididae) [30][31]. Aphicidal effect was also displayed by formulations based on emulsions of culture filtrates of an endophytic strain of C. oxysporum endowed with proteolytic activity, which were more active than conidial suspensions against the black bean aphid (Aphis fabae: Hemiptera, Aphididae) [17]. In a subsequent experiment, formulations based on culture filtrates of this strain and two more endophytic isolates of C. echinulatum and Cladosporium sp. showed activity against the green peach aphid (Myzus persicae: Hemiptera, Aphididae), which increased at increasing concentrations. A significant reduction in the number of colonizing aphids and a relative increase in the number of winged adults were recorded. Moreover, the pretreatment of plants negatively influenced embryonic development, thus affecting fertility [32]. In the same study, consistent chitinolytic activity was determined in the culture filtrates of Cladosporium sp.; indeed, chitinases are considered a main factor in the bioactivity of fungal culture filtrates, as also documented for other strains of Cladosporium spp. [24], C. cladosporioides [12][33], C. tenuissimum and C. xanthocromaticum [12].
Even more, the anti-insectan effects of culture filtrates may depend on the presence of toxic compounds (Figure 1). Fungi in the genus Cladosporium are known as prolific producers of bioactive secondary metabolites [34], some of which have been detected as possible determinants of detrimental effects on insects. This is the case of bassianolide, a cycloligomer depsipeptide identified as a product of a strain related to the C. cladosporioides s.c. [35]. The alkaloid 3-(4β-hydroxy-6-pyranonyl)-5-isopropylpyrrolidin-2-one was identified in the ethyl acetate extracts of another strain of C. cladosporioides displaying aphicidal activity [30]. Another alkaloid, hydroxyquinoline, was identified as the potentially active product in the extracts of a strain of C. subuliforme [25]. The novel compound citreoviridin A was extracted from an isolate of C. herbarum from a marine sponge and found to inhibit the growth of larvae of the cotton leafworm (Spodoptera littoralis: Lepidoptera, Noctuidae) [36]. Chlorogenic acid, purified from an endophytic isolate of C. velox, displayed insecticidal activity by inducing significant mortality in the larvae of S. litura or adversely prolonging their developmental period. This phenolic compound, previously known to cause gut toxicity in lepidopterans [37], was characterized as an α-glucosidase inhibitor, performing a non-competitive type of inhibition in vitro; it also inhibited the activity of α-glycosidases in the gut of the larvae [38][39].
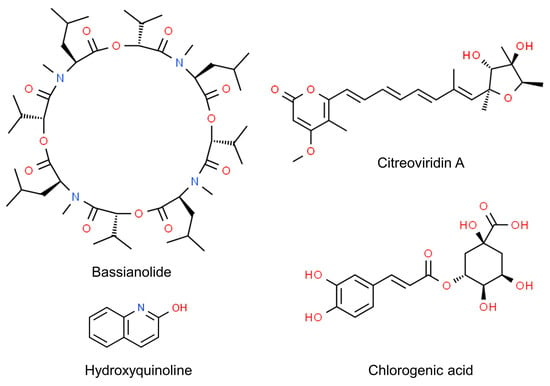
Figure 1. Chemical structure of Cladosporium secondary metabolites displaying anti-insectan effects.
The importance of secondary metabolites for entomopathogenic aptitude in Cladosporium has been further affirmed after a study carried out on strains associated with the Chinese white wax scale (Ericerus pela: Hemiptera, Coccidae). This insect is known to be infected by Cladosporium spp. related to C. sphaerospermum and C. langeronii, which kill the scales after dramatically altering their microbiome [40]. However, the scales were later found to also harbor a non-infective Cladosporium. Genome sequencing showed that the non-infective strain is related to C. cladosporioides and has a larger genome size than a pathogenic one, which is more related to C. sphaerospermum. Particularly, the former has specific genes involved in nutrition pathways that are absent in the pathogen. Conversely, the latter possesses genes participating in the biosynthetic pathways of mycotoxins, such as asperfuranone, emericellamide and fumagillin. These genes were not found in the non-pathogenic strain, which, on the other hand, presented genes associated with reduced virulence [3].
2. Interactions with Biocontrol Agents
Reports on the occurrence of an association with predatory and parasitoid insects introduce the question of whether the insecticidal properties of Cladosporium may also affect the performances of biocontrol agents employed in crop protection. Indeed, this association can be more than merely occasional, considering that Cladosporium were the most abundant fungi detected in the gut of the multicolored Asian lady beetle (Harmonia axyridis: Coleoptera, Coccinellidae) feeding on the pea aphid (Acyrthosiphon pisum: Hemiptera, Aphididae) [41]. Concerning this issue, a previously mentioned strain of Cladosporium sp. from H. armigera was found not to induce significant harmful effects on a panel of beneficial predatory insects, including the red and blue beetle (Dicranolaius bellulus: Coleoptera, Melyridae), the transverse ladybird (Coccinella transversalis: Coleoptera, Coccinellidae), the green lacewing (Mallada signatus: Neuroptera, Chrysopidae) and the damsel bug (Nabis kinbergii: Hemiptera, Nabidae) [21]. Conversely, laboratory assays carried out in Egypt showed that treatment with C. uredinicola affected the biocontrol of the silverleaf whitefly (Bemisia tabaci: Hemiptera, Aleyrodidae) by the eleven-spotted ladybird (Coccinella undecimpunctata: Coleoptera, Coccinellidae) and the parasitoid Eretmocerus mundus (Hymenoptera, Aphelinidae) in various ways. In fact, all larval stages of the coccinellid were sensitive to the fungus and tended to avoid feeding on the infected whiteflies. As for the parasitoid, although mortality of the exposed individuals was low, most females avoided laying eggs on treated nymphs; nevertheless, the combined use of C. uredinicola and E. mundus was found to synergistically increase the suppression of nymphs [42].
Olfactory experiments carried out in the laboratory indicated that the parasitoid wasp Lysiphlebus fabarum (Hymenoptera, Braconidae) can detect cues from aphids (A. fabae) infected by a pathogenic strain of Cladosporium sp. and avoid them; hence, the employment of this strain in the field could not affect the performance of the parasitoid, implying compatibility between these, and possibly more, biological control agents of aphids [14].
3. Plant-Mediated Interactions
In addition to arising after direct contact or ingestion of conidia, the entomopathogenic effects of Cladosporium can also be exerted in planta, as promoted by strains able to develop endophytically. Indeed, it is known that endophytic fungi may improve plant resistance to biotic adversities through various mechanisms, including general effects on fitness and growth promotion eventually exerted in synergistic relationships with other components of the plant microbiome [43][44]. The belief is gaining ground that these valuable properties could be exploited for improving yields while reducing the input of chemicals in crop management [45][46].
Cauliflower plants artificially infected with an endophytic strain of C. uredinicola did not show any disease symptoms, and the vigor of endophyte-infected plants also did not differ from untreated plants. Interestingly, larvae of S. litura feeding on leaves from treated plants were sluggish and underwent significantly higher mortality than the control. Most of the larvae died at the time of molting to the last instar, while the survivors took a significantly longer time to pupate and further suffered significantly higher mortality at the pupal stage. In the end, fewer adults emerged from larvae on endophyte-supplemented plants; some adults exhibited morphological deformities, such as crumpled and unequal wings, and survived for a very short time. Inhibitory effects were also observed on the reproductive potential and the hatchability of eggs. The life span of females that emerged from larvae fed on plants hosting C. uredinicola reduced significantly, while male longevity remained unaffected [47]. All these effects were assumed to depend on physiological changes induced by the endophyte. In fact, further studies disclosed cytotoxic effects on hemocytes of S. litura fed on endophyte-supplemented cauliflower plants, which showed changes in shape, extensive vacuolization and necrosis. Moreover, these abnormalities increased along with the feeding duration and ultimately resulted in adverse consequences on the fitness and survival of the insect [48].
However, it is quite intuitive to consider that plant-mediated relationships should be examined case by case, as the outcome of the interaction is not necessarily unfavorable to the insects. When inoculated in perennial thistle (Cirsium arvense), where it is known to develop endophytically, C. cladosporioides increased feeding of the thistle tortoise beetle (Cassida rubiginosa: Coleoptera, Chrysomelidae), while it had no effect on the cabbage moth (Mamestra brassicae: Lepidoptera, Noctuidae). Nevertheless, dual infection with C. cladosporioides and Trichoderma viride greatly reduced beetle feeding [49]. These findings indicate that the promoting effects of C. cladosporioides, as well as of other endophytes, depend on both the degree of specialization of the herbivore and the species assortment in the plant microbiome, which in turn may induce chemical changes in the host. Undoubtedly, these fungi deserve higher attention in the study of insect–plant interactions, considering that their endophytic occurrence could remarkably influence insect growth and even pest population dynamics.
References
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401.
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but different: The expanding realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74.
- Liu, W.; Yu, S.H.; Zhang, H.P.; Fu, Z.Y.; An, J.Q.; Zhang, J.Y.; Yang, P. Two Cladosporium fungi with opposite functions to the Chinese white wax scale insect have different genome characters. J. Fungi 2022, 8, 286.
- Chen, H.; Chen, J.; Qi, Y.; Chu, S.; Ma, Y.; Xu, L.; Lv, S.; Zhang, H.; Yang, D.; Zhu, Y.; et al. Endophytic fungus Cladosporium tenuissimum DF11, an efficient inducer of tanshinone biosynthesis in Salvia miltiorrhiza roots. Phytochemistry 2022, 194, 113021.
- Kishore Varma, P.; Chandra Sekhar, V.; Bhavani, B.; Upendhar, S. Cladosporium cladosporioides: A new report of parasitism on sugarcane woolly aphid, Ceratovacuna lanigera Zehntner. J. Entomol. Zool. Stud. 2019, 7, 1122–1126.
- Abdel-Baky, N.F.; Arafat, N.S.; Abdel-Salam, A.H. Three Cladosporium spp. as promising biological control candidates for controlling whiteflies (Bemisia spp.) in Egypt. Pak. J. Biol. Sci. 1998, 1, 188–195.
- Ibrahim, H.Y. Biodiversity of entomopathogenic fungi naturally infecting cabbage aphid, Brevicoryne brassicae. L. J. Plant Prot. Pathol. Mansoura Univ. 2017, 8, 631–634.
- Turky, W.; Hassoon, A.; Al-Taee, M. Isolation and diagnosis of fungi associated with the larvae of the mosquitoes Culex quinquefasciatus and its biological control. J. Multidiscip. Engin. Sci. Technol. 2018, 5, 9028–9035.
- Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.; Dalzoto, P.; Zawadeneak, M.A.; Pimentel, I.C. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller) (Lepidoptera: Crambidae). Braz. J. Biol. 2017, 78, 429–435.
- Pan, W.Y.; Chen, S.L.; Lian, J.H.; Qiu, H.Z.; Lan, G. A preliminary report on control of Hemiberlesia pitysophila using Cladosporium cladosporioides. Forest Pest Dis. 1989, 3, 22–23.
- Adam, J.; Overton, B. Forgotten fungi that could be used to control the spread of the spotted lanternfly (Hemiptera: Fulgoridae). Fungi 2023, 15, 41–50.
- Al-Shindah, R.S.; Hassan, A.A.; Mansour, M.S. Isolation and identification of entomopathogenic fungi from of green peach aphid Myzus persicae and evaluation of their activity for insect control. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1060, p. 012093.
- Islam, T.; Gupta, D.R.; Surovy, M.Z.; Mahmud, N.U.; Mazlan, N.; Islam, T. Identification and application of a fungal biocontrol agent Cladosporium cladosporioides against Bemisia tabaci. Biotechnol. Biotechnol. Equipment 2019, 33, 1698–1705.
- Mousavi, K.; Rajabpour, A.; Parizipour, M.H.G.; Yarahmadi, F. Biological and molecular characterization of Cladosporium sp. and Acremonium zeylanicum as biocontrol agents of Aphis fabae in a tri-trophic system. Entomol. Exp. Appl. 2022, 170, 877–886.
- Khan, H.A.A.; Khan, T. Efficacy of entomopathogenic fungi against three major stored insect pests, Rhyzopertha dominica, Sitophilus zeamais and Trogoderma granarium. J. Stored Prod. Res. 2023, 104, 102188.
- Ouidad, A.; Senoussi, M.M.; Oufroukh, A.; Birgücü, A.K.; Karaca, İ.; Kouadri, F.; Bensegueni, A. Pathogenicity of three entomopathogenic fungi, to the aphid species, Metopolophium dirhodum (Walker) (Hemiptera: Aphididae), and their alkaline protease activities. Egypt. J. Biol. Pest Control 2018, 28, 24.
- Bensaci, O.A.; Daoud, H.; Lombarkia, N.; Rouabah, K. Formulation of the endophytic fungus Cladosporium oxysporum Berk. and MA Curtis, isolated from Euphorbia bupleuroides subsp. luteola, as a new biocontrol tool against the black bean aphid (Aphis fabae Scop.). J. Plant Prot. Res. 2015, 55, 80–87.
- Renuka, S.; Ramanujam, B. Fungal endophytes from maize (Zea mays L.): Isolation, identification and screening against maize stem borer, Chilo partellus (Swinhoe). J. Pure Appl. Microbiol. 2016, 10, 523–529.
- Samways, M.J. Interrelationship between an entomogenous fungus and two ant-homopteran (Hymenoptera: Formicidae-Hemiptera: Pseudococcidae & Aphididae) mutualisms on guava trees. Bull. Entomol. Res. 1983, 73, 321–331.
- Saranya, S.; Ushakumari, R.; Jacob, S.; Philip, B.M. Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J. Biopesticides 2010, 3, 138–142.
- Bahar, M.H.; Backhouse, D.; Gregg, P.C.; Mensah, R. Efficacy of a Cladosporium sp. fungus against Helicoverpa armigera (Lepidoptera: Noctuidae), other insect pests and beneficial insects of cotton. Biocontrol Sci. Technol. 2011, 21, 1387–1397.
- Idrees, A.; Afzal, A.; Qadir, Z.A.; Li, J. Virulence of entomopathogenic fungi against fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. Front. Physiol. 2023, 14, 1107434.
- Abdel-Baky, N.F.; Abdel-Salam, A.H. Natural incidence of Cladosporium spp. as a bio-control agent against whiteflies and aphids in Egypt. J. Appl. Entomol. 2003, 127, 228–235.
- Abdel-Baky, N.F.; Fadaly, H.A.; EI-Nagar, M.E.; Arafat, N.S.; Abd-Allah, R.H. Virulence and enzymatic activities of some entomopathogenic fungi against whiteflies and aphids. J. Agric. Sci. Mansoura Univ. 2005, 30, 1153–1167.
- Wang, N.; Zhang, S.; Li, Y.J.; Song, Y.Q.; Lei, C.Y.; Peng, Y.Y.; Wang, J.J.; Lou, B.H.; Jiang, H.B. Novel isolate of Cladosporium subuliforme and its potential to control Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae). Egypt. J. Biol. Pest Control 2023, 33, 37.
- Idrees, A.; Qadir, Z.A.; Akutse, K.S.; Afzal, A.; Hussain, M.; Islam, W.; Waqas, M.S.; Bamisile, B.S.; Li, J. Effectiveness of entomopathogenic fungi on immature stages and feeding performance of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Insects 2021, 12, 1044.
- Ragab, M.; Abdel-Baky, N.F. Cladosporium uredinicola Speg. and Alternaria infectoria EG Simmons, as promising biocontrol agents for Bemisia argentifolii Bellows & Perring and Aphis gossypii Glov. on tomatoes. J. Agric. Sci. Mansoura Univ. 2004, 29, 5897–5906.
- Abbas, H.K.; Mulrooney, J.E. Effect of some phytopathogenic fungi and their metabolites on growth of Heliothis virescens (F.) and its host plants. Biocontrol Sci. Technol. 1994, 4, 77–87.
- Thakur, A.; Singh, V.; Kaur, A.; Kaur, S. Insecticidal potential of an endophytic fungus, Cladosporium uredinicola, against Spodoptera litura. Phytoparasitica 2013, 41, 373–382.
- Shaker, N.O.; Ahmed, G.M.M.; El-Sayed Ibrahim, H.Y.; El-Sawy, M.M.; El-Hoseiny Mostafa, M.; Abd El-Rahman Ismail, H.N. Secondary metabolites of the entomopathogenic fungus, Cladosporium cladosporioides and its relation to toxicity of cotton aphid, Aphis gossypii Glov.). Int. J. Entomol. Nematol. 2019, 5, 115–120.
- Shaker, N.O.; Ahmed, G.M.M.; El-Sawy, M.M.; El-Sayed Ibrahim, H.Y.; Abd El-Rahman Ismail, H.N. Isolation, characterization and insecticidal activity of methylene chloride extract of Cladosporium cladosporioides secondary metabolites against Aphis gossypii (Glov.). J. Plant Prot. Pathol. 2019, 10, 115–119.
- Bensaci, O.A.; Rouabah, K.; Aliat, T.; Lombarkia, N.; Plushikov, V.G.; Kucher, D.E.; Dokukin, P.A.; Temirbekova, S.K.; Rebouh, N.Y. Biological pests management for sustainable agriculture: Understanding the influence of Cladosporium-bioformulated endophytic fungi application to control Myzus persicae (Sulzer, 1776) in potato (Solanum tuberosum L.). Plants 2022, 11, 2055.
- Badran, R.A.; Aly, M.Z.Y. Studies on the mycotic inhabitants of Culex pipiens collected from fresh water ponds in Egypt. Mycopathologia 1995, 132, 105–110.
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 26, 3959.
- Mousavi, K.; Rajabpour, A.; Parizipour, M.H.G.; Yarahmadi, F. Insecticidal bioactive compounds derived from Cladosporium cladosporioides (Fresen.) GA de Vries and Acremonium zeylanicum (Petch) W. Gams & HC Evans. J. Plant Dis. Prot. 2023, 130, 543–549.
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733.
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compoundsin plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994, 24, 943–953.
- Singh, B.; Kaur, T.; Kaur, S.; Manhas, R.K.; Kaur, A. An alpha-glucosidase inhibitor from an endophytic Cladosporium sp. with potential as a biocontrol agent. Appl. Biochem. Biotechnol. 2015, 175, 2020–2034.
- Singh, B.; Kaur, T.; Kaur, S.; Manhas, R.K.; Kaur, A. Insecticidal potential of an endophytic Cladosporium velox against Spodoptera litura mediated through inhibition of alpha glycosidases. Pestic. Biochem. Physiol. 2016, 131, 46–52.
- Sun, T.; Wang, X.Q.; Zhao, Z.L.; Yu, S.H.; Yang, P.; Chen, X.M. A lethal fungus infects the Chinese white wax scale insect and causes dramatic changes in the host microbiota. Sci. Rep. 2018, 8, 5324.
- Huang, Z.; Zhu, L.; Lv, J.; Pu, Z.; Zhang, L.; Chen, G.; Hu, X.; Zhang, Z.; Zhang, H. Dietary effects on biological parameters and gut microbiota of Harmonia axyridis. Front. Microbiol. 2021, 12, 818787.
- Abdel-Baky, N.F. Interactions among the entomopathogenic fungus, Cladosporium uridenicolla Speg., and two whiteflies beneficial insects, Eretmocerus mundus Mercert and Coccienlla undecimpunctata L.: From an IPM prospective. J. Agric. Sci. Mansoura Univ. 2005, 30, 4217–4236.
- Shankar Naik, B. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis 2019, 79, 99–115.
- Nicoletti, R.; Beccaro, G.L.; Sekara, A.; Cirillo, C.; Di Vaio, C. Endophytic fungi and ecological fitness of chestnuts. Plants 2021, 10, 542.
- Bamisile, B.S.; Afolabi, O.G.; Siddiqui, J.A.; Xu, Y. Endophytic insect pathogenic fungi-host plant-herbivore mutualism: Elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 2023, 39, 326.
- Picciotti, U.; Araujo Dalbon, V.; Ciancio, A.; Colagiero, M.; Cozzi, G.; De Bellis, L.; Finetti-Sialer, M.M.; Greco, D.; Ippolito, A.; Lahbib, N.; et al. “Ectomosphere”: Insects and microorganism interactions. Microorganisms 2023, 11, 440.
- Thakur, A.; Kaur, S.; Kaur, A.; Singh, V. Enhanced resistance to Spodoptera litura in endophyte infected cauliflower plants. Environ. Entomol. 2013, 42, 240–246.
- Thakur, A.; Singh, V.; Kaur, A.; Kaur, S. Suppression of cellular immune response in Spodoptera litura (Lepidoptera: Noctuidae) larvae by endophytic fungi Nigrospora oryzae and Cladosporium uredinicola. Ann. Entomol. Soc. Am. 2014, 107, 674–679.
- Gange, A.C.; Eschen, R.; Wearn, J.A.; Thawer, A.; Sutton, B.C. Differential effects of foliar endophytic fungi on insect herbivores attacking a herbaceous plant. Oecologia 2012, 168, 1023–1031.
More
Information
Subjects:
Entomology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
693
Revisions:
2 times
(View History)
Update Date:
02 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




