
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Feroci | + 1534 word(s) | 1534 | 2020-04-08 09:52:45 | | | |
| 2 | Rita Xu | Meta information modification | 1534 | 2020-04-10 10:49:08 | | | | |
| 3 | Rita Xu | -12 word(s) | 1522 | 2020-10-29 09:46:54 | | | | |
| 4 | Rita Xu | Meta information modification | 1522 | 2020-10-29 09:47:35 | | |
Video Upload Options
The possibility of using an IL to enhance WO3 dispersion on an inorganic support, ensuring the formation of composites with a very large number of exposed catalytic sites, is reported. In addition, in some cases the IL was demonstrated to be able to direct the morphology of WO3 particles.
1. WO3 and Ionic Liquids in Fuel Desulfurization
One of the main problems in fuel combustion (beside CO2 formation) is the production of polluting and toxic gases, such as SO2 and SO3. In particular, naphtha shows the highest sulfur and olefine content, with a high octane number. The target of the desulfurization process is thus the elimination of the highest possible sulfur amount, while preserving a high olefine content (maintaining a high octane rating), in order to have good fuel performances during combustion, lowering to a minimum level the emissions of sulfurated compounds.
Of the organic sulfur compounds present in petroleum derivatives, thiols, sulfides, disulfides and thiophenes are among the most important (Figure 1).
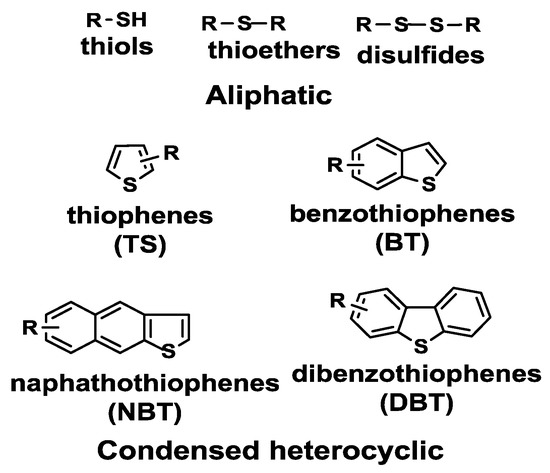
Figure 1. Main sulfur-containing compounds in petroleum fuels. Reproduced with permission [[1]]. Copyright 2016, Elsevier.
Regarding the available methods to carry out liquid fuel desulfurization, hydrodesulfurization (HDS) and oxidative desulfurization (ODS) are among the most used.
1.1. WO3 and Ionic Liquids in Hydrodesulfurization (HDS)
Hydrodesulfurization (HDS) is the removal of sulfur compounds from liquid petroleum derivatives by reaction with molecular hydrogen at a high temperature (300–350 °C). High pressures of H2 are needed for this reaction (20–100 atm), and sulfur compounds are transformed into H2S and hydrocarbons in the presence of catalysts. H2S is at the end removed from the reaction mixture and transformed into elemental sulfur [1]. This process is quite efficient to remove aliphatic compounds (thiols, sulfides, disulfides), while thiophene derivatives are quite recalcitrant, needing more drastic conditions, which influence the cost of the process, among other things. The difficulty of removing thiophenes seems to be related to a lower adsorption on the catalyst surface.
ILs can play an important role in catalyst synthesis, in order to obtain compounds with improved performances. To this regard, it should be remembered that HDS catalysts promote both sulfur transformation into H2S and olefine saturation, which is a highly undesired side reaction (lowering the octane number of the produced fuel). Daage and Chianelli [2] and Topsøe [3] elaborated a model to understand the selectivity of desulfuration over olefine saturation using supported metal sulfide catalysts (the brim-edge model). In particular, the brim sites of a multistack metal sulfide HDS catalyst (the top and bottom layers) catalyze both hydrodesulfuration and olefine saturation, while the edge sites catalyze only hydrodesulfuration. The conclusion is that a good HDS catalyst contains a high edge to brim ratio, and this ratio can be in part controlled by controlling the interactions between the catalyst and its support (necessary to disperse the metal catalyst, enhancing its surface). Such an interaction should be a compromise in order to have good metal dispersion, minimizing side reactions. Moreover, the incorporation of a “promoting element” (usually Co or Ni) can increase the number of edge sites.
Bao, Yuan and coworkers reported that the use of an IL, tetraethylammonium bromide (TEAB), in an aqueous solution at room temperature allowed an organic-inorganic nanocomposite (TEA2W6O19) to be formed [4]. The characteristic of such a composite was its core-shell structure (W6O19= core and TEA+ shell), which led to monodispersion, useful to deposit it onto alumina (the support) in the presence of a Ni promoter. The amount of dispersed Ni, along with the kind of dispersion, created catalyst structures with different edge to brim ratios, as evidenced in Figure 2 For a useful comparison, a catalyst obtained using the conventional impregnation method is also reported (Figure 2d).
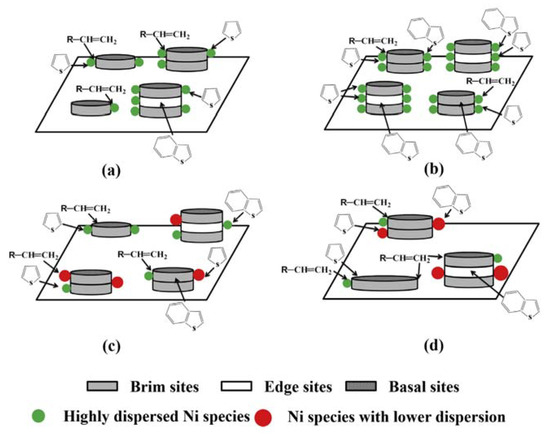
Figure 2. Schematic representation of different supported Ni promoted WO3/Al2O3 catalysts. NiO content: (a) < 4.8 wt.%, (b) 4.8 wt.%, (c) > 4.8 wt.%, (d) 4.8 wt.% (impregnation method). Reproduced with permission [4]. Copyright 2015, Elsevier.
The catalysts thus obtained, with a compromise between metal dispersion and stacking, yielded enhanced performances, of which the improvement of HDS selectivity, minimizing olefine saturation, was of noteworthy importance. The ability of ILs to induce the formation of particular structures (in this case a core-shell one) allowed the improvement of the catalyst performances.
1.2. WO3 and Ionic Liquids in Oxidative Desulfurization (ODS)
As previously said, thiophene derivatives are less prone to HDS reaction. In order to improve thiophenes abatement, while maintaining acceptable process costs, oxidative desulfurization (ODS) can be considered a promising method, due to its simplicity and high efficiency [1][5][6]. ODS is the chemical oxidation of sulfur compounds in liquid fuels (using as an example H2O2 as oxidant), yielding products which can be easily removed from the reaction mixture using a non-miscible solvent. In this regard, ILs can be efficiently used as extractive solvents of both starting and oxidized sulfur compounds and can be considered “greener” alternatives to conventional volatile organic compounds (VOCs).
As an example, Li and coworkers efficiently carried out the oxidative desulfurization of fuel using H2O2 as the oxidant agent in the presence of a WO3/C composite catalyst [5]. The extraction of sulfur compounds was carried out using an imidazolium IL (1-ethyl-3-methylimidazolium ethyl sulfate), added to the fuel as a non-miscible solvent (biphasic reaction medium). The WO3/C composite was oxidized to the complex H2[W2O3(O2)4(H2O)2]2 in the IL phase, which also extracted from fuel the aromatic sulfur compounds; the complex then oxidized dibenzothiophene (DBT) to its sulfone (DBTO2), which remained in the IL phase, allowing an easy separation. The same process could be carried out using 1-butyl-3-methylimidazolium tetrafluoroborate as DBTO2 extraction solvent [6].
In addition, Zhu, Li and coworkers reported the ability of an imidazolium IL (C16MImBr) to direct the synthesis of a WO3-SiO2 composite towards a mesoporous material (W-SiO2-20, Figure 3), which exhibited a high dispersion of tungsten throughout the structure (enhancing the catalytic activity) [7]. The synthesis of the mesoporous catalyst was carried out starting from a polyoxometalate compound ([C16mim]3PW12O40) in a one-pot gel of tetraethyl orthosilicate, which was then calcinated at 550 °C.
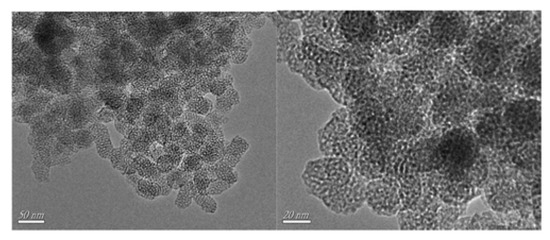
Figure 3. TEM images of the W-SiO2-20 catalyst. Reproduced with permission [7]. Copyright 2016, Elsevier.
The mesoporous catalyst was characterized using the usual techniques and efficiently used in ODS reactions (Figure 4) at the low temperature of 60 °C. The reaction times were quite short and very good yields were obtained after only 30 min. Moreover, the process did not require additional organic solvents as extractants.
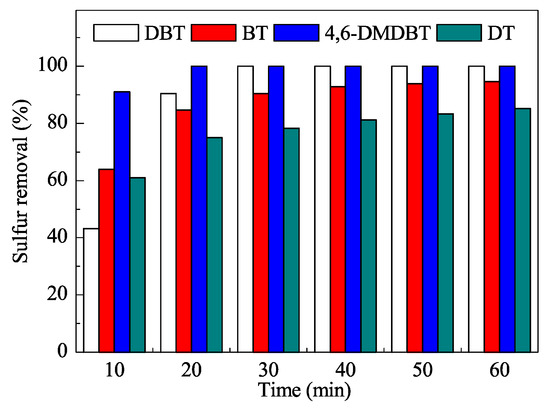
Figure 4. Removal of sulfur compounds in oxidative desulfurization (ODS) with the W-SiO2-20 catalyst. DBT: dibenzothiophene; BT: benzothiophene; 4,6-DMDBT: 4,6-dimethyldibenzothiophene; DT: 1-dodecanethiol. Reproduced with permission [7]. Copyrights 2016, Elsevier.
The same authors reported a similar synthesis, utilizing a different support (in this case mesoporous ZrO2) evidencing as both IL and calcination temperature, influenced the morphology and the dispersion of WO3 [8]. The best obtained catalyst (calcinated at 700 °C, using a C16-ammonium IL, 700-C16-WO3/ZrO2) performed very well in oxidation desulfurization. Dibenzothiophene (DBT) could be completely oxidized to DBT sulfone (DBTO2). Moreover, the catalyst could be recycled ten times with very low efficiency loss.
A functional IL ([(C16H33)2N(CH3)2]2W2O11) acted as WO3 nanoparticle precursor and a large surface area (203 m2/g) few-layer g-C3N4 support was used to disperse them, yielding a supported catalyst [9]; this composite was characterized using SEM (Figure 5), TEM, FT-IR, XRD and XPS, showing highly dispersed nanoparticles. The analysis showed that during the synthetic process of WO3 dispersion on the support, the structure of few-layer g-C3N4 was not destroyed (Figure 5D vs C), leading to a WO3 catalyst with a very high surface, not obtainable using pure WO3, whose structure showed agglomerates (Figure 5A).
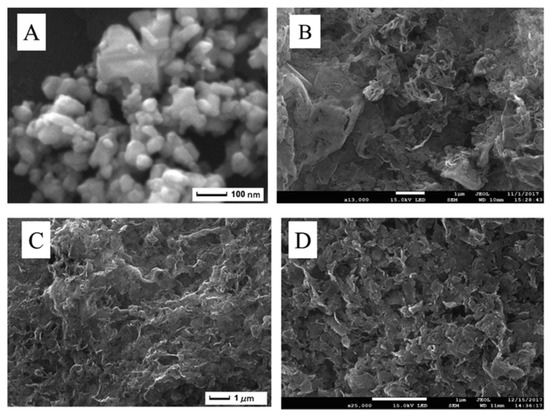
Figure 5. SEM images of (A) WO3, (B) bulk g-C3N4, (C) few-layer g-C3N4, (D) WO3/few-layer g-C3N4. Reproduced with permission [9]. Copyright 2019, Elsevier.
The enormous amount of exposed active sites rendered the composite an excellent catalyst in ODS processes, with the removal of 100% refractory sulfur-containing molecules at 50 °C in 1 h. Moreover, the catalyst was recycled up to six times without efficiency loss. A possible reaction mechanism is depicted in Figure 6.
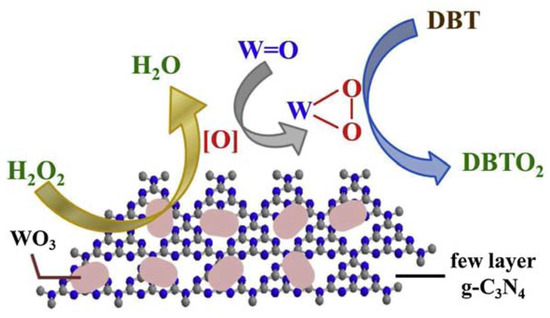
Figure 6. Possible ODS mechanism using WO3/few-layer g-C3N4 as catalyst. Reproduced with permission [9]. Copyright 2019, Elsevier.
Last, tungsten trioxide-carbon nanotubes composite (WO3/CNT) was demonstrated to be a very good catalyst in ODS of recalcitrant aromatic sulfur compounds, as reported by Li and coworkers [10]. The catalyst synthesis was quite easy and was carried out in the presence of an imidazolium IL (C16MImCl), using phosphotungstic acid (HPW) as tungsten source (Figure 7).
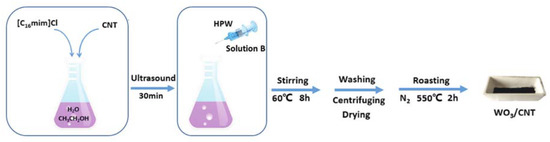
Figure 7. Schematic description of WO3/CNT catalyst preparation. Reproduced with permission [10]. Copyright 2019, Wiley and Society of Chemical Industry.
The characterization of such a composite showed that the IL played a crucial role in determining the dispersion degree and the crystal phase of WO3 on the carrier (carbon nanotube, CNT). In fact, the IL improved the transformation of tungsten trioxide from monoclinic to tetragonal, inhibiting at the same time the growth of metal oxide grains. In this way, high WO3 dispersion was obtained, enhancing the catalytic activity. Moreover, a comparison of catalytic activity of different supported tungsten oxide forms was carried out, demonstrating the following activity order in sulfur oxidative removal: tetrahedral > tetragonal > monoclinic.
References
- Abdul Waheed Bhutto; Rashid Abro; Shurong Gao; Tauqeer Abbas; Xiaochun Chen; Guangren Yu; Oxidative desulfurization of fuel oils using ionic liquids: A review. Journal of the Taiwan Institute of Chemical Engineers 2016, 62, 84-97, 10.1016/j.jtice.2016.01.014.
- M. Daage; R.R. Chianelli; Structure-Function Relations in Molybdenum Sulfide Catalysts: The "Rim-Edge" Model. Journal of Catalysis 1994, 149, 414-427, 10.1006/jcat.1994.1308.
- Henrik Topsøe; The role of Co–Mo–S type structures in hydrotreating catalysts. Applied Catalysis A: General 2007, 322, 3-8, 10.1016/j.apcata.2007.01.002.
- Shufeng Shan; Pei Yuan; Wei Han; Gang Shi; Xiaojun Bao; Supported NiW catalysts with tunable size and morphology of active phases for highly selective hydrodesulfurization of fluid catalytic cracking naphtha. Journal of Catalysis 2015, 330, 288-301, 10.1016/j.jcat.2015.06.019.
- Rongxiang Z.; Xiuping L.; Jianxun S.; Weiwei S., X.G.; Preparation of WO3/C Composite and Its Application in Oxidative Desulfurization of Fuel.. China Pet. Process. Petrochem. Technol. 2017, 19, 65.
- Xiuping L.; R.Z.; Chunfeng M.; Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil.. China Pet. Process. Petrochem. Technol. 2019, 21, 36.
- Ming Zhang; Wenshuai Zhuc; Hongping Li; Suhang Xun; Meng Li; Yanan Li; Yanchen Wei; Huaming Li; Fabrication and characterization of tungsten-containing mesoporous silica for heterogeneous oxidative desulfurization. Chinese Journal of Catalysis 2016, 37, 971-978, 10.1016/s1872-2067(15)61103-2.
- Suhang Xun; Caozheng Hou; Hongping Li; Minqiang He; Ruliang Ma; Ming Zhang; Wenshuai Zhuc; Huaming Li; Synthesis of WO3/mesoporous ZrO2 catalyst as a high-efficiency catalyst for catalytic oxidation of dibenzothiophene in diesel. Journal of Materials Science 2018, 53, 15927-15938, 10.1007/s10853-018-2720-7.
- Ruliang Ma; Jieru Guo; Nghui Wang; Minqiang He; Suhang Xun; Jinyang Gu; Wenshuai Zhuc; Huaming Li; Preparation of highly dispersed WO3/few layer g-C3N4 and its enhancement of catalytic oxidative desulfurization activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 572, 250-258, 10.1016/j.colsurfa.2019.04.006.
- Cheng Wang; Airong Li; Jingfan Xu; Jie Wen; Hui Zhang; Lianhong Zhang; Preparation of WO 3 /CNT catalysts in presence of ionic liquid [C 16 mim]Cl and catalytic efficiency in oxidative desulfurization. Journal of Chemical Technology & Biotechnology 2019, 94, 3403-3412, 10.1002/jctb.6154.




