Vanillin, one of the most widely used and appreciated flavoring agents worldwide, is the main constituent of vanilla bean extract, obtained from the seed pods of various members belonging to the Orchidaceae family. Due to the great demand in the food confectionery industry, as well as in the perfume industry, medicine, and more, the majority of vanillin used today is produced synthetically, and only less than one percent of the world’s vanilla flavoring market comes directly from the traditional natural sources. The increasing global demand for vanillin requires alternative and overall sustainable new production methods, and the recovery from biobased polymers, like lignin, is an environmentally friendly alternative to chemical synthesis.
1. Introduction
Vanillin (4-hydroxy-3-methoxybenzaldehyde, CAS Number 121-33-5) is an aromatic aldehyde with different functional groups, such as carbonyl, ether and aromatic alcohol (see Figure 1). It is a white solid, is soluble in water and constitutes the most important aroma component present in natural vanilla, providing its sweet and creamy odor.
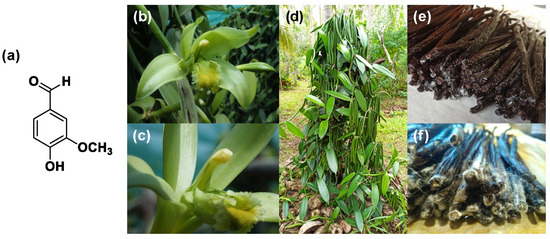
Figure 1. (a) Vanillin molecular structure; (b) Vanilla Planifolia Orchidacea after pollination; (c) vanilla Orchidacea after pollination flower details; (d) vanilla plant; (e) vanilla “frosted” pods; (f) vanilla crystallization details (original pictures kindly provided by Julien Pascal, New Caledonia).
The vanilla plant is originally from Mexico and is a tropical orchid of the Orchidaceae family, which includes more than 100 different species. As far as vanillin extraction is concerned, only three sources are relevant:
Vanilla planifolia,
Vanilla pompona and
Vanilla tahitensis [1]. In particular, the first one is the most cultivated by the food industries because of its pod quality and yield, whereas the last one is the rarest and therefore the most expensive
[2][3].
Vanillin is the key constituent of natural vanilla flavoring whose fragrance profile is composed of more than 200 components, and it is one of the most diffused and expensive flavors around the world after saffron, with a widespread use not only in the food and beverage industry, but also in the pharmaceutical industry as masking agents, fragrances and cosmetic sectors
[4]. Recently, the bioactive properties of vanillin such as neuroprotection and antioxidant, anti-inflammatory and anticarcinogenic activities have gained attention and increased its possible applications
[5]. Moreover, vanillin has demonstrated great potential as a building block for polymer preparation and, in particular, for those which are composed of aromatic moieties
[6][7].
2. Types of Vanillin
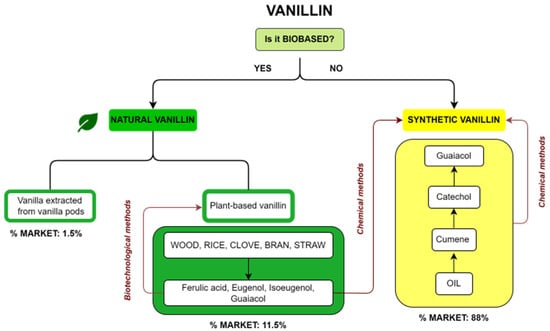
Commercial vanillin can be categorized on the basis of its origin. A summary diagram of the main types of commercial vanillin including sources and market share is reported in Figure 2. The first question about vanillin production is whether it is a biobased product or not. In the first case, vanillin is defined as natural when the source is vanilla extract or vanilla pods or when it is a plant-based vanillin coming from the biotechnological treatment of compounds such as ferulic acid, eugenol, isoeugenol and guaiacol, obtained from natural fonts such as wood, rice, cloves or straw. On the other hand, if vanillin is not biobased, it is a synthetic vanillin obtained via chemical methods from products recovered from oil or from plant-based precursors. The market share for the different types of vanillin is reported in Figure 2. Synthetic vanillin accounts for 88% of the global market, whereas natural vanillin from pods accounts for only 1.5% and plant-based vanillin 11.5%.
Figure 2. Diagram of the different types of vanillin, their sources and their respective market share.
2.1. Natural Vanillin
Current legislation aims to define what is considered “natural” based on the method of production. The criteria for naturalness may vary from state to state. In the past, flavors have been divided into three classes: natural flavors (extracted from natural sources or prepared from natural precursors using natural methods), nature-identical flavors (flavors produced via synthesis but chemically identical to natural ones), and artificial flavors (flavors produced via synthesis and not present in nature). Some countries, such as India and Brazil, still follow this classification. However, the natural flavoring industry is mainly influenced by the legislation of the United States and the European Union, which follow the international CODEX classification system. According to the Codex Guidelines for Flavoring CAC-GL 66/2008, natural flavoring substances are obtained through physical processes, such as distillation and solvent extraction, or enzymatic and microbiological processes from plant or animal material. These substances may be in their natural state or processed by traditional food preparation methods like drying, roasting, and fermentation. On the other hand, synthetic flavoring substances are formed through chemical synthesis. The European Union also classifies flavors into two categories: natural flavors and flavors. The reference legislation for this is Regulation (EC) No. 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavorings and certain food ingredients with flavoring properties for use in and on foods and amending Council Regulation (EEC) No. 1601/91, Regulations (EC) No. 2232/96 and (EC) No. 110/2008 and Directive 2000/13/EC. (Official Journal of European Union 2008, L 354/34). According to Article 3, paragraph c of this regulation, a “natural flavoring substance” is obtained through appropriate physical, enzymatic or microbiological procedures from material of vegetal, animal or microbiological origin. This means that natural flavoring substances are those that are typically present and identified in nature or are produced from natural precursors through natural methods.
Natural vanillin comprises both the vanillin extracted from the traditional source (e.g.,
V. planifolia) and the vanillin that is obtained starting from biological sources using selected processes that in several countries are considered, from a regulatory point of view, analogues to biological processes.
2.1.1. Extraction from Vanilla Pods
Natural vanillin is recovered from the pod of a tropical orchid, especially the
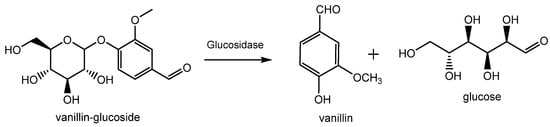
Vanilla planifolia, and is mostly produced in Indonesia, Madagascar, China and, to a lower extent, in Réunion, Guadeloupe, Turkey and Comores. Inside the green bean, vanillin is present as vanillin glucoside which is enzymatically hydrolyzed in vanillin and glucose during the curing process as shown in
Figure 3 [8]. This process allows for the release of various vanilla flavor components with the ratio of vanillin accounting for 20 g/kg of vanilla beans. Thanks to this procedure, the resulting vanillin can be used without restriction for edible and pharmaceutical purposes and can be labelled as “natural vanilla flavor”. In this case, due to the high cost of this process, the price of natural vanillin can range from USD 1200/kg to more than USD 4000/kg (2023).
Figure 3. Production of natural vanillin from vanillin glucoside extracted from Vanilla planifolia.
2.1.2. Plant-Based Vanillin
Plant-based vanillin is the vanillin obtained biotechnologically using enzymatic or microbial transformations of non-oil-based precursors as carbon sources, such as ferulic acid, eugenol or glucose
[9][10]. However, this type of vanillin can be considered natural or not, depending on the type of process utilized
[8][11]. In fact, in the literature, only vanillin biotechnologically produced is considered natural and can satisfy both the US and the EU regulatory requirements. This vanillin is also frequently called “bio-vanillin”. The first example was reported in 1988, in which the precursor vanillic acid was obtained from a simple carbon source, glucose, using recombinant
E. coli via the shikimic acid pathway
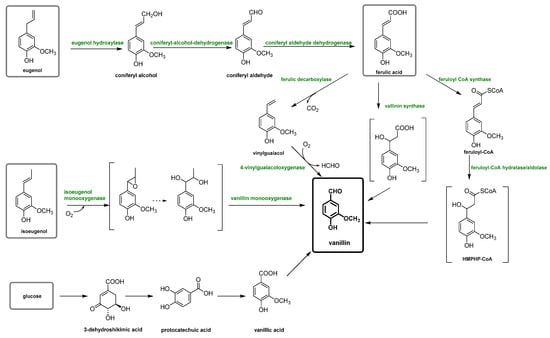
[12]. However, recently, different biotechnological vanillin production technologies have been extensively investigated in order to obtain bio-vanillin. These techniques (summarized in
Figure 4) include the transformation of eugenol, isoeugenol, ferulic acid and glucose with fermentation technology (solid-state fermentation), microorganisms bioengineering, enzymatic production, biosynthetic systems, bioconversion of agro-industrial wastes and production by microorganisms
[13][14][15].
Figure 4. Scheme of vanillin production pathways through a biotechnological process.
The price of vanillin obtained via the fermentation of ferulic acid is about USD 700/kg
[16].
2.2. Synthetic Vanillin
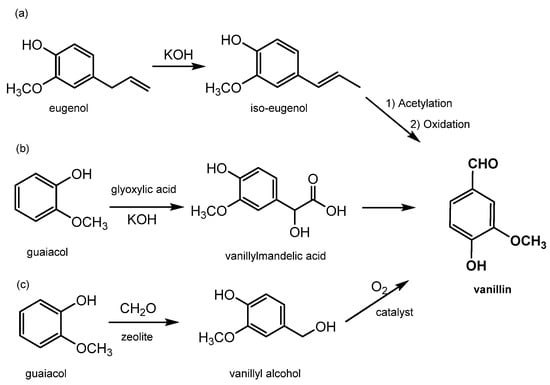
Synthetic vanillin constitutes around 88% of the global vanillin demand, and its production starts from petrol-based intermediates, specifically eugenol and guaiacol; for this reason, it has to be labelled “synthetic or artificial vanilla flavor”. Additionally, the adjective “artificial” or “synthetic” makes it unappealing to consumers. Its price is relatively low, around 10–20 USD/kg
[16], and it is sold mainly to ice-cream and chocolate manufacturers as well as in the form of fragrances to flavor companies. There are currently three main industrial processes to produce synthetic vanillin (reported in
Figure 5). The first synthetic method produces vanillin from eugenol by exploiting the isomerization of eugenol to isoeugenol using KOH in diethylene glycol. Isoeugenol is then converted into an acetate for OH protection and then finally oxidized to form vanillin using nitrobenzene or potassium dichromate (as shown in
Figure 5a)
[2]. In the second method, which accounts for 85% of the total production, vanillin is prepared from guaiacol using the Riedel process which includes the condensation of glyoxylic acid into guaiacol to generate vanillylmandelic acid and then vanillin (see
Figure 5b). This reaction is highly regio-selective towards the para position, thereby avoiding the formation of side products. The third method, known as Solvay’s route, involves the transformation of guaiacol, with a two-step reaction using sequentially HCHO and O
2, into vanillyl alcohol at first and then into vanillin (see
Figure 5c)
[8].
Figure 5. Main processes for chemical production of vanillin. (
a) synthetic method to produce vanillin from eugenol by eugenol isomerization in isoeugenol and its oxidation; (
b) vanillin production by Riedel process on guaiacol; (
c) vanillin synthesis by guaiacol Solvay’s route.
2.3. Vanillin Authentication
2.3.1. Chemical Fingerprint
Food or flavor preparations are considered authentic when they do not contain adulterants and their preparation method conforms to what is declared. This aspect is very relevant for any food and beverage containing vanillin or vanilla extracts. Indeed, vanilla extracts contain mainly vanillin and a few minor chemical components
[17]. Although these compounds are present in very minute amounts, they contribute to the unique flavor of the natural raw material. Therefore, the chemical fingerprint of a given vanilla pod extract depends on many factors such as vanilla cultivar, geographic origin and method of production and can be employed as a reference standard for analytic purposes.
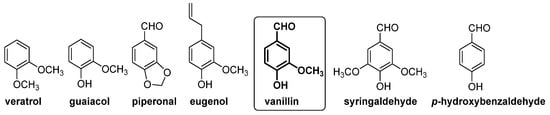
In this context, gas chromatography–vacuum ultraviolet spectroscopy (GC-VUV)
[18] and capillary electrophoresis
[19] have been used for the determination of natural and artificial flavoring compounds in natural extract samples. In these studies, guaiacol, veratrol, piperonal, eugenol, 4-hydroxybenzaldehyde, vanillic alcohol and vanillic acid were selected as chemical markers for the authentication of vanilla extract or vanilla aromatized foods (see
Figure 6).
Figure 6. Compounds present in extract of vanilla pods (left side) and in barrel-aged alcoholic beverages (right side), which have been used as markers for vanillin authentication.
A similar approach has been proposed for the authentication of the origin of vanillin present in barrel-aged alcoholic beverages. It is generally recognized that this aldehyde gives an important contribution to the flavor of aged distillates
[20] and, to a lesser extent, to aged wine
[21]. In these products, vanillin originates from the degradation of the lignin present in barrels wood. During aging, lignin macromolecules release the monomers coniferyl,
p-coumaryl and sinapyl alcohols. Coniferyl alcohol gives rise to coniferaldehyde, which is converted into vanillin and, in turn, is oxidized to form vanillic acid. Similarly,
p-coumaryl and sinapyl alcohols generate the corresponding aldehydes, which are transformed into
p-hydroxybenzaldehyde and syringaldehyde, respectively, which are further oxidized to their benzoic acid derivatives. Overall, the aging process affords a complex mixture of lignin-derived phenolic compounds, whose composition depends on several factors such as the kind of wood used, temperature storage, the alcohol content in the beverage and the duration of aging. Therefore, the analytic determination of these compounds in wines and distillates has turned out to be a useful tool for the assessment of their quality as well as to spot the fraudulent addition of synthetic vanillin.
2.3.2. Isotopic Fingerprint
Obviously, the authentication of purified vanillin cannot be accomplished via an analysis of its compositional profile. In this case, the use of isotopic profiling has proven to be the most effective approach. Indeed, in an organic molecule, the distribution of stable isotopes is not statistical but depends on the synthetic/biosynthetic path of its formation.
Chemical and biochemical reactions proceed with a small but defined kinetic isotope effect, namely, the change in the reaction rate when one of the atoms in the reactants is replaced by one of its isotopes. Therefore, any chemical reaction can increase/decrease the content of a given isotope in the newly formed molecule, depending on the isotope effect of the specific transformation. Close to the most abundant isotopes 12C, 1H and 16O, vanillin contains the isotopes 13C, 2H and 18O, whose abundance and distribution in the molecular frame is strictly related to the synthetic method of its production.
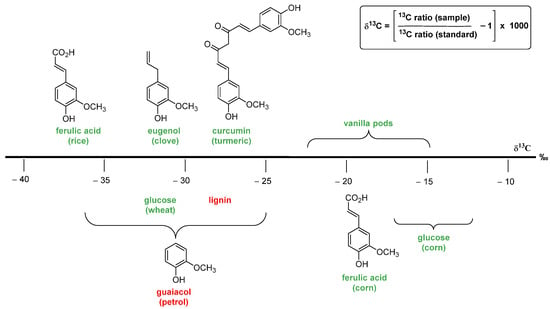
The authentication methods based on the carbon stable isotope ratio are widely used to assess the natural/artificial origin of vanillin. These analytic procedures are based on the evaluation of the
13C/
12C ratio. For natural materials (plants, animals and minerals), the ratio value is approximately 0.0112, and only the last digit varies. Therefore, in order to have a more suitable index, ratio values are converted into δ
13C value, calculated using the formula in
Figure 7, and expressed in ‰ values. A more negative δ
13C means more
12C, and a more positive δ
13C means more
13C. Organic carbon contains less of the
13C, relative to the initial inorganic carbon from the atmosphere, because photosynthetic carbon fixation involves several fractionating reactions with kinetic isotope effects
[22].
Figure 7. The fundamental precursors used for vanillin synthesis and the corresponding δ13C range of values measured for vanillin. The chemical precursors are reported in red; while the natural ones in green.
Although isotope ratio mass spectrometry (IRMS)
[23][24][25][26] still remains the most employed method for vanillin authentication, several new analytic approaches have been developed to overcome the above-mentioned issues. Taking into account that even
2H and
18O are incorporated in vanillin molecule with a measurable kinetic isotope effect, the combined measurement of the δ
13C value with the δ
2H value
[27][28][29] or with δ
18O values
[30][31][32] have proven to be a useful tool in authentication. These approaches have turned out to be particularly effective when exploited in combination with NMR techniques. Indeed, the isotopic content of vanillin varies not only in total, but more importantly, as a function of the different atomic sites within the molecule
[33]. This effect is the result of the specific synthetic/biological steps that have been involved in vanillin formation. Firstly,
2H
[34][35] and recently
13C-NMR
[36][37][38][39][40] have shown that it is possible to measure the isotope ratio of the specific atomic sites to obtain a complete isotopic fingerprint of the molecule. In this context, the SNIF-NMR (site-specific natural isotopic fractionation via nuclear magnetic resonance) methodology has been proposed to be the most valid NMR technique. SNIF-NMR and IRMS can be regarded as complementary analytic methods, and their combined use affords reliable results.
As a final point, it is worthy to describe the case of positional δ
18O values. The sources of oxygen needed to build up every organic compound are CO
2, atmospheric O
2 and ground water. The δ
18O values of these infinite reservoirs are very different from each other, ranging from +42.5‰ to +40.3‰ (CO
2), from +23.8‰ to +23.5‰ (atmospheric O
2) and from +2‰ to −10‰ (water)
[41]. Moreover, three different oxygen atoms are placed in three different positions in the molecular framework of vanillin. Therefore, the positional δ
18O values are strictly connected to the origin of the oxygen atoms that have been supplied in the synthesis/biosynthesis process.
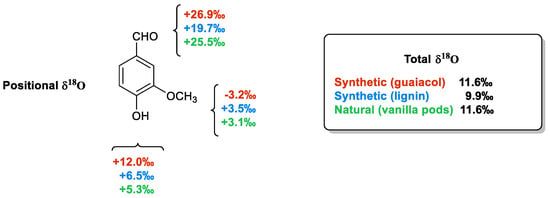
As shown in
Figure 8 [30], the total δ
18O values measured from vanillin samples of different origins are very similar to each other and do not allow for their authentication. On the contrary, the positional δ
18O values are able to differentiate samples of synthetic origin from those extracted from vanilla plants or produced from lignin via chemical oxidation. The main difference between the samples derived from guaiacol and those possessing the aromatic moiety of natural origin is in the value of the oxygen atoms linked to the aromatic ring. Otherwise, the extractive materials from pods are distinguished from those derived from lignin based on the carbonyl oxygen δ
18O values ranging from +26.2‰ to +25.5‰ in the natural material to +19.7‰ in the lignin-based sample.
Figure 8. The positional δ
18O values in three vanillin samples, two synthetic (from guaiacol and lignin) and one natural (from vanilla pods).
3. Vanillin Production from Lignin
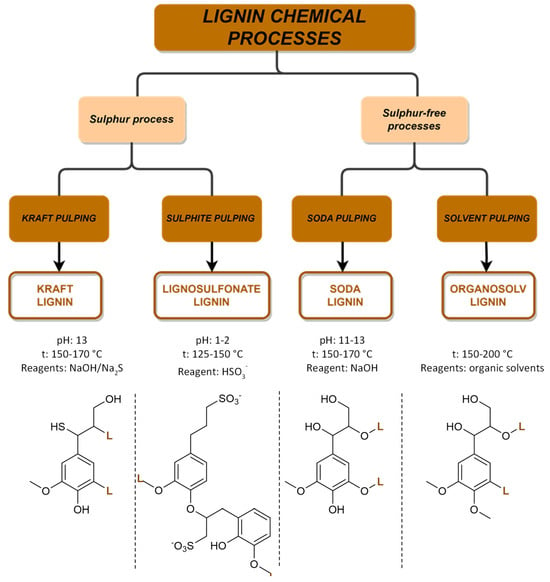
Every year, over 50 million tons of lignin are extracted via wood pulping and other biorefinery industries, but only a small fraction of around 2% is utilized in various applications. However, with the growing adoption of extraction technologies and a shift towards biorefinery processes, lignin is increasingly being recognized as a ‘green’ feedstock for fuels, chemicals, and materials. Traditionally, lignin has been considered an industrial residue of pulp and paper factories, with most of the annual production being used as a low-cost fuel for power and heat generation. However, lignin-derived products can play an important role in increasing our reliance on renewable-based chemicals, fuels, and materials and in reducing the carbon footprint of products and processes. The structure of lignin is complex and varies depending on plant species, tissue type, and extraction methods. Research is ongoing to explore the potential of the different lignin sources with the challenge to identify the best starting materials along with a set-up of new technologies. The most common industrial available lignins and their extraction processes are summarized in Figure 9. The chemical processes are divided into two main groups related to the presence of sulfur in the extraction processes.
Figure 9. Overview of the four main chemical processes for lignin extraction and the structure of lignin.
As the global economy continues to shift towards renewable feedstocks, there is a growing interest in developing new applications for lignin, which is driving commercial efforts. Moreover, from a circular economy perspective, the fractionation of lignocellulosic waste biomasses is also currently being highly investigated in order to recover and valorize the main components constituted by polysaccharides and lignin [59,60].
3.1. Lignin Oxidation
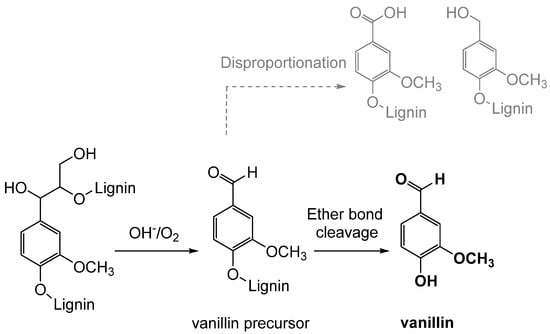
Oxidation is the classical process of converting lignin into vanillin through the use of oxidizing agents, such as oxygen, hydrogen peroxide, or ozone (see
Figure 10). Industrially, the oxidative depolymerization of lignin is the most advanced and widely used technology for the production of aromatic compounds, including vanillin
[42]. This process involves the oxidation of an aqueous solution of lignin, mainly lignosulfonate, which represents less than 10% of the total amount of lignins extracted worldwide
[8].
Figure 10. Simplified mechanism of lignin oxidation to form vanillin.
3.2. Biotechnological Lignin Transformations
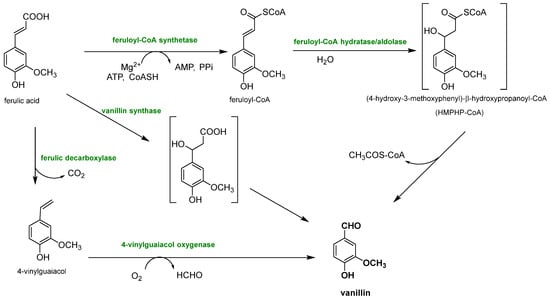
Biotechnological approaches have gained significant relevance for vanillin production from lignin, due to their sustainability and cost-effectiveness, because they permit the production of natural-identical vanillin which is in high demand due to the growing interest in natural and environmentally friendly compounds. This purpose is perfectly achieved by exploiting the biotechnological approaches, which involve the use of microorganisms, such as bacteria, fungi and yeasts, to transform lignin into the desired chemical products that perfectly fit this need. The main way to produce natural-identical vanillin through biotechnology consists of the microbial degradation of lignin to release ferulic acid which can be converted into vanillin (see Figure 11).
Figure 11. Different enzymatic reaction sequences for biovanillin production from ferulic acid recovered from lignocellulosic biomasses.
Ferulic acid can be dissociated from lignin using feruloyl esterases (EC 3.1.1.73), a subclass of carboxylic ester hydrolases that catalyze the cleavage of ester bonds between ferulic acid and lignocellulose from common agricultural waste
[43]. Feruloyl esterase activity was first discovered in 1987, and since then, it has been found in a wide range of microorganisms, such as bacteria (
Streptomyces olivochromogens and
Fribrobacter succinogenes) and fungi (
Penicillium and
Aspergillus)
[44], and to date, over 30 microbial cinnamoyl esterases have been identified. It has been found that the distance between the phenolic ring and the ester bond, as well as the number and the position of the methoxyl and hydroxyl groups, influences the enzyme activity.
Since ferulic acid is so abundant in different agricultural wastes, Chattopadhyay et al. tried to extract it from wheat bran and then tried to consequently convert it into vanillin using
Streptomyces sannanensi [45]. At first, ferulic acid was obtained using a ferulic acid esterase that cleaved the ester bonds present between the acid and the lignocellulosic biomass. Then, the acid was converted into vanillin (708 mg/L) through a CoA-dependent non-β-oxidative reaction sequence occurring via a CoA-dependent retro-aldol mechanism.
Although lignin degradation in nature is carried out by fungi, in particular, basidiomycetes, they are not widely used for commercial purposes. Instead, bacteria are preferred, and the complexity of lignin requires the utilization of metabolic engineering and/or microbial consortia. In this way, different bacteria with different enzymatic activities can catalyze the cleavage of several bonds present in lignin and release the desired chemicals, avoiding the challenges associated with lignin heterogeneity
[46]. Lignin-degrading bacteria can be found in nature in lignin-enriched environments, such as leaf litter, decomposing woods, compost soils, etc. Through bacteria-screening methods, some microorganisms belonging to phyla Proteobacteria, Actinobacteria and Firmicutes show a great ability to degrade lignin. These bacteria are able to depolymerize lignin using and combining the action of different enzymes, such as laccases, manganese peroxidases, Cyt P450, dioxygenase and others
[47].
Another example of utilizing natural bacteria consortia has been reported by Harshvardhan et al.
[48]. They were able to produce vanillin and a few other products using bamboo chips (
B. tulda) as a substrate for a natural bacterial consortium. They tested 14 natural bacteria consortia developed on bamboo chips using enrichment techniques, different media and different temperatures. Among them, only one bacteria consortia (H3) produced vanillin without any chemical pre-treatment. The consortium was composed of 28 strains identified by using the 16S rRNA sequencing method. Although vanillin was not the only product, it was the majority with a production of 0.9 ± 0.3 mg/mL vanillin, which can be compared to the vanillin yield obtained via the ferulic acid bio-conversion.
3.3. Lignin Hydrothermal Liquefaction
Hydrothermal liquefaction (HTL) has been widely studied in recent years as a promising technology for biomass processing
[49] and, more specifically, for lignin depolymerization
[50]. HTL is based on the use of compressed water under subcritical (200–374 °C) and, less frequently, supercritical (>374 °C) conditions, at pressures typically in the 15–220 bar range. These conditions allow for the exploitation of the wide interval of dielectric constant exhibited by water, which drops from 80.3 to 17.5 in the 22 °C to 327 °C temperature range (at 200 bar), enabling the fine-tuning of its solvating capabilities with respect to a very wide range of organic compounds. To put this into perspective, the dielectric constants (at 20 °C) of methanol, ethanol, and acetone are 33.0, 25.3 and 21.0, respectively. Moreover, the water pK
W is also strongly dependent on physical conditions, decreasing from 14.0 (at 25 °C, ambient pressure) to 11.1 (at 300 °C, 250 bar) and thus resulting in a reaction ambient richer in hydroxide and hydronium ions. The combination of these properties allows for the establishment of a reaction medium that combines some characteristics of organic solvents with a certain degree of acid–base catalytic properties
[51].
HTL has been applied to lignin to produce bio-oils and/or phenolics
[50]. The possibility to recover phenolic compounds indicates that, during HTL lignin depolymerization, hydrolysis is an important factor. For example, in bio-oils obtained from organosolv lignin, 80% of the products are monomeric and also dimeric phenolic compounds
[50]. Lignin HTL is a promising technique, due to its ability to maintain functional groups with high selectivity using water as a solvent, which is not always possible with other processes. In particular, the utilization of supercritical water allows for the retrieval of bifunctional aromatic compounds
[52]. The yield of the conversion of lignin into phenolic compounds also depends on factors not directly related to the HTL process itself, such as the source of lignin and the type of pre-treatment used. In fact, phenols, guaiacols and catechols are obtained from softwood lignin, whereas syringol is mainly produced from grass lignin.
The production of vanillin from lignin via HTL has typically been studied in the more general context of phenolic derivatives production. A selection of the most interesting approaches is presented here, with particular focus on the more recent literature.
3.4. Lignin Electrochemical Depolymerization
Electrochemical depolymerization is considered one of the most promising techniques for producing aromatic fine chemicals via a lignin cleavage. In fact, this process, which is an anodic degradation, is particularly environmentally friendly because it does not need intensive energy-consuming pre-treatments, such as high temperature and pressure, and can use renewable energy as a power source
[53]. Moreover, this method overcomes the major issues associated with the production of bio-based vanillin linked to the use of toxic reagents and the presence of waste by-products that could contaminate the desired final vanillin. However, this technique is not widely exploited due to the low yield it produces which is conditioned by the nature of the electrode responsible for mass transport and flow distribution and by the electrochemically active area. Active nickel-based electrodes were tested in 2016 by Stiefel et al. on Kraft lignin
[53]. They experimented with these electrodes at room temperature, ambient pressure and using a current of 8 A, obtaining a lignin degradation of 81% in 2 h, 87% in 3 h and 96% in 11 h
[53]. The reduction of lignin molecular weight to 220 Da was achieved, but they recovered, using a membrane module containing polymeric tight ultrafiltration membranes in a constant-flow cross-flow mode, vanillin, acetovanillone and different carboxylic acids with an individual yield of these products lower than 0.5%. The authors reported that this method was still in development and the yields could be improved in order to use the technique on an industrial scale.
An interesting possibility is to combine hydrogen production and lignin depolymerization. In the ordinary electrocatalytic water splitting for hydrogen production, the anodic product stream is oxygen. By replacing the oxygen evolution reaction (OER) with a less energy-intensive process such as biomass depolymerization, it is possible to implement a process with less energy requirement and with an anodic product stream of greater value than oxygen (e.g., phenolic in the case of lignin depolymerization). Ghahremani et al.
[54] studied the simultaneous hydrogen production and lignin depolymerization using NiSn electrodes and lignin dissolved in 1 M NaOH solution. The authors studied different Ni/Sn ratios and cell potentials and found the highest vanillin production rate (about 300 mg/min of lignin at 10 g/L lignin concentration) using the NiSn
20% at 1.4 V. Unfortunately, no yield data were provided for a complete batch process example. Higher cell voltages result in a lower lignin production rate because of the activation of competitive anodic OER.
3.5. Lignin Photocatalytic Depolymerization
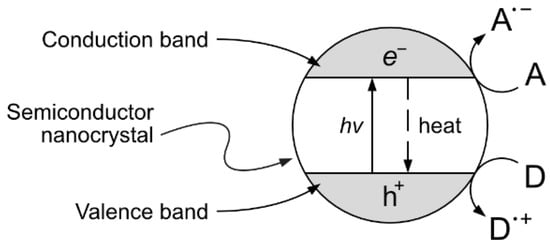
One interesting approach, although still at the experimental stage, is the production of vanillin via controlled oxidation of lignin using photocatalytic techniques. The implementation of this strategy is particularly appealing because of the potential sustainability of the resulting process. The selection of studies given in the following paragraphs can provide a good starting point for the development of further research. Heterogeneous photocatalysis is based on the photon-driven promotion of an electron from the valence band to the conduction band, resulting in a catalytic activated semiconductor crystal. The formed positive hole (h+) and free electron (e−) can then migrate from the bulk to the surface of the catalyst crystal, becoming available for redox reactions with suitable electron donors and acceptors (see Figure 12).
Figure 12. Heterogeneous photocatalytic process. The adsorption of a photon on a semiconductor crystal causes the promotion of an electron from the valence band to the conduction band, generating an e−/h+ pair in the bulk of the photocatalyst. The generated electron and hole can then migrate to the surface and become available for redox reactions with an electron acceptor (A) or donor (D) species or they can thermally recombine in a competitive process.
The possible (and usually favored) electron–hole recombination in the catalyst structure is, however, a direct concurrent result of the desired surface redox processes. Therefore, great research efforts have been devoted to ease the redox reaction (e.g., by reducing the photocatalyst crystal size in order to reduce the length of the charge migration path from the bulk to the surface) and/or stabilize the generated e
−/h
+ pair enhancing the charge separation (e.g., by synthesizing composite photocatalysts with tailored band structures that favor the physical displacement of electrons and holes)
[55]. A substantial role in water-based heterogeneous photocatalytic oxidation processes can be also given by the hydroxyl radical HO·, which can be generated from the direct oxidation of water at the catalyst surface and other secondary processes
[56].
Because of the photon contribution to the reach of the reaction activation energy, photocatalytic reactions can be easily carried out in mild conditions at ambient temperature, typically using water as a solvent and without resorting to toxic oxidizing or reducing agents. Photocatalytic processes are thus considered a very interesting option in the context of green chemistry. Moreover, photocatalytic reactions can be potentially carried out directly exploiting natural solar radiation as a photon source, further reducing the environmental impact of the implemented process
[57]. Several applications of photocatalysis have been studied, including water splitting for hydrogen production
[58], carbon dioxide reduction
[59] and implementations such as advanced oxidation processes (AOPs) for air
[60] or water
[61] depollution and sustainable chemical process development
[62][63].
3.5.1. Photocatalysis of Pulping Black Liquor
Prado et al.
[64] reported an example of photocatalytic lignin depolymerization directly performed on the pulping black liquor (i.e., without lignin separation) for the recovery of derived compounds. The authors used two different black liquors deriving respectively from organosolv pulping (with 60% ethanol at 180 °C for 90 min, solid/liquid ratio 1:4) and from ionic liquid (IL) pulping (with [Bmim][MeSO
4] under microwave, 200 °C for 30 min, solid/liquid ratio 1:10). The photocatalytic oxidation was carried out adding the photocatalyst (sol–gel synthesized TiO
2) to the pulping black liquor (2 g/L for organosolv-derived black liquor and 4 g/L for the IL-derived one). After photocatalysis, lignin was separated via acid precipitation and centrifugation while the liquid fraction was extracted with ethyl acetate to obtain the derived oil. The analysis of the precipitated lignin demonstrated a better degradation from the photocatalysis of organosolv black liquor in comparison to the photocatalyzed IL black liquor. This was attributed to the different pH values of the two reaction media (4.8 for organosolv vs. 7.0 for IL). In the extracted oil, the authors observed lignin-derived compounds and also furfural and other sugar degradation products. The yields of lignin-derived phenolics were definitely higher for the organosolv pulping medium. One of the main recovered compounds in the extracted oil was syringaldehyde, which accounted for up to 14.2% (
w/
w) of the oil fraction for organosolv black liquor and 1.2% for IL black liquor (both obtained after 0.5 h photocatalysis). The maximum recovered vanillin was 0.9% (organosolv) and 0.1% (IL), always as the oil fraction extracted after 0.5 h of photocatalytic treatment. Longer photocatalysis reaction times resulted in lower yields. The remarkably lower yields from IL black liquor photocatalysis were attributed to the formation of nitrogen-containing compounds derived from the photocatalytic degradation of the ionic liquid components. Although this is interesting from the point of view of process economy (no lignin separation must be performed), the reported results indicate that the direct photocatalytic treatment of pulping black liquors could be critical in most aspects, including the sub-optimal condition dictated by the pulping medium itself (e.g., pH) and the presence of critical amounts of extraneous compounds (e.g., sugars and pulping reagents) that can interfere with the photocatalytic process, both lowering the yields and generating undesirable byproducts.
3.5.2. Photocatalysis of Separated Lignins
The problems encountered in the direct photocatalysis of black liquor can be overcome by operating on lignins purified from the pulping medium. The application of photocatalysis to the synthesis of vanillin from sodium lignosulfonate (SLS) has been recently reported by Qiu et al.
[65]. The authors used a mesoporous, high specific surface area titanium dioxide photocatalyst obtained from the calcination in air of MIL-125, a Ti-based metal–organic framework. The photocatalytic conversion experiments were carried out with Xenon lamp irradiation (6 h) under air at room temperature. Of the three different catalysts prepared, calcinated at 400, 500 and 600 °C, respectively, the one calcinated at 400 °C demonstrated the highest specific surface area (174 m
2/g) and the highest vanillin yields (2.1 mg per g of SLS).
Ahmad et al.
[66] described the formation of vanillin and 4-hydroxybenzaldehyde in the photocatalytic degradation of lignin obtained via the delignification of rice straw residues. The lignin extraction was performed in 1 M NaOH solution at 150 °C for 1 h. The photocatalytic alkaline lignin extraction was performed in a stirred reactor using TiO
2 or ZnO-suspended photocatalyst particles. While the lignin underwent a steady degradation during the photocatalytic process, the concentration of both vanillin and 4-hydroxybenzaldehyde demonstrated, after an initial induction phase where none of the two products were detected, a rise and a following decrease after they reached the maximum value. This was attributed to the activation of the concomitant photocatalytic degradation of both products, confirmed by the authors who measured the degradation kinetics of pure vanillin and 4-hydroxybenzaldehyde in separate experiments using the same photocatalytic conditions. The maximum lignin degradation rate was found for ZnO with 2 g/L catalyst loading and, in these conditions, a higher vanillin concentration was also recorded (51.2 mg/L at 8 h process time). The 4-hydroxybenzaldehyde was formed in lower amounts, with the maximum concentration (20.4 mg/L) recorded using the TiO
2 photocatalyst at 1.5 g/L catalyst loading at 10 h process time.
3.5.3. Photocatalysis with Combined Processes
A possible strategy for enhancing the overall performance of photocatalytic oxidation is the combination with other complementary approaches developing an integrated process with optimized performances. Few works involving the specific production of vanillin are available in the literature on this topic. An example targeted at lignin oxidation was proposed by Tian et al.
[67] which described a combination of photocatalytic and electrochemical processes for the degradation of Kraft lignin. The authors studied various combinations of a Ti/TiO
2 nanotube electrode and a Ti/TaO
5-IrO
2 electrode in electrochemical and/or photocatalytic operation modes. The counter electrode was a Pt foil, while an Ag/AgCl electrode was used as a reference electrode. The authors studied the kinetics of the lignin degradation process measuring the lignin concentration with UV spectrophotometry (295 nm). All studied processes resulted in pseudo-first-order kinetics. The highest rate constant (0.021 min
−1) was demonstrated by the combined photocatalytic/electrochemical process obtained and by connecting both electrodes at +600 mV (vs. the Ag/AgCl reference electrode) under UV irradiation. The separate electrochemical oxidation, performed with the Ti/TaO
5-IrO
2 electrode held at +600 mV without UV irradiation resulted in less than half the degradation rate constant (0.009 min
−1).
The combined photocatalytic and biocatalytic degradation of Kraft lignin was studied by Kamwilaisak et al.
[68] using TiO
2 and laccase. The authors studied both a combined dual-step process (photocatalysis followed by biocatalysis) and a combined single-step process (photocatalysis and biocatalysis running concurrently in the same batch). Separate photocatalysis and biocatalysis processes were performed as references. All batches were carried out at 50 °C and pH 5 for 24 h using 1 g/L lignin and 3 g/L TiO
2 (for photocatalytic processes) and 2.5 units/mL laccase (for biocatalytic processes). The photocatalytic processes were carried out under UV-A light by fluorescent tubes at 5.3 µE cm
−2 s
−1 (~1.7 W/cm
2 at 370 nm). The authors studied also the effect of the addition of H
2O
2 (5.55 g/L) in all experiments. The lignin degradation yield of the different systems was biocatalysis << photocatalysis ≈ combined single-step < combined dual-step. The addition of H
2O
2 increased the degradation yield of all the processes with the sole exception of the combined single-step. Several degradation products were identified, mainly, organic acids and carbohydrates. Vanillin was identified but not quantified. This study indicated the potentialities of combined strategies but also the possible problems due to the interference of different processes running simultaneously in the same reactor.
3.5.4. Perspectives for Vanillin Production by Controlled Photocatalytic Lignin Degradation
Although currently still at the experimental laboratory stage, the production of vanillin by the controlled photocatalytic oxidation of lignin appears to be a potentially very interesting goal, particularly considering the increasing focus on the sustainability of industrial chemical processes. However, several problems must still be resolved, and more research is needed for an effective deployment of the technology. Despite the overall mild operating conditions, the typically high amount of hydroxyl radicals generated in the photocatalytic process can establish a quite reactive environment that possibly causes a significant degradation of the generated vanillin. Catalytic systems with enhanced selectivity are needed for an effective production of the target molecule, reducing competitive degradation pathways of the original lignin and of the product itself. In this regard, it is interesting to note that the mild operating conditions potentially enable the use of tailored on-process separating systems such as membranes or adsorbing resins in order to separate the neo-formed vanillin from the reaction medium, thus avoiding possible product degradation. Original approaches on the reactor construction may therefore be interesting research topics
[69].
4. Conclusions
Vanillin has been known, for a long time, for its properties as a flavoring agent for food, beverages and pharmaceuticals. Moreover, its belonging to the large class of phenolic compounds has pushed the research more recently on its antioxidant properties and its use as a natural building block for many biobased polymers. In this framework, vanillin obtained from a biobased waste residue such as lignin offers various significant environmental and economic benefits including waste reduction and natural resource recycling. The development of new technologies and processes for the production of vanillin from lignin thus represents a promising approach to valorize an underutilized renewable resource and also address the growing global demand for natural vanillin. Chemical, enzymatic, microbial and photochemical conversion methods have been described here and have shown great potentialities in providing a more sustainable and cost-effective source of vanillin. However, it appears that highly significant challenges remain in terms of vanillin yield, selectivity, and process efficiency.