Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamir Pitton Rissardo | -- | 1535 | 2024-01-31 14:41:26 | | | |
| 2 | Rita Xu | Meta information modification | 1535 | 2024-02-01 02:56:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pitton Rissardo, J.; Caprara, A.L.F. Neuroimaging Techniques in Differentiating Parkinson’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/54595 (accessed on 07 February 2026).
Pitton Rissardo J, Caprara ALF. Neuroimaging Techniques in Differentiating Parkinson’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/54595. Accessed February 07, 2026.
Pitton Rissardo, Jamir, Ana Letícia Fornari Caprara. "Neuroimaging Techniques in Differentiating Parkinson’s Disease" Encyclopedia, https://encyclopedia.pub/entry/54595 (accessed February 07, 2026).
Pitton Rissardo, J., & Caprara, A.L.F. (2024, January 31). Neuroimaging Techniques in Differentiating Parkinson’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/54595
Pitton Rissardo, Jamir and Ana Letícia Fornari Caprara. "Neuroimaging Techniques in Differentiating Parkinson’s Disease." Encyclopedia. Web. 31 January, 2024.
Copy Citation
Neuroimaging can provide significant benefits in evaluating patients with movement disorders associated with drugs. The dopaminergic radiotracers already reported to assess patients with drug-induced parkinsonism are [123I]-FP-CIT, [123I]-β-CIT, [99mTc]-TRODAT-1, [18F]-DOPA, [18F]-AV-133, and [18F]-FP-CIT. The most studied one and the one with the highest number of publications is [123I]-FP-CIT. Fludeoxyglucose (18F) revealed a specific pattern that could predict individuals susceptible to developing drug-induced parkinsonism.
dopamine transporter
DAT
dopaminergic imaging
1. Introduction
Drug-induced movement disorders impact a significant portion of the population. At least one percent of the population is estimated to suffer from tremors or ataxia secondary to medications [1][2]. In this context, there is a significant burden related to the high cost of extensive diagnostic workup, hospitalization, increased healthcare expenditures, and lost workdays due to drug-induced movement disorders. More than five percent of subjects initially presenting with Parkinson’s disease are commonly later diagnosed with drug-induced parkinsonism [3].
Neuroleptics are the most common class of medications associated with drug-induced parkinsonism [4]. They may block dopaminergic D2 receptors in the postsynaptic neurons. The prevalence of drug-induced parkinsonism in individuals managed with neuroleptics in the literature widely varies from 15% to 60% [2][5]. In this context, the duration of neuroleptic therapy, neuroleptic doses, and genetic predisposition of individuals may significantly influence the development of drug-induced parkinsonism [6]. Drug-induced parkinsonism can occur secondary to many agents, including antibiotics, antidepressants, antiseizure medications, and calcium channel blockers [7][8].
An inaccurate or delayed diagnosis of drug-induced parkinsonism may result in ineffective treatment and expose patients to side effects, impacting their quality of life. In this context, the clinical differentiation between Parkinson’s disease and drug-induced parkinsonism usually requires discontinuing the offending medication for a long period, which is often challenging and, in some cases, not feasible, such as in active neuropsychiatric disorders. Moreover, a levodopa trial could be effective in suspected subclinical parkinsonism, especially in parkinsonism secondary to dopamine-blocking agents [9].
Neuroimaging could provide significant benefits for patients presenting with drug-induced parkinsonism, mainly in those individuals with similar and undifferentiated clinical manifestations to Parkinson’s disease. Neuroimaging techniques have different parameters for assessing brain regions’ structure, function, metabolism, and receptor sites (Figure 1).
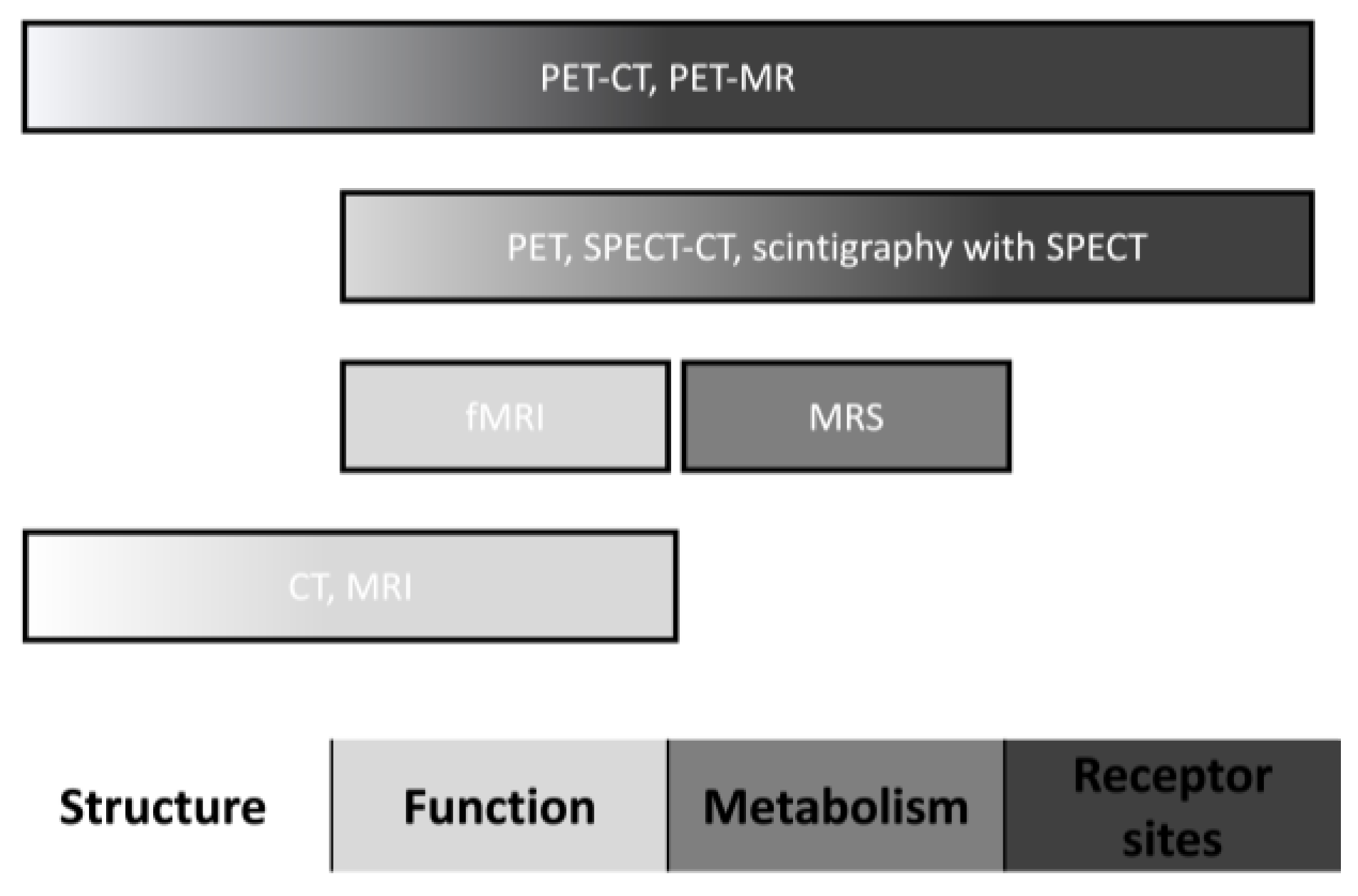
Figure 1. Neuroimaging characteristics. Abbreviations: CT, computed tomography; fMRI, functional magnetic resonance imaging; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; PET, positron emission tomography; and SPECT, single-photon emission computed tomography.
2. [18F]-Fluorodeoxyglucose ([18F]-FDG) PET
[18F]-FDG is a radiotracer that can mark the tissue uptake of glucose, which is closely correlated with some metabolism pathways. Several studies already evaluated the use of [18F]-FDG in supporting the diagnosis of Parkinson’s disease, in which the specific pattern encountered is an increased uptake of the striatum, thalamus, motor cortex, and cerebellum. On the other hand, the temporoparietooccipital cortex is believed to have a lower uptake [10].
Kotomin et al. studied the metabolic brain imaging approach using the 18F-FDG PET and spatial covariance analysis to find possible factors that could predict drug-induced parkinsonism. They found that the expression of a Parkinson’s-disease-related pattern on 18F-FDG was commonly related to the development of parkinsonism secondary to drugs. However, this pattern was also observed in patients receiving antipsychotics without motor symptoms [11].
3. [123I]-MIBG Cardiac Imaging
[123I]-MIBG scintigraphy assesses the integrity of the cardiac sympathetic nerve terminals. Studies showed that this neuroimaging technique can be used to differentiate Parkinson’s disease from other forms of parkinsonism [12]. A limited number of studies assessing [123I]-MIBG scintigraphy in parkinsonism secondary to drugs have already been published in the literature.
Lee et al. evaluated 52 individuals with parkinsonism, of which 20 were diagnosed with drug-induced parkinsonism. Ten percent of the subjects with a drug-induced parkinsonism diagnosis showed a reduced uptake compared to patients with Parkinson’s disease. The two individuals with drug-induced parkinsonism and a reduced uptake also had no improvement in their motor symptoms with drug discontinuation. However, both patients significantly improved motor symptoms with the levodopa trial [13].
Lee et al. performed a second study with cross-cultural smell identification (CCSI) testing in 54 individuals with parkinsonism, of which 15 were diagnosed with drug-induced parkinsonism. One of the participants had low CCSI scores and a reduced uptake of [123I]-MIBG, which can suggest that olfactory tests may help distinguish between parkinsonism secondary to drugs and subclinical Parkinson’s disease. It is noteworthy that the CCSI test can be performed quickly in the outpatient clinic and is inexpensive compared to scintigraphy [14].
Kim et al. studied the combination of [123I]-MIBG and [123I]-FP-CIT SPECT in 36 individuals with parkinsonism, of which 20 had a diagnosis of drug-induced parkinsonism. In this study, 80% of the individuals with drug-induced parkinsonism had normal cardiac imaging and DAT imaging studies. Interestingly, two individuals presented with normal [123I]-FP-CIT and decreased [123I]-MIBG uptakes. After two years, these individuals had worsened parkinsonian symptoms. A second imaging sequence showed a reduced uptake of [123I]-FP-CIT and [123I]-MIBG. Therefore, these findings suggest cardiac abnormalities are found before striatal region lesions. In this way, it is possible that those patients with probable drug-induced parkinsonism and normal DAT scans with less improvement after drug discontinuation will benefit significantly from cardiac imaging [15].
Shafie et al. studied 44 patients with parkinsonism secondary to drugs and 32 patients with idiopathic Parkinson’s disease. The authors found that the difference [123I]-MIBG uptake between the Parkinson’s disease and drug-induced parkinsonism groups was significant. Moreover, Shafie et al. reported that [123I]-MIBG scans could be used to determine the prognosis of people with parkinsonism secondary to drugs. The subjects with drug-induced parkinsonism that did not improve motor symptoms after offending drug discontinuation had a low heart-to-mediastinum ratio [16].
4. Magnetic Resonance Imaging (MRI)—The Swallowtail Appearance
Nigrosomes are small clusters of dopaminergic cells within the healthy substantia nigra. They can have a hypersignal in the axial section, with either a linear or comma appearance. They are bordered anteriorly, laterally, and medially by a low-intensity signal, giving it a swallow-tailed appearance. A loss of the normal swallowtail appearance of the susceptibility signal pattern in the substantia nigra on axial imaging is one of the diagnostic signs for Parkinson’s disease [17].
Sung et al. studied 20 individuals with drug-induced parkinsonism and 29 with Parkinson’s disease. The individuals were first assessed with [18F]-FP-CIT imaging after nigrosome-1 3T imaging was evaluated. Then, 85% of the patients with parkinsonism secondary to drugs were interpreted as normal 3T imaging findings, in which the sensitivity was 100%, specificity 85%, and accuracy 93.9% [18].
Studies with an ultra-high-field MRI (7T) showed significant sensitivity and specificity for diagnosing Parkinson’s disease based on the loss of the swallowtail appearance [19]. Therefore, future investigations with high-quality neuroimaging could be a promising field for supporting the diagnosis of non-degenerative causes of parkinsonism, such as parkinsonism secondary to drugs.
5. Transcranial Ultrasound
B-mode transcranial ultrasonography was already studied to support the diagnosis of Parkinson’s disease. This imaging method, when compared to other techniques, has significant advantages, such as relatively low costs, broad availability, and a noninvasive approach. The characteristic finding in patients with Parkinson’s disease is an increased echogenicity of the mesencephalic substantia nigra region, which is probably related to iron deposition [20]. The presence of this sign is highly specific to the diagnosis of a degenerative form of parkinsonism. Nevertheless, the sensitivity depends on the exact cut-off value of the substantia nigra area used and the type of ultrasound machine [21].
Bouwmans et al. assessed 196 individuals with parkinsonism of unclear etiology. After two years of follow-up, seven individuals were diagnosed with drug-induced parkinsonism. All the individuals were evaluated with [123I]-FP-CIT and B-mode transcranial ultrasonography. Ultrasonography accurately identified drug-induced parkinsonism in 86% of the subjects [22].
Olivares Romero et al.’s study enrolled 20 subjects diagnosed with possible drug-induced parkinsonism in which the offending agent was discontinued. The authors found a sensitivity of 80% and a negative predictive value of 87.5% with the evaluation of echogenicity in the substantia nigra and the lentiform nucleus regions [23].
Oh et al. studied the significance of early transcranial ultrasound in diagnosing drug-induced parkinsonism. They found pure drug-induced parkinsonism has different echogenicity patterns than unmasked Parkinson’s disease. The substantia nigra hyperechogenicity in patients with unmasked Parkinson’s disease showed a sensitivity of 75% and a specificity of 91.1%. Therefore, early transcranial ultrasonography findings may be useful in predicting unmasked Parkinson’s disease in individuals presenting with possible parkinsonism secondary to drugs [24].
6. Optical Coherence Tomography
Patients with Parkinson’s disease commonly present visual symptoms, especially perceptual disturbances such as impairment in stereopsis, visual illusions, and visual hallucinations. Patients with Parkinson’s disease have a decreased average capillary retinal nerve fiber layer in every quadrant [25]. Moreover, Jimenez et al. proposed an equation to determine the Parkinson’s disease progression based on the Unified Parkinson’s Disease Rating Scale (UPDRS) total score and the retinal nerve fiber layer thickness measured by optical coherence tomography [26].
Suh et al. assessed 97 individuals with Parkinson’s disease and 27 with parkinsonism secondary to drugs using optical coherence tomography and [18F] N-(3-fluoropropyl)-2b-carbon ethoxy-3b-(4-iodophenyl) nortropane (FP-CIT). They compared the two groups’ peripapillary retinal nerve fiber layer and macular retinal thickness. There were no significant differences in peripapillary and macular retinal thickness values [27]. Suh et al.’s study is important because it showed that, in the early stages of drug-induced parkinsonism, there is no benefit in measuring these optic parameters to differ from early Parkinson’s disease.
References
- Baizabal-Carvallo, J.F.; Morgan, J.C. Drug-Induced Tremor, Clinical Features, Diagnostic Approach and Management. J. Neurol. Sci. 2022, 435, 120192.
- Rissardo, J.P.; Vora, N.; Mathew, B.; Kashyap, V.; Muhammad, S.; Fornari Caprara, A.L. Overview of Movement Disorders Secondary to Drugs. Clin. Pract. 2023, 13, 959–976.
- Esper, C.D.; Factor, S.A. Failure of Recognition of Drug-Induced Parkinsonism in the Elderly. Mov. Disord. 2008, 23, 401–404.
- de Germay, S.; Montastruc, F.; Carvajal, A.; Lapeyre-Mestre, M.; Montastruc, J.-L. Drug-Induced Parkinsonism: Revisiting the Epidemiology Using the WHO Pharmacovigilance Database. Park. Relat. Disord. 2020, 70, 55–59.
- Feldman, M.; Marmol, S.; Margolesky, J. Updated Perspectives on the Management of Drug-Induced Parkinsonism (DIP): Insights from the Clinic. Ther. Clin. Risk Manag. 2022, 18, 1129–1142.
- Rissardo, J.P.; Caprara, A.L.F. Predictors of Drug-Induced Parkinsonism. APIK J. Intern. Med. 2023.
- Rissardo, J.P.; Fornari Caprara, A.L. Lamotrigine-Associated Movement Disorder: A Literature Review. Neurol. India 2021, 69, 1524–1538.
- Rissardo, J.P.; Caprara, A.L.F. Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines 2023, 10, 33.
- Hardie, R.J.; Lees, A.J. Neuroleptic-Induced Parkinson’s Syndrome: Clinical Features and Results of Treatment with Levodopa. J. Neurol. Neurosurg. Psychiatry 1988, 51, 850–854.
- Meyer, P.T.; Frings, L.; Rücker, G.; Hellwig, S. (18)F-FDG PET in Parkinsonism: Differential Diagnosis and Evaluation of Cognitive Impairment. J. Nucl. Med. 2017, 58, 1888–1898.
- Kotomin, I.; Korotkov, A.; Solnyshkina, I.; Didur, M.; Cherednichenko, D.; Kireev, M. Parkinson’s Disease-Related Brain Metabolic Pattern Is Expressed in Schizophrenia Patients during Neuroleptic Drug-Induced Parkinsonism. Diagnostics 2022, 13, 74.
- Pagano, G.; Niccolini, F.; Politis, M. Imaging in Parkinson’s Disease. Clin. Med. 2016, 16, 371–375.
- Lee, P.H.; Kim, J.S.; Shin, D.H.; Yoon, S.-N.; Huh, K. Cardiac 123I-MIBG Scintigraphy in Patients with Drug Induced Parkinsonism. J. Neurol. Neurosurg. Psychiatry 2006, 77, 372–374.
- Lee, P.H.; Yeo, S.H.; Yong, S.W.; Kim, Y.J. Odour Identification Test and Its Relation to Cardiac 123I-Metaiodobenzylguanidine in Patients with Drug Induced Parkinsonism. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1250–1252.
- Kim, J.-S.; Oh, Y.-S.; Kim, Y.-I.; Yang, D.-W.; Chung, Y.-A.; You, I.-R.; Lee, K.-S. Combined Use of 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy and Dopamine Transporter (DAT) Positron Emission Tomography (PET) Predicts Prognosis in Drug-Induced Parkinsonism (DIP): A 2-Year Follow-up Study. Arch. Gerontol. Geriatr. 2013, 56, 124–128.
- Shafie, M.; Mayeli, M.; Saeidi, S.; Mirsepassi, Z.; Abbasi, M.; Shafeghat, M.; Aghamollaii, V. The Potential Role of the Cardiac MIBG Scan in Differentiating the Drug-Induced Parkinsonism from Parkinson’s Disease. Clin. Park. Relat. Disord. 2022, 6, 100130.
- Prasuhn, J.; Neumann, A.; Strautz, R.; Dreischmeier, S.; Lemmer, F.; Hanssen, H.; Heldmann, M.; Schramm, P.; Brüggemann, N. Clinical MR Imaging in Parkinson’s Disease: How Useful Is the Swallow Tail Sign? Brain Behav. 2021, 11, e02202.
- Sung, Y.H.; Noh, Y.; Lee, J.; Kim, E.Y. Drug-Induced Parkinsonism versus Idiopathic Parkinson Disease: Utility of Nigrosome 1 with 3-T Imaging. Radiology 2016, 279, 849–858.
- Cosottini, M.; Frosini, D.; Pesaresi, I.; Costagli, M.; Biagi, L.; Ceravolo, R.; Bonuccelli, U.; Tosetti, M. MR Imaging of the Substantia Nigra at 7 T Enables Diagnosis of Parkinson Disease. Radiology 2014, 271, 831–838.
- Berg, D.; Roggendorf, W.; Schröder, U.; Klein, R.; Tatschner, T.; Benz, P.; Tucha, O.; Preier, M.; Lange, K.W.; Reiners, K.; et al. Echogenicity of the Substantia Nigra: Association with Increased Iron Content and Marker for Susceptibility to Nigrostriatal Injury. Arch. Neurol. 2002, 59, 999–1005.
- Mehnert, S.; Reuter, I.; Schepp, K.; Maaser, P.; Stolz, E.; Kaps, M. Transcranial Sonography for Diagnosis of Parkinson’s Disease. BMC Neurol. 2010, 10, 9.
- Bouwmans, A.E.P.; Vlaar, A.M.M.; Mess, W.H.; Kessels, A.; Weber, W.E.J. Specificity and Sensitivity of Transcranial Sonography of the Substantia Nigra in the Diagnosis of Parkinson’s Disease: Prospective Cohort Study in 196 Patients. BMJ Open 2013, 3, e002613.
- Olivares Romero, J.; Arjona Padillo, A.; Barrero Hernández, F.J.; Martín González, M.; Gil Extremera, B. Utility of Transcranial Sonography in the Diagnosis of Drug-Induced Parkinsonism: A Prospective Study. Eur. J. Neurol. 2013, 20, 1451–1458.
- Oh, Y.-S.; Kwon, D.-Y.; Kim, J.-S.; Park, M.-H.; Berg, D. Transcranial Sonographic Findings May Predict Prognosis of Gastroprokinetic Drug-Induced Parkinsonism. Park. Relat. Disord. 2018, 46, 36–40.
- Lee, J.-Y.; Ahn, J.; Kim, T.W.; Jeon, B.S. Optical Coherence Tomography in Parkinson’s Disease: Is the Retina a Biomarker? J. Park. Dis. 2014, 4, 197–204.
- Jiménez, B.; Ascaso, F.J.; Cristóbal, J.A.; López del Val, J. Development of a Prediction Formula of Parkinson Disease Severity by Optical Coherence Tomography. Mov. Disord. 2014, 29, 68–74.
- Suh, W.; Baek, S.U.; Oh, J.S.; Seo, S.Y.; Kim, J.S.; Han, Y.M.; Kim, M.S.; Kang, S.Y. Retinal Thickness and Its Interocular Asymmetry Between Parkinson’s Disease and Drug-Induced Parkinsonism. J. Korean Med. Sci. 2023, 38, e86.
More
Information
Subjects:
Neuroimaging
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
541
Revisions:
2 times
(View History)
Update Date:
01 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




