1. Dopaminergic Hypothesis of Addiction—Wise (1980)
A significant portion of both animal and human behavior is governed by mechanisms of positive reinforcement. The initial steps to identify the underlying brain circuits of reward were taken by Olds and Milner
[1]. These researchers found that rats acquired the behavior of pressing a lever only when this action resulted in intracranial stimulation of certain brain regions, such as the medial forebrain bundle and the mesolimbic cortical dopaminergic pathway, referred to as “pleasure centers” by the researchers. They asserted that these “pleasure” regions would be the same as those activated by the satisfaction of basic needs such as food, thirst, and sex, explaining the reinforcement–maintenance of these behaviors. In the subsequent years, numerous intracranial electrical stimulation studies were conducted to “map” the different pleasure centers in the brain
[2].
Concurrently, pharmacological studies were conducted to determine which neurotransmitter was mediating reward signaling in these circuits. Initially, it was believed that noradrenaline pathways controlled reward, as the administration of noradrenergic antagonists decreased the acquisition of intracranial self-stimulation behavior
[3]. However, it was later established that this effect was due to the drowsiness and impairment caused by the blockade of this neurotransmitter rather than an effect on the reward response
[4]. As soon as the first selective dopamine antagonists were developed, it was confirmed that their administration had a specific effect on reward. Wise and collaborators studied the pharmacodynamics of drugs such as cocaine and amphetamine, discovering that both increased dopamine in the mesolimbic dopaminergic system, while dopamine antagonists were capable of blocking the rewarding effects of these psychoactive drugs
[5][6]. These authors also confirmed that other drugs like alcohol, benzodiazepines, and barbiturates produced their reinforcement through a similar mechanism, disinhibiting dopamine neurons rather than directly exciting them
[4]. Finally, they demonstrated that dopaminergic antagonists blocked natural reward associated with food
[7], reinforcing the theory that natural rewards, intracranial self-stimulation reward, and drugs act on a common dopamine-dependent substrate.
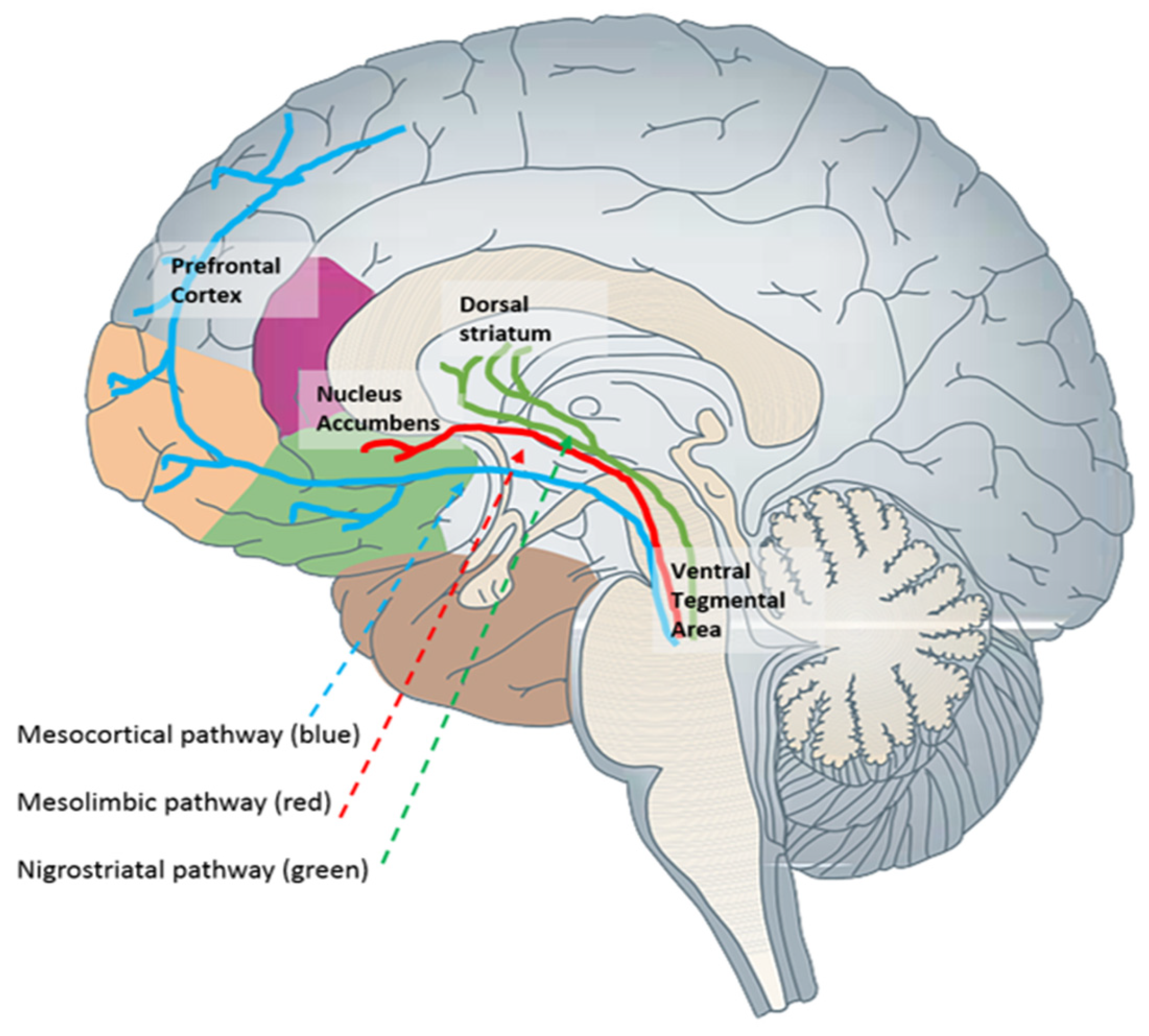
This substrate corresponds to the brain’s reward system, which aligns with the mesolimbic cortical dopaminergic pathway. This pathway includes projections originating from the ventral tegmental area and terminating in the nucleus accumbens, with additional connections to other structures such as the hippocampus, prefrontal cortex, amygdala, olfactory tubercle, and lateral septal nucleus (
Figure 1). Subsequent studies confirmed that the reward level induced by a drug is directly related to the phasic increase in dopamine levels in the nucleus accumbens
[8].
Figure 1. Main dopaminergic pathways. The brain reward system is primarily associated with the mesolimbic (red) and mesocortical (blue) pathways.
According to the “Dopaminergic Hypothesis of Addiction” drugs act through a common mechanism of increasing dopamine in the brain’s reward system, promoting positive reinforcement, and motivating drug consumption and addiction
[9]. In addition to an explanatory model of the mechanism of action of all drugs, this theory provides a preliminary neurophysiological understanding of the phenomenon of vulnerability to addiction.
Numerous preclinical and clinical studies supported that low dopamine release was a predictor of vulnerability to addiction, while an adequate dopaminergic tone played a role in the resilience of individuals who would not escalate in drug consumption
[9]. In the same vein, it was also described that the level of expression of D2/D3 dopamine receptors in the striatum modulated the subjective response to different substances
[10] Individuals with fewer dopamine receptors, with no prior drug use, reported greater pleasure or “liking” after the consumption of psychostimulants
[10][11]. Animal studies showed that these individuals engaged in more intense drug self-administration behaviors
[12]. Therefore, dopaminergic hypofunction in the mesolimbic system would, according to this theory, be a risk factor by enhancing the euphoric response and reward obtained from drugs.
However, this theory, by exclusively considering drug-induced positive reinforcement in its explanation, lacks explanatory value for various phenomena in the addictive process, such as withdrawal syndrome or craving, which would be better elucidated using a negative-reinforcement mechanism. Subsequently, based on this theory, others emerged asserting that the role of dopamine was not solely to mediate the experience of euphoria and reward but also to promote the formation of motivational salience, the establishment of habits, and reward expectations associated with cues
[13]. These new insights into the functions of dopamine led to the development of new neurobiological theories of addiction, such as Robinson and Berridge’s “Incentive Sensitization Theory”
[13].
2. Incentive Sensitization Theory—Robinson and Berridge (1993)
Drugs of abuse induce a highly intense and prolonged activation of the reward system, with dopamine increases between 3 and 10 times greater than those observed with natural rewards
[14]. After repeated consumption, elevations beyond “natural levels” will lead to alterations in the functioning of the reward system.
As mentioned earlier, dopamine’s role in this system extends beyond encoding reward; it also participates in the memory consolidation, habit formation, and motivational salience associated with the drug. Therefore, neuroadaptations in this system will compromise all these processes. In this regard, Robinson and Berridge
[13] suggested that neuroadaptive changes following repeated consumption make individuals hypersensitive to environmental cues associated with drug reward. These authors state that the reward system becomes sensitized; therefore, in the presence of environmental cues associated with the drug, individuals are involuntarily motivated to invest resources and energy in approaching the substance, which is perceived as relevant or necessary. This incentive salience could be understood as an attentional bias toward stimuli associated with drug consumption, highly charged with the power to motivate consummatory behavior. Therefore, these authors conclude that after repeated use, motivation shifts from an initial state of pleasure-driven consumption (liking) to pathological craving (wanting), reflecting a process of sensitization and conditioning of the reward system.
This theory is very interesting as it enables the explanation of craving, drug seeking, and drug consumption when the addict is exposed to environments and cues associated with drug use. However, it is limited since the authors have not delved into the neuroadaptive processes that would explain this hypersensitivity to cues, nor have they connected these processes with a greater or lesser susceptibility to addiction development.
3. Habit and Compulsion Theory—Robbins and Everitt (1999); Everitt and Robbins (2005)
The central idea of the “Habit and Compulsion” theory is that, in the addictive process, the initial substance consumption occurs voluntarily, driven by its recreational effects (positive reinforcement). However, with repeated consumption, the individual progressively loses control over the consumption behavior, which turns into a compulsive behavior (stimulus response) that is difficult to extinguish.
Building on the incentive sensitization theory
[13], Robbins and Everitt
[15] acknowledge that repeated overactivation of the dopaminergic system would lead to alterations that would cause drugs to acquire a high-incentive motivational value, that stimulates drug craving in the presence of associated cues
[13]. However, they assert that this theory would not explain why addicts find it impossible to control consumption behavior and why they persist despite the severe consequences of their addiction. Everitt and Robbins address this limitation by stating that neuroplastic processes also impact dopaminergic circuits controlling goal-directed behaviors and habit formation.
Thus, in the transition from voluntary and occasional consumption to compulsive consumption, a progressive shift in the locus of control of drug-associated behaviors would be observed, moving from top-down control to a regulation of the behavior controlled by the basal ganglia. Behavior would cease to be controlled by ventral striatal regions, extensively connected with the prefrontal cortex and amygdala, to be controlled by dorsal striatal regions, specialized in the maintenance of motor sequences. Therefore, the prefrontal cortex would increasingly have less inhibitory control over drug-associated motor behaviors, which would become compulsive and disinhibited.
The activation threshold necessary to initiate these motor habits would be progressively reduced, making the exposure to environmental cues enough to trigger drug-seeking and -consumption behaviors. Robbins and Everitt
[15] assert that this loss of control would not occur in all individuals who repeatedly consume a drug; only a percentage of vulnerable individuals would progress to compulsive consumption. Through studies with animal models, they found that rodents exhibiting more impulsive behavior robustly acquired drug self-administration behavior. In fact, in these preclinic studies researchers found that impulsive animals continued performing drug-seeking behaviors even when contextual cues indicated that the drug was not present or that an electric shock (punishment) would be administered if they pressed the lever
[16][17]. For these authors, impulsivity is the underlying factor of the susceptibility to escalate drug consumption, relapse, and ultimately lose control over substance use. Other researchers have conducted clinical studies confirming that the activation threshold required to initiate these motor habits would gradually decrease, making exposure to environmental cues sufficient enough to trigger drug-seeking and -consumption behaviors. According to Robbins and Everitt
[15], this loss of control would not occur in all individuals who repeatedly consume a drug; only a subset of vulnerable individuals would progress to compulsive consumption. Their research using animal models revealed that rodents displaying more impulsive behavior robustly acquired drug self-administration behavior. Notably, in these preclinical studies, impulsive animals persisted in drug-seeking behaviors even when contextual cues indicated the absence of the drug or the administration of an electric shock (punishment) upon lever pressing
[16]. For these authors, impulsivity is the underlying factor of the susceptibility to escalating drug consumption, relapse, and eventual loss of control over substance use. Additional clinical studies conducted by other researchers have supported that the construct of impulsivity is a predisposing factor associated with vulnerability to substance-use disorders, and also it is a consequence of chronic consumption
[18].
4. Allostasis Theory of Addiction—Koob and Le Moal (1997)
The “Allostasis” theory, developed by Koob and Le Moal
[19] shares with previous theories the idea that in the initial phases of the addictive process, consumption is motivated by the expectation of positive reinforcement. However, after intense and chronic consumption, behavior maintenance should be attributed to a process of negative reinforcement, as only the drug can alleviate the activation and discomfort that occurs during periods of abstinence. To explain this transition, Koob and Le Moal
[19][20] formulated a theory that integrates the foundations of the opponent-process theory
[21] and the concept of homeostasis.
Living organisms seek to actively regulate and maintain a stable internal environment, allowing them to adapt to changes in the external environment (homeostasis). As previously discussed, the consumption of drugs and their abuse leads to an activation of the reward system above its “natural” levels, making this aberrant activation a threat to the homeostasis of this system. This triggers the activation of two corrective mechanisms that aim to counteract the drug’s effects: on one hand, there is a loss of function in the reward system, resulting in an increase in its activation thresholds; on the other hand, there is hyperactivation of stress or anti-reward systems.
Koob and Le Moal
[19] assert that chronic activation of these corrective mechanisms can strengthen them to the point of counteracting and masking the rewarding effects of drugs. In fact, these corrective mechanisms would strengthen to such an extent that they would produce an overcompensation beyond the initial homeostatic level. Therefore, a new set point or “allostasis” would be established, as an attempt of anticipatory compensation for future drug consumption.
While the establishment of this new set point is an adaptive response aimed at anticipating and favoring the stability of the reward system, it has a negative impact on individual affect and motivation. This is because the person undergoes a chronic state of reward-system hypofunction and a state of stress-system hyperactivity. In other words, a constant state of stress and anti-reward dominates the subject’s motivational balance. In this state, basal dopaminergic tone and even natural rewards (such as food or sex) prove insufficient to return the system to its natural level. Only drugs abused will be capable of offsetting the negative consequences of this allostatic state, leading to their consumption not for their reinforcing effects but for their ability to alleviate this dysphoric state during abstinence.
This theory is the first to include structures outside the reward system to explain dysphoria and craving during abstinence. According to the authors, neuroadaptations within the reward system would underlie the diminished pleasurable effects of the drug. Additionally, they propose that the hyperactivity of the brain stress system, along with the subsequent increase in its neuroendocrine products (CRF and noradrenaline), accounts for anxiety and discomfort states during abstinence. This theory is the first to incorporate structures outside the reward system to elucidate dysphoria and craving during abstinence. According to the authors, neuroadaptations within the reward system underlie the diminished pleasurable effects of the drug.
Ultimately, the Allostasis Theory of addiction would help in understanding why stress acts as a risk factor in all stages of the addictive process, being a key factor in relapse. As previously mentioned, the neuroendocrine stress response actively participates in modulating the reward system, generating an anti-reward response that would amplify the rewarding value associated with the drug. In this sense, individuals that are more susceptible to stress, and those presenting a more pronounced and lasting endocrine response, would be particularly vulnerable to these negative effects.