
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Chool Boo | -- | 5013 | 2024-01-31 08:46:22 | | | |
| 2 | Mona Zou | Meta information modification | 5013 | 2024-02-01 08:26:18 | | |
Video Upload Options
Fibrosis, which causes structural hardening and functional degeneration in various organs, is characterized by the excessive production and accumulation of connective tissue containing collagen, alpha-smooth muscle actin (α-SMA), etc. In traditional medicine, extracts of medicinal plants or herbal prescriptions have been used to treat various fibrotic diseases. RA, as well as the extracts of Glechoma hederacea, Melissa officinalis, Elsholtzia ciliata, Lycopus lucidus, Ocimum basilicum, Prunella vulgaris, Salvia rosmarinus (Rosmarinus officinalis), Salvia miltiorrhiza, and Perilla frutescens, have been shown to attenuate fibrosis of the liver, kidneys, heart, lungs, etc. in experimental animal models. Their antifibrotic effects were associated with the attenuation of oxidative stress, inflammation, cell activation, epithelial–mesenchymal transition, and fibrogenic gene expression.
1. Introduction
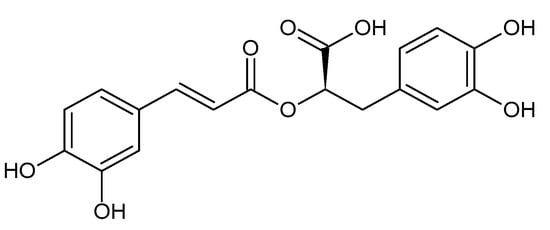
2. Liver Fibrosis
3. Kidney Fibrosis
4. Heart Fibrosis
5. Lung Fibrosis
6. Post-Surgical Abdominal Adhesion
7. Fibrosis in the Salivary Glands
8. Skin Wounds
9. Pterygium in the Eyes
10. Fibrosis of Autologous Fat Grafts
References
- Lee, S.I.; Kim, H.J.; Baek, M.C.; Park, K.M.; Park, Y.; Yoon, C.H.; Boo, Y.C. Wen-pi-tang-Hab-Wu-ling-san, an oriental herbal prescription, attenuates epithelial-mesenchymal transdifferentiation stimulated by TGF-beta1 in kidney cells. Phytother. Res. 2007, 21, 548–553.
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566.
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004.
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Asp. Med. 2023, 92, 101191.
- Gyorfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol. 2018, 68–69, 8–27.
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730.
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176.
- Sato, S.; Yanagihara, T.; Kolb, M.R.J. Therapeutic targets and early stage clinical trials for pulmonary fibrosis. Expert Opin. Investig. Drugs 2019, 28, 19–28.
- Rayego-Mateos, S.; Valdivielso, J.M. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin. Ther. Targets 2020, 24, 655–670.
- Park, S.; Nguyen, N.B.; Pezhouman, A.; Ardehali, R. Cardiac fibrosis: Potential therapeutic targets. Transl. Res. 2019, 209, 121–137.
- Latief, U.; Ahmad, R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J. Tradit. Complement. Med. 2018, 8, 352–360.
- Xu, Y.; Chen, J.; Wang, H.; Lu, Y. Research and application of herbal medicine in the treatment of chronic kidney disease since the 21st century: A visualized bibliometric analysis. Front. Pharmacol. 2022, 13, 971113.
- Wu, X.; Huang, J.; Wang, J.; Xu, Y.; Yang, X.; Sun, M.; Shi, J. Multi-Pharmaceutical Activities of Chinese Herbal Polysaccharides in the Treatment of Pulmonary Fibrosis: Concept and Future Prospects. Front. Pharmacol. 2021, 12, 707491.
- Wang, L.; Zhu, T.; Feng, D.; Li, R.; Zhang, C. Polyphenols from Chinese Herbal Medicine: Molecular Mechanisms and Therapeutic Targets in Pulmonary Fibrosis. Am. J. Chin. Med. 2022, 50, 1063–1094.
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell Longev. 2018, 2018, 8394818.
- Zhou, Z.; Qiao, Y.; Zhao, Y.; Chen, X.; Li, J.; Zhang, H.; Lan, Q.; Yang, B. Natural products: Potential drugs for the treatment of renal fibrosis. Chin. Med. 2022, 17, 98.
- Alberti, A. Importance of dietary hydroxycinnamic acids in the therapy of liver fibrosis. Orv. Hetil. 2012, 153, 948–953.
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600.
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292.
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98.
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421.
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153.
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228.
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410.
- Azhar, M.K.; Anwar, S.; Hasan, G.M.; Shamsi, A.; Islam, A.; Parvez, S.; Hassan, M.I. Comprehensive Insights into Biological Roles of Rosmarinic Acid: Implications in Diabetes, Cancer and Neurodegenerative Diseases. Nutrients 2023, 15, 4297.
- Bansal, R.; Poelstra, K. Hepatic Stellate Cell Targeting Using Peptide-Modified Biologicals. Methods Mol. Biol. 2023, 2669, 269–284.
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine 2010, 17, 282–288.
- Miao, C.G.; Yang, Y.Y.; He, X.; Huang, C.; Huang, Y.; Zhang, L.; Lv, X.W.; Jin, Y.; Li, J. Wnt signaling in liver fibrosis: Progress, challenges and potential directions. Biochimie 2013, 95, 2326–2335.
- Xu, C.Y.; Wang, J.; Zhu, T.J.; Shen, Y.; Tang, X.S.; Fang, L.; Xu, Y.Z. Cross-Talking Between PPAR and WNT Signaling and its Regulation in Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 247–254.
- Yang, M.D.; Chiang, Y.M.; Higashiyama, R.; Asahina, K.; Mann, D.A.; Mann, J.; Wang, C.C.; Tsukamoto, H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology 2012, 55, 1271–1281.
- Zhang, J.J.; Wang, Y.L.; Feng, X.B.; Song, X.D.; Liu, W.B. Rosmarinic acid inhibits proliferation and induces apoptosis of hepatic stellate cells. Biol. Pharm. Bull. 2011, 34, 343–348.
- De Smet, V.; Eysackers, N.; Merens, V.; Kazemzadeh Dastjerd, M.; Halder, G.; Verhulst, S.; Mannaerts, I.; van Grunsven, L.A. Initiation of hepatic stellate cell activation extends into chronic liver disease. Cell Death Dis. 2021, 12, 1110.
- Lu, C.; Zou, Y.; Liu, Y.; Niu, Y. Rosmarinic acid counteracts activation of hepatic stellate cells via inhibiting the ROS-dependent MMP-2 activity: Involvement of Nrf2 antioxidant system. Toxicol. Appl. Pharmacol. 2017, 318, 69–78.
- Wang, Y.Y.; Lin, S.Y.; Chen, W.Y.; Liao, S.L.; Wu, C.C.; Pan, P.H.; Chou, S.T.; Chen, C.J. Glechoma hederacea extracts attenuate cholestatic liver injury in a bile duct-ligated rat model. J. Ethnopharmacol. 2017, 204, 58–66.
- Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Liao, S.L.; Chou, S.T.; Yang, C.P.; Chen, C.J. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. 2017, 108, 214–223.
- Yang, T.; Shen, D.P.; Wang, Q.L.; Tao, Y.Y.; Liu, C.H. Investigation of the absorbed and metabolized components of Danshen from Fuzheng Huayu recipe and study on the anti-hepatic fibrosis effects of these components. J. Ethnopharmacol. 2013, 148, 691–700.
- Yang, T.; Liu, S.; Wang, C.H.; Tao, Y.Y.; Zhou, H.; Liu, C.H. Comparative pharmacokinetic and tissue distribution profiles of four major bioactive components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J. Pharm. Biomed. Anal. 2015, 114, 152–158.
- El-Lakkany, N.M.; El-Maadawy, W.H.; Seif El-Din, S.H.; Hammam, O.A.; Mohamed, S.H.; Ezzat, S.M.; Safar, M.M.; Saleh, S. Rosmarinic acid attenuates hepatic fibrogenesis via suppression of hepatic stellate cell activation/proliferation and induction of apoptosis. Asian Pac. J. Trop. Med. 2017, 10, 444–453.
- Kim, M.; Yoo, G.; Randy, A.; Son, Y.J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients 2020, 12, 1166.
- Lyu, C.; Kong, W.; Liu, Z.; Wang, S.; Zhao, P.; Liang, K.; Niu, Y.; Yang, W.; Xiang, C.; Hu, X.; et al. Advanced glycation end-products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nat. Biomed. Eng. 2023, 7, 1437–1454.
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352.
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84.
- Jung, H.W.; Yoon, C.H.; Kim, Y.H.; Boo, Y.C.; Park, K.M.; Park, Y.K. Wen-Pi-Tang-Hab-Wu-Ling-San extract inhibits the release of inflammatory mediators from LPS-stimulated mouse macrophages. J. Ethnopharmacol. 2007, 114, 439–445.
- Jung, H.W.; Jung, J.K.; Ramalingam, M.; Yoon, C.H.; Bae, H.S.; Park, Y.K. Anti-diabetic effect of Wen-pi-tang-Hab-Wu-ling-san extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2012, 44, 97–102.
- Seok, Y.M.; Kim, J.; Choi, K.C.; Yoon, C.H.; Boo, Y.C.; Park, Y.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney ischemia/reperfusion injury in mice A role for antioxidant enzymes and heat-shock proteins. J. Ethnopharmacol. 2007, 111, 333.
- Seok, Y.M.; Kim, J.; Park, M.J.; Boo, Y.C.; Park, Y.K.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney fibrosis induced by ischemia/reperfusion in mice. Phytother. Res. 2008, 22, 1057.
- Jung, K.J.; Kim, J.; Park, Y.K.; Yoon, Y.R.; Park, K.M. Wen-pi-tang-Hab-Wu-ling-san reduces ureteral obstructive renal fibrosis by the reduction of oxidative stress, inflammation, and TGF-beta/Smad2/3 signaling. Food Chem. Toxicol. 2010, 48, 522–529.
- Kim, T.W.; Kim, Y.J.; Seo, C.S.; Kim, H.T.; Park, S.R.; Lee, M.Y.; Jung, J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ss and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339.
- Hsieh, Y.H.; Tsai, J.P.; Ting, Y.H.; Hung, T.W.; Chao, W.W. Rosmarinic acid ameliorates renal interstitial fibrosis by inhibiting the phosphorylated-AKT mediated epithelial-mesenchymal transition in vitro and in vivo. Food Funct. 2022, 13, 4641–4652.
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027.
- Yao, Y.; Yang, J.; Wang, D.; Zhou, F.; Cai, X.; Lu, W.; Hu, C.; Gu, Z.; Qian, S.; Guan, X.; et al. The aqueous extract of Lycopus lucidus Turcz ameliorates streptozotocin-induced diabetic renal damage via inhibiting TGF-beta1 signaling pathway. Phytomedicine 2013, 20, 1160–1167.
- Wang, C.; Zhou, J.; Wang, S.; Liu, Y.; Long, K.; Sun, T.; Zhi, W.; Yang, Y.; Zhang, H.; Zhao, Y.; et al. Guanxining injection alleviates fibrosis in heart failure mice and regulates SLC7A11/GPX4 axis. J. Ethnopharmacol. 2023, 310, 116367.
- Su, Z.; Wang, X.; Gao, X.; Liu, Y.; Pan, C.; Hu, H.; Beyer, R.P.; Shi, M.; Zhou, J.; Zhang, J.; et al. Excessive activation of the alternative complement pathway in autosomal dominant polycystic kidney disease. J. Intern. Med. 2014, 276, 470–485.
- Fathiazad, F.; Matlobi, A.; Khorrami, A.; Hamedeyazdan, S.; Soraya, H.; Hammami, M.; Maleki-Dizaji, N.; Garjani, A. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimum basilicum L. (basil) against isoproterenol induced myocardial infarction in rats. Daru 2012, 20, 87.
- Wei, J.; Leng, L.; Sui, Y.; Song, S.; Owusu, F.B.; Li, X.; Cao, Y.; Li, P.; Wang, H.; Li, R.; et al. Phenolic acids from Prunella vulgaris alleviate cardiac remodeling following myocardial infarction partially by suppressing NLRP3 activation. Phytother. Res. 2024, 38, 384–399.
- Fei, Q.; Ma, H.; Zou, J.; Wang, W.; Zhu, L.; Deng, H.; Meng, M.; Tan, S.; Zhang, H.; Xiao, X.; et al. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J. Mol. Cell Cardiol. 2020, 145, 1–13.
- Liu, Q.; Tian, J.; Xu, Y.; Li, C.; Meng, X.; Fu, F. Protective Effect of RA on Myocardial Infarction-Induced Cardiac Fibrosis via AT1R/p38 MAPK Pathway Signaling and Modulation of the ACE2/ACE Ratio. J. Agric. Food Chem. 2016, 64, 6716–6722.
- Zhang, L.; Bei, Z.; Li, T.; Qian, Z. An injectable conductive hydrogel with dual responsive release of rosmarinic acid improves cardiac function and promotes repair after myocardial infarction. Bioact. Mater. 2023, 29, 132–150.
- Zhang, X.; Ma, Z.G.; Yuan, Y.P.; Xu, S.C.; Wei, W.Y.; Song, P.; Kong, C.Y.; Deng, W.; Tang, Q.Z. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKalpha/Smad3 signaling. Cell Death Dis. 2018, 9, 102.
- Zhang, X.; Zhu, J.X.; Ma, Z.G.; Wu, H.M.; Xu, S.C.; Song, P.; Kong, C.Y.; Yuan, Y.P.; Deng, W.; Tang, Q.Z. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 2019, 15, 556–567.
- Rahbardar, M.G.; Eisvand, F.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity. Nutr. Cancer 2022, 74, 747–760.
- Bahri, S.; Ben Ali, R.; Gasmi, K.; Mlika, M.; Fazaa, S.; Ksouri, R.; Serairi, R.; Jameleddine, S.; Shlyonsky, V. Prophylactic and curative effect of rosemary leaves extract in a bleomycin model of pulmonary fibrosis. Pharm. Biol. 2017, 55, 462–471.
- Bahri, S.; Mies, F.; Ben Ali, R.; Mlika, M.; Jameleddine, S.; Mc Entee, K.; Shlyonsky, V. Rosmarinic acid potentiates carnosic acid induced apoptosis in lung fibroblasts. PLoS ONE 2017, 12, e0184368.
- Bahri, S.; Ali, R.B.; Abdennabi, R.; Nahdi, A.; Mlika, M.; Jameleddine, S. Industrial Elimination of Essential Oils from Rosmarinus officinalis: In Support of the Synergic Antifibrotic Effect of Rosmarinic and Carnosic Acids in Bleomycin Model of Lung Fibrosis. Nutr. Cancer 2021, 73, 2376–2387.
- Zhang, T.; Ma, S.; Liu, C.; Hu, K.; Xu, M.; Wang, R. Rosmarinic Acid Prevents Radiation-Induced Pulmonary Fibrosis through Attenuation of ROS/MYPT1/TGFbeta1 Signaling Via miR-19b-3p. Dose Response 2020, 18, 1559325820968413.
- Luo, J.; Zhang, L.; Zhang, X.; Long, Y.; Zou, F.; Yan, C.; Zou, W. Protective effects and active ingredients of Salvia miltiorrhiza Bunge extracts on airway responsiveness, inflammation and remodeling in mice with ovalbumin-induced allergic asthma. Phytomedicine 2019, 52, 168–177.
- Shakeri, F.; Eftekhar, N.; Roshan, N.M.; Rezaee, R.; Moghimi, A.; Boskabady, M.H. Rosmarinic acid affects immunological and inflammatory mediator levels and restores lung pathological features in asthmatic rats. Allergol. Immunopathol. 2019, 47, 16–23.
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104.
- Oelhafen, K.; Shayota, B.J.; Muhleman, M.; Klaassen, Z.; Shoja, M.M.; Tubbs, R.S.; Loukas, M. Peritoneal bands: A review of anatomical distribution and clinical implications. Am. Surg. 2012, 78, 377–384.
- Kakanezhadi, A.; Rezaei, M.; Raisi, A.; Dezfoulian, O.; Davoodi, F.; Ahmadvand, H. Rosmarinic acid prevents post-operative abdominal adhesions in a rat model. Sci. Rep. 2022, 12, 18593.
- Peng, H.H.; Chen, Y.M.; Lee, C.I.; Lee, M.W. Synthesis of a disulfide cross-linked polygalacturonic acid hydrogel for biomedical applications. J. Mater. Sci. Mater. Med. 2013, 24, 1375–1382.
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188.
- Castelli, J.; Simon, A.; Louvel, G.; Henry, O.; Chajon, E.; Nassef, M.; Haigron, P.; Cazoulat, G.; Ospina, J.D.; Jegoux, F.; et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat. Oncol. 2015, 10, 6.
- Zhang, T.; Liu, C.; Ma, S.; Gao, Y.; Wang, R. Protective Effect and Mechanism of Action of Rosmarinic Acid on Radiation-Induced Parotid Gland Injury in Rats. Dose Response 2020, 18, 1559325820907782.
- Kuba, M.C.; Turkoglu, A.; Oguz, A.; Tuncer, M.C.; Kaya, S.; Basol, O.; Bilge, H.; Tatli, F. Comparison of local rosmarinic acid and topical dexpanthenol applications on wound healing in a rat experimental wound model. Folia Morphol. 2021, 80, 618–624.
- Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules 2023, 28, 4034.
- Shahraki, T.; Arabi, A.; Feizi, S. Pterygium: An update on pathophysiology, clinical features, and management. Ther. Adv. Ophthalmol. 2021, 13, 25158414211020152.
- Chen, Y.Y.; Tsai, C.F.; Tsai, M.C.; Chen, W.K.; Hsu, Y.W.; Lu, F.J. Anti-fibrotic effect of rosmarinic acid on inhibition of pterygium epithelial cells. Int. J. Ophthalmol. 2018, 11, 189–195.
- Cin, B.; Ciloglu, N.S.; Omar, S.; Kaya Terzi, N. Effect of Rosmarinic Acid and Alcohol on Fat Graft Survival in Rat Model. Aesthetic Plast. Surg. 2020, 44, 177–185.




