
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zeinab Ezzeddine | -- | 3208 | 2024-01-31 08:01:43 | | | |
| 2 | Sirius Huang | Meta information modification | 3208 | 2024-02-01 01:37:32 | | |
Video Upload Options
The opportunistic pathogen Klebsiella pneumoniae (K. pneumoniae) can colonize mucosal surfaces and spread from mucosae to other tissues, causing fatal infections. Medical equipment and the healthcare setting can become colonized by Klebsiella species, which are widely distributed in nature and can be found in water, soil, and animals. Moreover, a substantial number of community-acquired illnesses are also caused by this organism worldwide. These infections are characterized by a high rate of morbidity and mortality as well as the capacity to spread metastatically. Hypervirulent Klebsiella strains are thought to be connected to these infections. Four components are critical to this bacterium’s pathogenicity—the capsule, lipopolysaccharide, fimbriae, and siderophores.
1. Introduction
2. Bacteriology of K. pneumoniae
3. Pathophysiology of K. pneumoniae
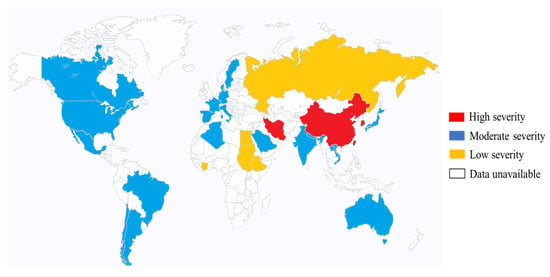
4. Epidemiology of K. pneumoniae (Mainly Hypervirulent K. pneumoniae in Eastern Asia)
References
- Choby, J.E.; Howard-Anderson, J.; Weiss, D.S. Hypervirulent Klebsiella pneumoniae-clinical and molecular perspectives. J. Intern. Med. 2020, 287, 283–300.
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017.
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744.
- Pomakova, D.K.; Hsiao, C.-B.; Beanan, J.M.; Olson, R.; MacDonald, U.; Keynan, Y.; Russo, T.A. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: An emerging and under-recognized pathogenic variant. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 981–989.
- Lan, P.; Shi, Q.; Zhang, P.; Chen, Y.; Yan, R.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Core genome allelic profiles of clinical Klebsiella pneumoniae strains using a random forest algorithm based on multilocus sequence typing scheme for hypervirulence analysis. J. Infect. Dis. 2020, 221, 263–271.
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118.
- Lee, H.C.; Chuang, Y.C.; Yu, W.L.; Lee, N.Y.; Chang, C.M.; Ko, N.Y.; Wang, L.R.; Ko, W.C. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: Association with invasive syndrome in patients with community-acquired bacteraemia. J. Intern. Med. 2006, 259, 606–614.
- Li, W.; Sun, G.; Yu, Y.; Li, N.; Chen, M.; Jin, R.; Jiao, Y.; Wu, H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin. Infect. Dis. 2014, 58, 225–232.
- Liu, Y.; Li, X.Y.; Wan, L.G.; Jiang, W.Y.; Yang, J.H.; Li, F.Q. Virulence and transferability of resistance determinants in a novel Klebsiella pneumonia sequence type 1137 in China. Microb. Drug Resist. 2014, 20, 150–155.
- Bergey, D.H.; David, R.; Boone, G.M.; Garrity, R.W.C.; Bergey, D.H. Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA; London, UK, 2001.
- Wen, Z.; Zhang, J.-R. Chapter 3—Bacterial Capsules. In Molecular Medical Microbiology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015.
- Domenico, P.; Salo, R.J.; Cross, A.S.; Cunha, B.A. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumonia. Infect. Immun. 1994, 62, 4495–4499.
- Brissac, T.; Martínez, E.; Kruckow, K.L.; Riegler, A.N.; Ganaie, F.; Im, H.; Bakshi, S.; Arroyo-Diaz, N.M.; Spencer, B.L.; Saad, J.S.; et al. Capsule Promotes Intracellular Survival and Vascular Endothelial Cell Translocation during Invasive Pneumococcal Disease. mBio 2021, 12, e0251621.
- Lawlor, M.S.; Handley, S.A.; Miller, V.L. Comparison of the host responses to wild-type and cpsB mutant Klebsiella pneumoniae infections. Infect. Immun. 2006, 74, 5402–5407.
- Pan, Y.J.; Lin, T.L.; Chen, C.T.; Chen, Y.Y.; Hsieh, P.F.; Hsu, C.R.; Wu, M.C.; Wang, J.T. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 2015, 5, 15573.
- Russo, T.A.; Olson, R.; Fang, C.T.; Stoesser, N.; Miller, M.; MacDonald, U.; Hutson, A.; Barker, J.H.; La Hoz, R.M.; Johnson, J.R. Identification of biomarkers for the differentiation of hypervirulent Klebsiella pneumonia from classical K. pneumoniae. J. Clin. Microbiol. 2018, 56, e00776-18.
- Wang, L.; Shen, D.; Wu, H.; Ma, Y. Resistance of hypervirulent Klebsiella pneumoniae to both intracellular and extracellular killing of neutrophils. PLoS ONE 2017, 12, e0173638.
- Shankar-Sinha, S.; Valencia, G.A.; Janes, B.K.; Rosenberg, J.K.; Whitfield, C.; Bender, R.A.; Standiford, T.J.; Younger, J.G. The Klebsiella pneumoniae O antigen contributes to bacteremia and lethality during murine pneumonia. Infect. Immun. 2004, 72, 1423–1430.
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Jhugroo, P. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis. Mon. 2020, 66, 100971.
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603.
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010, 10, 179.
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical Epidemiology of the Global Expansion of Klebsiella pneumoniae Carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796.
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661.
- Hsu, C.R.; Lin, T.L.; Pan, Y.J.; Hsieh, P.F.; Wang, J.T. Isolation of a Bacteriophage specific for a new capsular type of Klebsiella pneumoniae and Characterization of its Polysaccharide Depolymerase. PLoS ONE 2013, 8, e70092.
- Ramos, P.I.; Picão, R.C.; Vespero, E.C.; Pelisson, M.; Zuleta, L.F.G.; Almeida, L.G.P.; Gerber, A.L.; Vasconcelos, A.T.R.; Gales, A.C.; Nicolás, M.F. Pyrosequencing-Based Analysis Reveals a Novel Capsular Gene Cluster in a KPC-Producing Klebsiella pneumoniae Clinical Isolate Identified in Brazil. BMC Microbiol. 2012, 12, 173.
- Li, B.; Zhao, Y.; Liu, C.; Chen, Z.; Zhou, D. Molecular Pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2014, 9, 1071–1081.
- Yang, F.L.; Yang, Y.L.; Liao, P.C.; Chou, J.C.; Tsai, K.C.; Yang, A.S.; Sheu, F.; Lin, T.L.; Hsieh, P.F.; Wang, J.T.; et al. Structure and Immunological Characterization of the Capsular Polysaccharide of a Pyrogenic Liver Abscess caused by Klebsiella pneumoniae: Activation of Macrophages through Toll-like Receptor 4. J. Biol. Chem. 2011, 286, 21041–21051.
- Gonzalez-Ferrer, S.; Peñaloza, H.F.; Budnick, J.A.; Bain, W.G.; Nordstrom, H.R.; Lee, J.S.; Van Tyne, D. Finding Order in the Chaos: Outstanding Questions in Klebsiella pneumoniae Pathogenesis. Infect. Immun. 2021, 89, e00693-20.
- Merino, S.; Altarriba, M.; Izquierdo, L.; Nogueras, M.M.; Regue, M.; Tomas, J.M. Cloning and Sequencing of the Klebsiella pneumoniae O5 wb Gene Cluster and its Role in Pathogenesis. Infect. Immun. 2000, 68, 2435–2440.
- Clements, A.; Tull, D.; Jenney, A.W.; Farn, J.L.; Kim, S.H.; Bishop, R.E.; McPhee, J.B.; Hancock, R.E.; Hartland, E.L.; Pearse, M.J.; et al. Secondary Acylation of Klebsiella pneumoniae Lipopolysaccharide Contributes to Sensitivity to Antibacterial Peptides. J. Biol. Chem. 2007, 282, 15569–15577.
- March, C.; Cano, V.; Moranta, D.; Llobet, E.; Pérez-Gutiérrez, C.; Tomás, J.M.; Suárez, T.; Garmendia, J.; Bengoechea, J.A. Role of Bacterial Surface Structures on the Interaction of Klebsiella pneumoniae with Phagocytes. PLoS ONE 2013, 8, e56847.
- Struve, C.; Bojer, M.; Krogfelt, K.A. Characterization of Klebsiella pneumoniae Type 1 Fimbriae by Detection of Phase Variation during Colonization and Infection and Impact on Virulence. Infect. Immun. 2008, 76, 4055–4065.
- Stahlhut, S.G.; Struve, C.; Krogfelt, K.A. Klebsiella pneumoniae Type 3 Fimbriae Agglutinate Yeast in a Mannose-Resistant Manner. J. Med. Microbiol. 2012, 61 Pt 3, 317–322.
- Struve, C.; Bojer, M.; Krogfelt, K.A. Identification of a Conserved Chromosomal Region Encoding Klebsiella pneumoniae Type 1 and Type 3 Fimbriae and Assessment of the Role of Fimbriae in Pathogenicity. Infect. Immun. 2009, 77, 5016–5024.
- Wu, C.C.; Huang, Y.J.; Fung, C.P.; Peng, H.L. Regulation of the Klebsiella pneumoniae Kpc Fimbriae by the Site-Specific Recombinase KpcI. Microbiology 2010, 156 Pt 7, 1983–1992.
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995.
- Sebghati, T.A.; Korhonen, T.K.; Hornick, D.B.; Clegg, S. Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect. Immun. 1998, 66, 2887–2894.
- Di Martino, P.; Livrelli, V.; Sirot, D.; Joly, B.; Darfeuille-Michaud, A. A New Fimbrial Antigen Harbored by CAZ-5/ SHV-4-Producing Klebsiella pneumoniae Strains Involved in Nosocomial Infections. Infect. Immun. 1996, 64, 2266–2273.
- Regueiro, V.; Moranta, D.; Frank, C.G.; Larrarte, E.; Margareto, J.; March, C.; Garmendia, J.; Bengoechea, J.A. Klebsiella pneumoniae Subverts the Activation of Inflammatory Responses in a NOD1-Dependent Manner. Cell Microbiol. 2011, 13, 135–153.
- Llobet, E.; March, C.; Gimenez, P.; Bengoechea, J.A. Klebsiella pneumoniae OmpA Confers Resistance to Antimicrobial Peptides. Antimicrob. Agents Chemother. 2009, 53, 298–302.
- Tsai, Y.K.; Fung, C.P.; Lin, J.C.; Chen, J.-H.; Chang, F.-Y.; Chen, T.-L.; Siu, L.K. Klebsiella pneumoniae Outer Membrane Porins OmpK35 and OmpK36 Play Roles in Both Antimicrobial Resistance and Virulence. Antimicrob. Agents Chemother. 2011, 55, 1485–1493.
- Padilla, E.; Llobet, E.; Domenech-Sanchez, A.; Martinez-Martinez, L.; Bengoechea, J.A.; Alberti, S. Klebsiella pneumoniae AcrAB Efflux Pump Contributes to Antimicrobial Resistance and Virulence. Antimicrob. Agents Chemother. 2010, 54, 177–183.
- Pollack, M.; Nieman, R.; Reinhardt, J.; Charache, P.; Jett, M.; Hardy, P., Jr. Factors influencing colonisation and antibiotic-resistance patterns of gram-negative bacteria in hospital patients. Lancet 1972, 300, 668–671.
- Chiu, C.T.; Lin, D.Y.; Liaw, Y.F. Metastatic Septic Endophthalmitis in Pyogenic Liver Abscess. J. Clin. Gastroenterol. 1988, 10, 524–527.
- Liu, Y.C. Klebsiella pneumoniae Liver Abscess Associated With Septic Endophthalmitis. Arch. Intern. Med. 1986, 146, 1913.
- Victor, L.Y.; Hansen, D.S.; Ko, W.C.; Sagnimeni, A.; Klugman, K.P.; Von Gottberg, A.; Goossens, H.; Wagener, M.M.; Benedi, V.J.; International Klebsiella Study Group. Virulence Characteristics of Klebsiella and Clinical Manifestations of K. pneumoniae Bloodstream Infections. Emerg. Infect. Dis. 2007, 13, 986–993.
- Coutinho, R.L.; Visconde, M.F.; Descio, F.J.; Nicoletti, A.G.; Pinto, F.C.; da Silva, A.C.R.; Rodrigues-Costa, F.; Gales, A.C.; Furtado, G.H. Community-acquired invasive liver abscess syndrome caused by a K1 serotype Klebsiella pneumoniae isolate in Brazil: A case report of hypervirulent ST23. Mem. Inst. Oswaldo Cruz 2014, 109, 970–971.
- Fazili, T.; Sharngoe, C.; Endy, T.; Kiska, D.; Javaid, W.; Polhemus, M. Klebsiella pneumoniae Liver Abscess: An Emerging Disease. Am. J. Med. Sci. 2016, 351, 297–304.
- Sturm, E.; Tai, A.; Lin, B.; Kwong, J.; Athan, E.; Howden, B.P.; Angliss, R.D.; Asaid, R.; Pollard, J. Bilateral osteomyelitis and liver abscess caused by hypervirulent Klebsiella pneumoniae—A rare clinical manifestation (case report). BMC Infect. Dis. 2018, 18, 1–5.
- Sánchez-López, J.; García-Caballero, A.; Navarro-San Francisco, C.; Quereda, C.; Ruiz-Garbajosa, P.; Navas, E.; Dronda, F.; Morosini, M.I.; Cantón, R.; Diez-Aguilar, M. Hypermucoviscous Klebsiella pneumoniae: A challenge in community acquired infection. ID Cases 2019, 17, e00547.
- Chung, D.R.; Lee, S.S.; Lee, H.R.; Kim, H.B.; Choi, H.J.; Eom, J.S.; Kim, J.S.; Choi, Y.H.; Lee, J.S.; Chung, M.H.; et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J. Infect. 2007, 54, 578–583.
- Guo, Y.; Wang, S.; Zhan, L.; Jin, Y.; Duan, J.; Hao, Z.; Lv, J.; Qi, X.; Chen, L.; Kreiswirth, B.N.; et al. Microbiological and Clinical Characteristics of Hypermucoviscous Klebsiella pneumoniae Isolates Associated with Invasive Infections in China. Front. Cell Infect. Microbiol. 2017, 7, 24.
- Cubero, M.; Grau, I.; Tubau, F.; Pallarés, R.; Dominguez, M.; Liñares, J.; Ardanuy, C. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013). Clin. Microbiol. Infect. 2016, 22, 154–160.
- Peirano, G.; Pitout, J.D.; Laupland, K.B.; Meatherall, B.; Gregson, D.B. Population-Based Surveillance for Hypermucoviscosity Klebsiella pneumoniae Causing Community-Acquired Bacteremia in Calgary, Alberta. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 61–64.
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumonia. Clin. Microbiol. Rev. 2019, 32, 10–1128.
- Albasha, A.M.; Osman, E.H.; Abd-Alhalim, S.; Alshaib, E.F.; Al-Hassan, L.; Altayb, H.N. Detection of several carbapenems resistant and virulence genes in classical and hyper-virulent strains of Klebsiella pneumoniae isolated from hospitalized neonates and adults in Khartoum. BMC Res. Notes 2020, 13, 312.
- Vasilyev, I.Y.; Nikolaeva, I.V.; Siniagina, M.N.; Kharchenko, A.M.; Shaikhieva, G.S. Multidrug-Resistant Hypervirulent Klebsiella pneumoniae Found Persisting Silently in Infant Gut Microbiota. Int. J. Microbiol. 2020, 2020, 4054393.
- Sewunet, T.; Asrat, D.; Woldeamanuel, Y.; Ny, S.; Westerlund, F.; Aseffa, A.; Giske, C.G. High prevalence of blaCTX-M-15 and nosocomial transmission of hypervirulent epidemic clones of Klebsiella pneumoniae at a tertiary hospital in Ethiopia. JAC Antimicrob. Resist. 2021, 3, dlab001.
- Emam, S.M.; Abdelrahman, S.; Hasan, A.A.; EL-Melouk, M.S. Hypervirulent Klebsiella pneumoniae at Benha University Hospitals. Egypt. J. Hosp. Med. 2023, 90, 3592–3597.
- Liu, B.T.; Zhang, X.Y.; Wan, W.S.; Hao, J.J.; De Jiang, R.; Song, F.J. Characteristics of carbapenem-resistant Enterobacteriaceae in ready-to-eat vegetables in China. Front. Microbiol. 2018, 9, 359969.
- Furlan, J.P.R.; Savazzi, E.A.; Stehling, E.G. Genomic insights into multidrug-resistant and hypervirulent Klebsiella pneumoniae co-harboring metal resistance genes in aquatic environments. Ecotoxicol. Environ. Saf. 2020, 201, 110782.
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Edwards, D.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; Pilcher, D.V.; et al. Gastrointestinal Carriage Is a Major Reservoir of Klebsiella pneumoniae Infection in Intensive Care Patients. Clin. Infect. Dis. 2017, 65, 208–215.
- Fung, C.P.; Lin, Y.T.; Lin, J.C.; Chen, T.L.; Yeh, K.M.; Chang, F.Y.; Chuang, H.C.; Wu, H.S.; Tseng, C.P.; Siu, L.K. Klebsiella pneumoniae in Gastrointestinal Tract and Pyogenic Liver Abscess. Emerg. Infect. Dis. 2012, 18, 1322.
- Harada, S.; Tateda, K.; Mitsui, H.; Hattori, Y.; Okubo, M.; Kimura, S.; Sekigawa, K.; Kobayashi, K.; Hashimoto, N.; Itoyama, S.; et al. Familial Spread of a Virulent Clone of Klebsiella pneumoniae Causing Primary Liver Abscess. J. Clin. Microbiol. 2011, 49, 2354–2356.
- Zhu, J.; Wang, T.; Chen, L.; Du, H. Virulence Factors in Hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 642484.
- Baker, J.L.; Hendrickson, E.L.; Tang, X.; Lux, R.; He, X.; Edlund, A.; McLean, J.S.; Shi, W. Klebsiella and Providencia Emerge as Lone Survivors Following Long-Term Starvation of Oral Microbiota. Proc. Natl. Acad. Sci. USA 2019, 116, 8499–8504.
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic Analysis of Diversity, Population Structure, Virulence, and Antimicrobial Resistance in Klebsiella pneumoniae, an Urgent Threat to Public Health. Proc. Natl. Acad. Sci. USA 2015, 112, 3574–3581.
- Hsieh, P.F.; Lu, Y.R.; Lin, T.L.; Lai, L.Y.; Wang, J.T. Klebsiella pneumoniae Type VI Secretion System Contributes to Bacterial Competition, Cell Invasion, Type-1 Fimbriae Expression, and in vivo Colonization. J. Infect. Dis. 2019, 219, 637–647.
- Dunn, S.J.; Connor, C.; McNally, A. The Evolution and Transmission of Multi-Drug Resistant Escherichia coli and Klebsiella pneumoniae: The Complexity of Clones and Plasmids. Curr. Opin. Microbiol. 2019, 51, 51–56.
- Wyres, K.L.; Wick, R.R.; Judd, L.M.; Froumine, R.; Tokolyi, A.; Gorrie, C.L.; Lam, M.M.C.; Duchêne, S.; Jenney, A.; Holt, K.E. Distinct Evolutionary Dynamics of Horizontal Gene Transfer in Drug Resistant and Virulent Clones of Klebsiella pneumoniae. PLoS Genet. 2019, 15, 1008114.
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Monnet, D.L. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281.
- Stojowska-Swędrzyńska, K.; Łupkowska, A.; Kuczyńska-Wiśnik, D.; Laskowska, E. Antibiotic Heteroresistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 2021, 23, 449.
- Prince, S.E.; Dominger, K.A.; Cunha, B.A.; Klein, N.C. Klebsiella pneumoniae Pneumonia. Heart Lung 1997, 26, 413–417.
- Pana, Z.D.; Zaoutis, T. Treatment of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae (ESBLs) Infections: What Have We Learned Until Now? F1000Research 2018, 7. F1000 Faculty Rev-1347.
- Fritzenwanker, M.; Imirzalioglu, C.; Herold, S.; Wagenlehner, F.M.; Zimmer, K.-P.; Chakraborty, T. Treatment Options for Carbapenem-Resistant Gram-Negative Infections. Dtsch. Arztebl. Int. 2018, 115, 345–352.




