
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thiago Moura Rocha | -- | 5185 | 2024-01-30 17:11:40 | | | |
| 2 | Mona Zou | Meta information modification | 5185 | 2024-02-01 08:17:00 | | | | |
| 3 | Mona Zou | Meta information modification | 5185 | 2024-02-01 08:17:50 | | |
Video Upload Options
Agriculture plays a major role on society, especially in developing countries which rely on commodity exportation markets. To maintain high crop productivity, the use of agrochemicals was once employed as the main strategy, which in turn affected soil, water, and human health. In order to aid this issue, identifying some alternatives, such as the implementation of biofertilizers and inoculants as bioinputs in modern agriculture, are imperative to improve ecosystem quality. Among these bioinputs, a few bioproducts have shown good performances, such as phytohormones (e.g., auxins and giberellins), biosurfactants, and other enzymes; thus, it is extremely important to assure the quality and feasibility of their production in biorefinery scenarios. These bioproducts can be synthesized through fermentation processes through utilizing plant biomasses and agricultural byproducts as carbon sources. In this sense, to increase the tecno-economical availability of these processes, the implementation of solid-state fermentation (SSF) has shown great potential due to its ease of operation and cost-attractiveness.
1. Inoculants and Biological Controlling Agents as Bioinputs
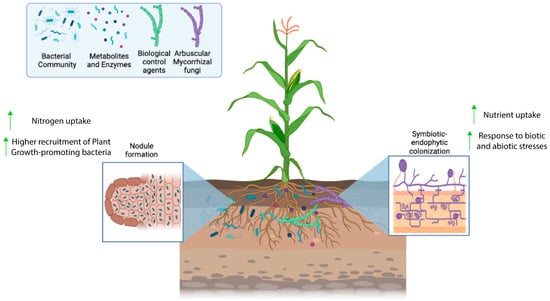
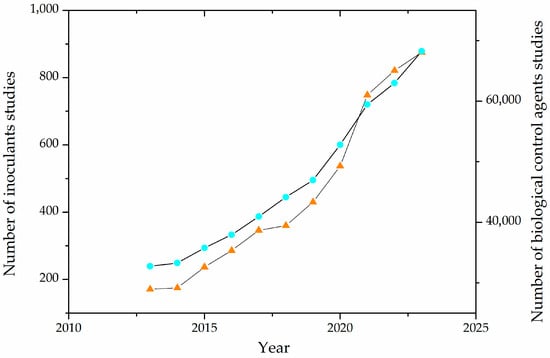
Production of Inoculants and Biological Control Agents by SSF
2. Biosurfactants: Promising and Emerging Agricultural Bioinputs
Production of Biosurfactants in SSF
3. Phytohormones as Agricultural Bioinputs
3.1. Gibberellic Acids
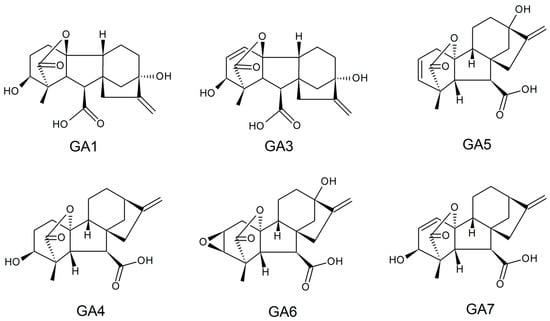
Production of Gibberellic Acids via SSF
3.2. Auxins
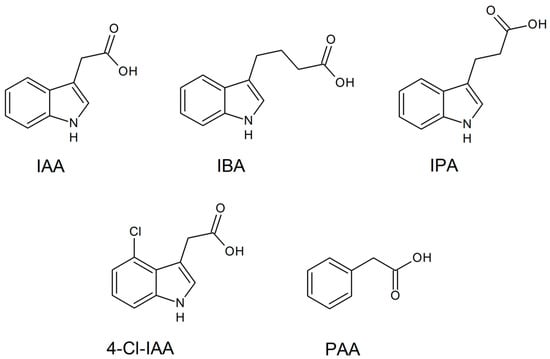
Production of Auxins in SSF
4. Other Important Agricultural Bioinputs Obtained by SSF
References
- Laditi, M.A.; Nwoke, O.C.; Jemo, M.; Abaidoo, R.C.; Ogunjobi, A.A. Evaluation of microbial inoculants as biofertilizers for the improvement of growth and yield of soybean and maize crops in savanna soils. Afr. J. Agric. Res. 2012, 7, 405–413.
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985.
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425.
- Abdullahi, I.N.; Chuwang, P.Z.; Isah, A.D. Effect of biofertilizer application on growth of Vitellaria paradoxa seedlings. J. Res. Environ. Sci. Toxicol. 2012, 1, 294–297.
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilizers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607.
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577.
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355.
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The Role of Microbial Inoculants on Plant Protection, Growth Stimulation, and Crop Productivity of the Olive Tree (Olea europea L.). Plants 2020, 9, 743.
- Kowalska, J.; Krzymińska, J.; Tyburski, J. Yeasts as a Potential Biological Agent in Plant Disease Protection and Yield Improvement—A Short Review. Agriculture 2022, 12, 1404.
- Savita Sharma, A. Fungi as Biological Control Agents. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55, pp. 395–411.
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408.
- Koul, B.; Chopra, M.; Lamba, S. Microorganisms as biocontrol agents for sustainable agriculture. In Relationship Between Microbes and the Environment for Sustainable Ecosystem Services; Samuel, J., Kumar, A., Singh, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 45–68.
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/agricultural-inoculants-market (accessed on 17 December 2023).
- Markets and Markets. Agricultural Inoculants Market. Available online: https://www.marketsandmarkets.com/Market-Reports/agricultural-inoculants-market-152735696.html (accessed on 17 December 2023).
- Marcelino, P.R.F.; Milani, K.M.L.; Mali, S.; dos Santos, O.J.A.P.; de Oliveira, A.L.M. Formulations of polymeric biodegradable low-cost foam by melt extrusion to deliver plant growth-promoting bacteria in agricultural systems. Appl. Microbiol. Biotechnol. 2016, 100, 7323–7338.
- Zhao, H.; Zhang, X.; Li, Z. Growth of Rhizobium leguminosarum in a periodic pressure oscillating, solid-state fermentation of wheat straw. Biotechnol. Lett. 2001, 23, 827–829.
- Molina-Peñate, E.; del Carmen Vargas-García, M.; Artola, A.; Sánchez, A. Filling in the gaps in biowaste biorefineries: The use of the solid residue after enzymatic hydrolysis for the production of biopesticides through solid-state fermentation. Waste Manag. 2023, 161, 92–103.
- Vassilev, N.; Baca, M.T.; Vassileva, M.; Franco, I.; Azcon, R. Rock phosphate solubilization by Aspergillus niger grown on sugar-beet waste medium. Appl. Microbiol. Biotechnol. 1995, 44, 546–549.
- Mikkelsen, R.L. Humic materials for agriculture. Better Crops 2005, 89, 6–10.
- Burlakovs, J.; Kļaviņš, M.; Osinska, L.; Purmalis, O. The impact of humic substances as remediation agents to the speciation forms of metals in soil. APCBEE Procedia 2013, 5, 192–196.
- Huelva López, R.; Martínez Balmori, D.; Calderín García, A.; Hernández González, O.L.; Guridi Izquierdo, F. Propiedades químicas y química-físicas de derivados estructurales de ácidos húmicos obtenidos de vermicompost. Actividad biológica. Rev. Cienc. Téc. Agropecu. 2013, 22, 56–60.
- Yel, E.; Ahmetli, G. Environmental dilemma of humic substances: Being adsorbents and being carcinogens. Int. J. Environ. Sci. Dev. 2015, 6, 73.
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974.
- Volpi, M.P.C.; Corzo, I.J.M.; Bastos, R.G.; Santana, M.H.A. Production of humic acids by solid-state fermentation of Trichoderma reesei in raw oil palm empty fruit bunch fibers. 3 Biotech 2019, 9, 393.
- Ji, J.L.; Chen, F.; Liu, S.; Yang, Y.; Hou, C.; Wang, Y.Z. Co-production of biogas and humic acid using rice straw and pig manure as substrates through solid-state anaerobic fermentation and subsequent aerobic composting. J. Environ. Manag. 2022, 320, 115860.
- Vassilev, N.; de Oliveira Mendes, G. Solid-state fermentation and plant-beneficial microorganisms. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Pandey, A., Larroche, C., Carlos Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 435–450.
- Mendes, G.O.; Dias, C.S.; Silva, I.R.; Júnior, J.I.R.; Pereira, O.L.; Costa, M.D. Fungal rock phosphate solubilization using sugarcane bagasse. World J. Microbiol. Biotechnol. 2013, 29, 43–50.
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Lobermann, B.; Malusa, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996.
- Cameotra, S.S.; Makkar, R.S. Biosurfactant-enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 2010, 82, 97–116.
- Marcelino, P.R.F.; Gonçalves, F.; Jimenez, I.M.; Carneiro, B.C.; Santos, B.B.; da Silva, S.S. Sustainable production of biosurfactants and their applications. In Lignocellulosic Biorefining Technologies, 1st ed.; Ingle, A.P., Chandel, A.K., Silva, S.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 1, pp. 159–183.
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for Sophorolipid biosynthesis. Biotechnol. Adv. 2022, 54, 107788.
- Zeng, Z.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.; Liu, Z.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.; et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds. Sci. Total Environ. 2018, 634, 1–11.
- Kallimanis, A.; Frillingos, S.; Drainas, C.; Koukkou, A.I. Taxonomic identification, phenanthrene uptake activity, and membrane lipid alterations of the PAH degrading Arthrobacter sp. strain Sphe3. Appl. Microbiol. Biotechnol. 2007, 76, 709–717.
- Murínová, S.; Dercová, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 873081.
- Sana, S.; Datta, S.; Biswas, D.; Sengupta, D. Assessment of synergistic antibacterial activity of combined biosurfactants revealed by bacterial cell envelop damage. Biochim. Biophys. Acta Biomembr. 2018, 1860, 579–585.
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35.
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406.
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693.
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13.
- Banat, I.M.; Carboue, Q.; Saucedo-Castaneda, G.; de Jesus Cazares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222.
- Franco Marcelino, P.R.; da Silva, V.L.; Rodrigues Philippini, R.; Von Zuben, C.J.; Contiero, J.; Dos Santos, J.C.; da Silva, S.S. Biosurfactants produced by Scheffersomyces stipitis cultured in sugarcane bagasse hydrolysate as new green larvicides for the control of Aedes aegypti, a vector of neglected tropical diseases. PLoS ONE 2017, 12, e0187125.
- Barbosa, F.G.; Ribeaux, D.R.; Rocha, T.; Costa, R.A.; Guzmán, R.R.; Marcelino, P.R.; Lacerda, T.M.; Silva, S.S.D. Biosurfactants: Sustainable and versatile molecules. J. Braz. Chem. Soc. 2022, 33, 870–893.
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320.
- Nalini, S.; Parthasarathi, R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Ann. Agrar. Sci. 2018, 16, 108–115.
- Sachdev, S.; Bauddh, K.; Singh, R.P. Prospective of biosurfactant in management of fusarium wilt and early blight of Lycopersicon esculentum. Plant Stress 2023, 7, 100126.
- Hoff, G.; Arguelles Arias, A.; Boubsi, F.; Pršić, J.; Meyer, T.; Ibrahim, H.M.; Steels, S.; Luzuriaga, P.; Legras, A.; Franzil, L.; et al. Surfactin stimulated by pectin molecular patterns and root exudates acts as a key driver of the Bacillus-plant mutualistic interaction. mBio 2021, 12, e01774-21.
- Çelik, P.A.; Manga, E.B.; Çabuk, A.; Banat, I.M. Biosurfactants’ Potential Role in Combating COVID-19 and Similar Future Microbial Threats. Appl. Sci. 2021, 11, 334.
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K. Biosurfactants: A Covid-19 perspective. Front. Microbiol. 2020, 11, 1341.
- Antony, S.; Sukumaran, T.U.; Rathinam, P.; Reshmy, R.; Binod, P.; Pandey, A.; Sindhu, R. Biosurfactants in respiratory viruses and the Coronavirus disease 2019 pandemic. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin Adetunji, C.O., Ahamed, M.I., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 439–450.
- Raza, Z.A.; Shahzad, Q.; Rehman, A.; Taqi, M.; Ayub, A. Biosurfactants in the sustainable eradication of SARS COV-2 from the environmental surfaces. 3 Biotech 2022, 12, 273.
- Rybicki, E.P. A Top Ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20.
- Islam, W.; Adnan, M.; Tayyab, M.; Hussain, M.; Islam, S.U. Phyto-metabolites; an impregnable shield against plant viruses. Nat. Prod. Commun. 2018, 13, 1934578X1801300131.
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M.; et al. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014.
- Manjunatha, L.; Rajashekara, H.; Uppala, L.S.; Ambika, D.S.; Patil, B.; Shankarappa, K.S.; Nath, V.S.; Kavitha, T.R.; Mishra, A.K. Mechanisms of Microbial Plant Protection and Control of Plant Viruses. Plants 2022, 11, 3449.
- Raheel, M.; Aatif, H.M.; Ali, S.; Shakeel, Q.; Ahmad, A.; Shaheen, M.R. Biological control activity of biosurfactant against plant pathogens. In Applications of Biosurfactant in Agriculture; Inamuddin Adetunji, C.O., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 17–28.
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology® 2023, 113, 117–141.
- Csukasi, F.; Merchante, C.; Valpuesta, V. Modification of plant hormone levels and signaling as a tool in plant biotechnology. Biotechnol. J. 2009, 4, 1293–1304.
- De Oliveira, J.; Rodrigues, C.; Vandenberghe, L.P.S.; Câmara, M.C.; Libardi, N.; Soccol, C.R. Gibberellic Acid Production by Different Fermentation Systems Using Citric Pulp as Substrate/Support. BioMed Res. Int. 2017, 2017, 5191046.
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398.
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. In Annual Plant Reviews; Hedden, P., Thomas, S.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 49, pp. 253–284.
- Bao, S.; Hua, C.; Shen, L.H.Y. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131.
- Pawełkowicz, M.E.; Skarzyńska, A.; Pląder, W.; Przybecki, Z. Genetic and molecular bases of cucumber (Cucumis sativus L.) sex determination. Mol. Breed. 2019, 39, 50.
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329.
- Ning, H.T.; Subroto, G. Effect of Hormone Concentration and frequency of administration of Gibberellins on Growth and Yield of Tomato Fruit. Agric. Sci. 2018, 1, 104–115.
- Regnault, T.; Davière, J.M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Diaz, I.L.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants 2015, 1, 15073.
- Pimenta Lange, M.J.; Lange, T. Ovary-derived precursor gibberellin A9 is essential for female flower development in cucumber. Development 2016, 143, 4425–4429.
- Band, L.R.; Úbeda-Tomás, S.; Dyson, R.J.; Middleton, A.M.; Hodgman, T.C.; Owen, M.R.; Jensen, O.E.; Bennett, M.J.; King, J.R. Growth-induced hormone dilution can explain the dynamics of plant root cell elongation. Proc. Natl. Acad. Sci. USA 2012, 109, 7577–7582.
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421.
- Li, H.; Wu, H.; Qi, Q.; Li, H.; Li, Z.; Chen, S.; Ding, Q.; Wang, Q.; Yan, Z.; Gai, Y.; et al. Gibberellins Play a Role in Regulating Tomato Fruit Ripening. Plant Cell Physiol. 2019, 60, 1619–1629.
- Mushtaq, S.; Amjad, M.; Ziaf, K.; Afzal, I. Gibberellins application timing modulates growth, physiology, and quality characteristics of two onion (Allium cepa L.) cultivars. Environ. Sci. Pollut. Res. 2018, 25, 25155–25161.
- Chen, S.; Wang, X.J.; Tan, G.F.; Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861.
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Park, J.M.; Lee, S.M.; Lee, I.J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 36–44.
- Lima, S.F.; Jesus, A.A.; Vendruscolo, E.P.; Oliveira, T.R.; Maria Andrade, G.O.; Simon, C.A. Development and production of sweet corn applied with biostimulant as seed treatment. Hortic. Bras. 2019, 6, 94–100.
- Oliveira, F.d.A.; de Medeiros, J.F.; de Cunha, R.C.; da Souza, M.W.d.L.; Lima, L.A. Use of biostimulants in relieving salt stress in popcorn. Rev. Ciênc. Agron. 2016, 47, 307–315.
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686.
- Peres, A.R.; Kappes, C.; Portugal, J.R.; de Sá, M.E.; Meirelles, F.C. Physiological quality of soybean seeds with application of biostimulant. Aust. J. Crop Sci. 2017, 11, 162–168.
- de Moraes, E.R.; Mageste, J.G.; Lana, R.M.Q.; da Silva, R.V.; de Camargo, R. Sugarcane: Organo-Mineral Fertilizers and Biostimulants. In Sugarcane—Technology and Research; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018.
- Nguyen, C.T.; Dang, L.H.; Nguyen, D.T.; Tran, K.P.; Giang, B.L.; Tran, N.Q. Effect of GA3 and Gly plant growth regulators on productivity and sugar content of sugarcane. Agriculture 2019, 9, 136.
- Hussain, N.; Anwar, A.; Yasmeen, A.; Arif, M.; Naz, S.; Bibi, M.; Iqbal, J.; Qadir, I.; Salim, M.N.; Latif, S. Resource use efficiency of cotton in improved vs conventional planting geometry with exogenous application of bio-stimulant and synthetic growth retardant. Braz. J. Biol. 2020, 6984, 18–26.
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533.
- Berli, F.J.; Pharis, R.P.; Bottini, A.R.; Alonso, R.E. Use of Gibberellin A5 to Increase the Yield and Quality of Wine Grapes. U.S. Patent Application No. 16/138,335, 24 January 2019.
- Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-gibberellins-market (accessed on 18 December 2023).
- Rodrigues, C.; Vandenberghe, L.P.D.S.; Teodoro, J.; Oss, J.F.; Pandey, A.; Soccol, C.R. A new alternative to produce gibberellic acid by solid state fermentation. Braz. Arch. Biol. Technol. 2009, 52, 181–188.
- Satpute, D.; Sharma, V.; Murarkar, K.; Bhotmange, M.; Dharmadhikari, D. Solid-state fermentation for production of gibberellic acid using agricultural residues. Int. J. Environ. Pollut. 2010, 43, 201–213.
- Kumar, P.K.R.; Lonsane, B.K. Solid state fermentation: Physical and nutritional factors influencing gibberellic acid production. Appl. Microbiol. Biotechnol. 1990, 34, 145–148.
- Piccoli, P.; Lucangeli, C.D.; Bottini, R.; Schneider, G. Hydrolysis of gibberellin A 20-glucoside and gibberellin A 20-glucosyl ester by Azospirillum lipoferum cultured in a nitrogen-free biotin-based chemically-defined medium. Plant Growth Regul. 1997, 23, 179–182.
- Dobert, R.C.; Rood, S.B.; Blevins, D.G. Gibberellins and the legume-rhizobium symbiosis: I. Endogenous gibberellins of lima bean (Phaseolus lunatus L.) stems and nodules. Plant Physiol. 1992, 98, 221–224.
- Kumar, P.K.R.; Lonsane, B.K. Immobilized growing cells of Gibberella fujikuroi P-3 for production of gibberellic acid and pigment in batch and semi-continuous cultures. Appl. Microbiol. Biotechnol. 1988, 28, 537–542.
- Pastrana, L.M.; Gonzalez, M.; Pintado, J.; Murado, M.A. Interactions affecting gibberellic acid production in solid-state culture: A factorial study. Enzym. Microb. Technol. 1995, 17, 784–790.
- Tomasini, A.; Fajardo, C.; Barrios-González, J. Gibberellic acid production using different solid-state fermentation systems. World J. Microbiol. Biotechnol. 1997, 13, 203–206.
- Machado, C.M.; Soccol, C.R.; de Oliveira, B.H.; Pandey, A. Gibberellic acid production by solid-state fermentation in coffee husk. Appl. Biochem. Biotechnol. 2002, 102, 179–191.
- Camara, M.C.; Vandenberghe, L.P.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA 3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062.
- Panchal, R.R.; Desai, P.V. Study of Gibberellic Acid Production by Solid State Fermentation Using Fusarium Moniliforme Sheldon. Int. J. Appl. Sci. Biotechnol. 2016, 4, 402–407.
- Azcón, R.; Barea, J.M. Synthesis of auxins, gibberellins and cytokinins by Azotobacter vinelandii and Azotobacter beijerinckii related to effects produced on tomato plants. Plant Soil 1975, 43, 609–619.
- Kasahara, H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42.
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196.
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58.
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 2017, 8, 1323.
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137.
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2009, 69, 437–449.
- Jing, H.; Strader, L.C. Interplay Auxin and Cytokinin in Lateral Root Development. Int. J. Mol. Sci. 2019, 20, 486.
- Cucinotta, M.; Manrique, S.; Guazzotti, A.; Quadrelli, N.E.; Mendes, M.A.; Benkova, E.; Colombo, L. Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 2016, 143, 4419–4424.
- Heisler, M.G.; Byrne, M.E. Progress in understanding the role of auxin in lateral organ development in plants. Curr. Opin. Plant Biol. 2020, 53, 73–79.
- Harmer, S.L.; Brooks, C.J. Growth-mediated plant movements: Hidden in plain sight. Curr. Opin. Plant Biol. 2018, 41, 89–94.
- Nakajima, Y.; Nara, Y.; Kobayashi, A.; Sugita, T.; Miyazawa, Y.; Fujii, N.; Takahashi, H. Auxin transport and response requirements for root hydrotropism differ between plant species. J. Exp. Bot. 2017, 68, 3441–3456.
- Barbier, F.F.; Dun, E.A.; Beveridge, C.A. Apical dominance. Curr. Biol. 2017, 27, R864–R865.
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036.
- Solangi, S.K.; Qureshi, S.T.; Khan, I.A.; Raza, S. Establishment of in vitro callus in sugarcane (Saccharum officinarum L.) varieties influenced by different auxins. Afr. J. Biotechnol. 2016, 15, 1541–1550.
- Ahmed, A.A.A.; Alkharpotly, A.A.; Gabal, A.A.A.; Abido, A.I.A. Potato growth and yield as affected by foliar application with NAA auxin and 6-BA cytokinin. J. Plant Prod. 2021, 12, 591–596.
- Pramanik, K.; Mohapatra, P.P. Role of auxin on growth, yield and quality of tomato-A review. Int. J. Curr. Microbiol. Appl. Sci 2017, 6, 1624–1636.
- Noor, A.; Ziaf, K.; Naveed, M.; Khan, K.S.; Ghani, M.A.; Ahmad, I.; Anwar, R.; Siddiqui, M.H.; Shakeel, A.; Khan, A.I. L-Tryptophan-Dependent Auxin-Producing Plant-Growth-Promoting Bacteria Improve Seed Yield and Quality of Carrot by Altering the Umbel Order. Horticulturae 2023, 9, 954.
- Balliu, A.B.; Sallaku, G. Exogenous auxin improves root morphology and restores growth of grafted cucumber seedlings. Hortic. Sci. 2017, 44, 82–90.
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms. Horticulturae 2023, 9, 193.
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528.
- Jankovska-Bortkevič, E.; Katerova, Z.; Todorova, D.; Jankauskienė, J.; Mockevičiūtė, R.; Sergiev, I.; Jurkonienė, S. Effects of Auxin-Type Plant Growth Regulators and Cold Stress on the Endogenous Polyamines in Pea Plants. Horticulturae 2023, 9, 244.
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysse, A.M.; Brewster, W.K.; Bryan, K.; Daeuble, J.F.; Fields, S.C.; Gast, R.E.; Green, R.A.; et al. The discovery of Arylex™ active and Rinskor™ active: Two novel auxin herbicides. Bioorganic Med. Chem. 2016, 24, 362–371.
- Matsuda, R.; Handayani, M.L.; Sasaki, H.; Takechi, K.; Takano, H.; Takio, S. Production of indoleacetic acid by strains of the epiphytic bacteria Neptunomonas spp. isolated from the red alga Pyropia yezoensis and the seagrass Zostera marina. Arch. Microbiol. 2018, 200, 255–265.
- do Prado, D.Z.; Okino-Delgado, C.H.; Zanutto-Elgui, M.R.; da Silva, R.B.G.; Pereira, M.S.; Jahn, L.; Ludwig-Muller, J.; da Silva, M.R.; Velini, E.D.; Fleuri, L.F. Screening of Aspergillus, Bacillus and Trichoderma strains and influence of substrates on auxin and phytases production through solid-state fermentation. Biocatal. Agric. Biotechnol. 2019, 19, 101165.
- Giri, R.; Meena, V.; Sharma, R.K. Production of indole acetic acid by a wood degrading fungus Phanerochaete chrysosporium. J. Food Chem. Nanotechnol. 2020, 6, 97–101.
- do Prado, D.Z.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Müller, J.; da Silva, M.R.; Fernandes, C.J.C.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus flavipes as a novel biostimulant for rooting-enhancement of Eucalyptus. J. Clean. Prod. 2019, 234, 681–689.
- Piotrowska-Długosz, A. Significance of enzymes and their application in agriculture. In Biocatalysis: Enzymatic Basics and Applications; Husain, Q., Ullah, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 277–308.
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. Ciênc. Solo 2021, 45, e0210109.
- Arya, P.S.; Yagnik, S.M.; Raval, V.H. Role of microbial enzymes in agricultural industry. In Biotechnology of Microbial Enzymes, Brahmachari; Academic Press: Cambridge, MA, USA, 2023; pp. 525–550.




