1. Inoculants and Biological Controlling Agents as Bioinputs
The advancement of green and circular economy concepts worldwide has intensified the search for sustainable and highly efficient products that can replace synthetic products derived from petroleum and mineral extraction. Recently, in the agricultural sector, the excessive use of NPK fertilizers (nitrogen, phosphate, and potassium compounds) and synthetic pesticides has shown the harmful effects of these toxic compounds in the reduction of the biodiversity present in soils, as well as the contamination of water sources, groundwater, and food, causing several health problems for organisms living on the planet [
30,
31]. An alternative to replace these synthetic products and restore the balance of soil biodiversity is the use of inoculants and biological control agents [
32].
Inoculants are biological products composed of living microorganisms that benefit the development of different plant species, increasing the availability of nutrients for plants and improving the absorption of nutrients by plants. They also induce plant immunity and favor soil quality [
33,
34]. The microorganisms used in the preparation of inoculant formulations are known as plant growth promoters, examples of which include bacteria and fungi which can be found adhered to the roots (rhizospheric), inside plant tissues (endophytic), or in plant leaves. These microorganisms stand out for being nitrogen fixers (diazotrophs); phosphate solubilizers; and producers of siderophores, phytohormones, antimicrobial compounds, and enzymes. Examples of microorganisms used in inoculant formulations include bacteria of the genera
Burkholderia,
Pantoea,
Enterobacter,
Pseudomonas,
Massilia,
Sphingobium,
Sphingomonas,
Agrobacterium,
Rhizobium,
Bradyrhizobium, and
Ochrobactrum and fungi of the genera
Penicillium and
Mycorrhiza [
12,
35,
36]. A schematic representation of the interactions among those microorganisms occurring in the rhizosphere is exhibited in
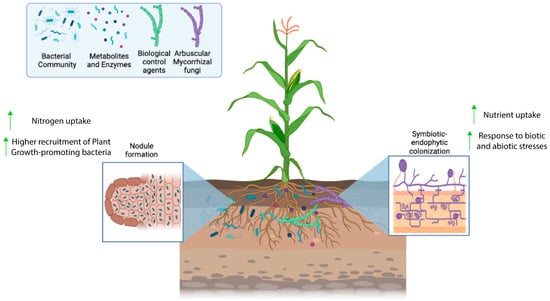
Figure 1.
Figure 1. Biological processes occurring in the rhizosphere, including the growth and colonization of the plant roots by different types of microorganisms.
Biological control agents are bioproducts composed of micro or macroorganisms that act to reduce the biotic and abiotic factors that may negatively interfere in agricultural production [
37]. They are used in important methods for protecting plants against attacks by arthropod pests and phytopathogenic microorganisms, proving to be effective without inflicting ecological damage [
38]. This study will focus on the production of microbial biological control agents or bioinsecticides, more specifically those composed of entomopathogenic bacteria and fungi, which infect insect pests and have antagonistic activity against phytopathogenic microorganisms.
Among the most common formulations of these biological control agents on the market, those containing bacteria from the genera
Agrobacterium,
Bacillus,
Pseudomonas,
Streptomyces, and
Paenibacillus and also fungi from the genera
Trichoderma,
Metarhizium, and
Beauveria are some examples of effective and well-studied agents that act against several plant diseases [
12,
16]. Biological control agents may act under hyperparasitism, competition, the secretion of lytic enzymes, and plant growth promotion, inducing systemic host resistance and antibiosis [
39].
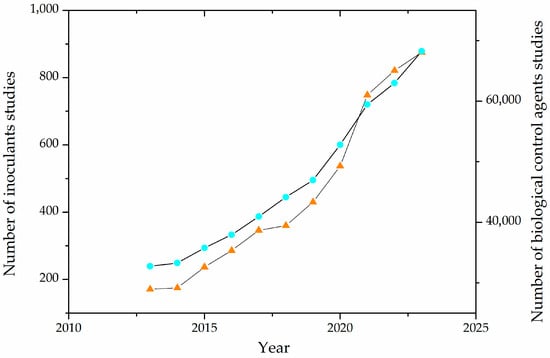
In recent years, there has been an increasing number of works regarding the production and effects of inoculants and biological control agents, as depicted in Figure 2.
Figure 2. Evolution of the number of publications on agricultural inoculants and biological control agents in the last decade. The light blue circles (○) represent studies of biological control agents, and the orange triangles (Δ) represent studies of inoculants.
From a technological point of view, the increasing study of inoculants and biological control agents is due to the advancements in fermentative processes and molecular biology techniques, the development of biorefinery concepts, and the imperative need for more sustainable agricultural inputs.
The main steps involved in the bioprocess to obtain inoculant formulations and biological control agents consist of the selection of suitable microbial strains; the characterization of their morphological, physiological, and biochemical properties; the evaluation of fermentation conditions (optimization of nutritional and physical parameters); and the development of biopinputs using cheap reagents, adjuvants, and vehicles which will stabilize the bioactive agents in the formulation.
Aiming to reduce production costs, combined with the sustainable concepts of biorefineries and green and circular economies, lignocellulosic biomass has started to be used as a raw material in the development of inoculant formulations and agricultural biological control agents, being considered a viable alternative.
Production of Inoculants and Biological Control Agents by SSF
Inoculants had a global market share of approximately USD 1.0 to USD 2.3 billion in 2020, and their market share is expected to reach USD 3.9 billion by 2025, with a CAGR ranging between 11 and 12.8%. Biological control agents currently have a market worth approximately USD 6.6 billion, and this market worth is expected to grow at a CAGR of 15.8% to reach USD 13.7 billion by 2027. The leading global inoculant and biological control agent manufacturers are Novozymes (Frederiksberg, Denmark), BASF SE (Ludwigshafen am Rhein, Germany), Premier Tech (Boizenburg, Germany), Bioceres Crop Solutions (Rosario, Argentine), Marrone Bio Innovations Inc. (Davis, CA, USA), Bayer CropScience AG (Monheim am Rhein, Germany), and Valent Biosciences (Libertyville, IL, USA) [
40,
41].
With the advancements in the use of inoculants and biological control agents, the productivity of these compounds is one of the main issues that need to be evaluated for their large-scale production and global implementation. The steps that need to be taken to choose the proper microbial strain in the bioinput strikingly rely on the cultivar species, the type of disease, and the desired effect upon the system, ranging from the induction of innate plant immunity and the direct antagonism of the pathogen to the production of enzymes or improvement of soil quality. Subsequently, process conditions and substrate composition alter the dynamics of microbial metabolism; thus, the microbial strain must be cautiously selected in order to drive the production to either biomass or metabolite excretion. Both strategies contribute to the suppression of pathogens; however, the best option must be examined in situ and will depend on the prevalence of their positive effects over time or over the cultivation period.
Regarding substrate type, medium composition influences microbial growth and metabolite production. The main carbon sources assimilated by microorganisms used in inoculant and biological control formulations are sugars and polyols such as glucose, sucrose, xylose, lactose, glycerol, mannitol, and others. These nutrients can be found in different substrates. Considering the cost and availability of raw materials and substrates, agro-industrial byproducts such as lignocellulosic biomasses mainly composed of a carbohydrate portion rich in cellulose and hemicellulose stand out. As previously reported, lignocellulosic materials are promising nutritional sources that can be used in SSF for the production of bioinputs such as inoculants and control agents. In addition to supports/substrates, lignocellulosic materials can also be used as vehicles for inoculant formulations and biological control agents [
42].
Zhao et al. [
43] reported the production of
Rhizobium leguminosarum biomass by SSF using fresh wheat straw and charcoal as supports. In SSF with wheat straw, it was observed that after 72 h of cultivation, there was approximately a thousand-fold increase in the number of viable cells, starting from an initial concentration of 7.3 to approximately 10.0 log cfu/g substrate. When charcoal was used, after 72 h of cultivation, a 10-fold increase in the number of viable cells was observed. The results obtained in this study showed that although charcoal is known as an excellent inert support for biofertilizer production, wheat straw presented a better performance.
Bacillus thuringensis is a type of bacteria present in the soil that produces certain types of proteins, known as
Cry, with an insecticidal effect on
Lepidoptera,
Coleoptera, and
Diptera; thus, it is normally used as a potential bioinsecticide. Within this context, Molina-Peñate et al. [
44] reported on the use of solid waste treated with lytic enzymes (e.g., cellulases, hemicellulases, pectinases, and amylases) as raw material for the mass growth of
Bacillus thuringiensis in SSF. After 100 h of cultivation, approximately 10
8 viable cells/g of substrate and 10
8 spores/g of substrate were obtained. The presence of
Cry protein crystals, essential for insecticidal action, was also observed. Accordingly, this strategy proved to be viable on the scale at which it was carried out, being an alternative process with a value-added bioinput for future biorefineries.
Regarding the production of biofertilizers, Vassilev et al. [
64] demonstrated the feasibility of using solid beet residues for the growth of microbial biomass of
Aspergillus niger, which, by producing organic acids during the fermentation process, solubilized the natural phosphate, making it available for the plant.
Solid biofertilizers obtained by SSF from lignocellulosic biomass when applied to the soil are also important for the formation of humic substances. These substances have long been recognized as the most widely distributed organic component on the planet. Humic substances are formed mainly from the biological degradation of plant and animal residues. Their structures have an abundance of carbonyl and phenolic groups that contribute to their complexation and have amphipathic characteristics that can bind to mineral soil surfaces [
67,
68,
69,
70]. The main types of humic substances found in soil are fulvic acids, humins, and humic acids, which act at different pH. Humic substances contribute to heat retention in the soil, which stimulates seed germination and plant root development; they act against erosion, preventing runoff due to the fact that they contain aggregates from combining with clays, enabling a better water retention capacity [
71]. When the solid biofertilizer obtained by SSF, still containing a significant lignin content, comes into contact with soil microorganisms such as rhizospheric bacteria, it will be degraded by the action of phenoloxidases and enzymes that produce hydrogen peroxide. The resulting phenolic compounds will be precursors of humic substances. Some studies have demonstrated the efficient production of humic acids by SSF using lignocellulosic agro-industrial byproducts such as raw empty fruit bunch and rice straw fibers [
72,
73].
In addition to lignocellulosic substrates, other materials can be used in the production of inoculants, biological control agents, and biofertilizers. Examples include cheese whey, beetroot and sugarcane molasses, glycerol, solid residues from olive oil mills, and vinasse, which can be used in SSF as a substrate/support or moistening solution [
21,
64,
74,
75].
In addition to inoculants and biological control agents, other bioinputs such as biosurfactants and phytohormones can also be produced by SSF, as reported in the next sections.
2. Biosurfactants: Promising and Emerging Agricultural Bioinputs
Biosurfactants (or biological surfactants) are microbial metabolites with an amphipathic structure that have outstanding physicochemical and biological properties such as the ability to reduce surface tension, emulsifying capacity, and antimicrobial potential. According to the biochemical groups present in their structures, biosurfactants can be classified as glycolipids, lipopeptides, lipoproteins, phospholipids, and similar molecules [
76]. Glycolipids and lipopeptides are the most common microbial biosurfactants.
Glycolipids are formed by the union of sugars (monosaccharides and polyols) and lipids, and they have molar masses between 476 and 848 Da and critical micellar concentrations (CMCs) between 20 and 366 mg/L [
77,
78]. Among the most common biosurfactant glycolipids are rhamnolipids, produced by bacteria, and sophorolipids, produced by yeast.
Lipopeptides are biosurfactants formed by the union between peptides and lipids and have molar masses greater than 1000 Da; due to this, they are called high-molar-mass biosurfactants, and they have CMCs around 10μmol/L or 23 mg/L. Surfactin is the lipopeptide produced by some bacteria best described in [
77].
In agriculture, microbial biosurfactants are commonly used as emulsifiers in agrochemical formulations. However, in recent years, with the search for “clean” methodologies for more sustainable agricultural production, it has been observed that biosurfactants can also be used as antimicrobials, germinating agents, and growth stimulants.
Due to their antimicrobial properties, in recent years biosurfactants have been the target of agronomic studies aiming to use them as biopesticides to control pathogens in crops. The antimicrobial activity of biosurfactants is due to their membranotropic effects. Upon contact with pathogen cells, biosurfactants cause the permeabilization of the target cell through interacting with lipids and membrane proteins, causing cell disruption and death. The interaction mechanisms of the interactions between biosurfactants and membrane molecules involve three processes: (I) a change in membrane hydrophobicity through the intermolecular interactions between different functional groups present in biosurfactants, lipids, and proteins [
79]; (II) the fluidization of the cell membrane, which alters the balance between anchored saturated and unsaturated fatty acids [
80,
81]; (III) increasing the exchange of charge between the anchored molecules and thus opening pores and channels that, in turn, favor greater permeabilization of the cell membrane [
82,
83]. In turn, these aforementioned mechanisms cause the efflux of bioactive intracellular compounds such as enzymes, lipopolysaccharides (LPS), polypeptides (PP), and functional effectors, namely Microbial Associated Molecular Patterns (MAMPs), which could stimulate or “sensitize” plants, leading to a faster response of systemic resistance to pathogens [
84].
Still, regarding their agricultural applications, there are also reports that biosurfactants produced by some endophytic microorganisms act as protective molecules, acting significantly on growth and against some biotic and abiotic factors [
85,
86].
Production of Biosurfactants in SSF
Despite being molecules of fundamental importance for the industrial and agricultural sectors, the production costs of biosurfactants are still high. This fact is due to the raw materials used and the purification processes adopted [
86]. In recent years, the use of agro-industrial byproducts for the production of biosurfactants has increased. Various lignocellulosic materials can be used in the production of biosurfactants via SSF.
As previously reported, obtaining biosurfactants by SSF is still carried out on small scales, with only 2% of studies reporting a yield of between 5 and 10 kg of bioproduct. Furthermore, 62% of the studies on obtaining biosurfactants by SSF reported the production of glycolipids (sophorolipids, rhamnolipids, and others) and lipopeptides (surfactin, iturin, and others), and 40% of the studies reported the use of packed beds or porcelain fully aerated and agitated bioreactors [
98].
When analyzing the advantages of obtaining biosurfactants by SSF when compared to SmF, a greater production of biosurfactant by SSF is observed. However, disadvantages such as the complexity in establishing mathematical models, as well as the difficulties in purification and scaling up, show that there are still many advances to be achieved in this promising bioprocess for industrial application, considering sustainable concepts for a green economy.
Glycolipids are a type of biosurfactant with antifungal, larvicidal, and mosquitocidal properties, thus acting as agricultural defenders against pests and fungal infections [
99,
100]. Glycolipid-based biopesticides are applied to agricultural crops to control phytopathogenic bacteria, fungi, and pests [
101].
The antibiotic properties of biosurfactants have been explored for some time and are well known. Antifungal properties stand out, since there is a more limited number of active ingredients for the treatment of these phytopathogens when compared to antibiotics. It is known that fungi such as mold/white rot (e.g., Sclerotinia sclerotiorum, Penicillium digitatum, Penicillium italicum) and black rot (e.g., Alternaria citri), among others, cause major losses for leguminous crops such as soybeans, beans, and citrus fruits. The routine use of a variety of synthetic fungicides, such as triazoles, triazolinthione, carbamates, dithiocarbamates, organochlorines, and organophosphates, has caused several environmental problems, such as the pollution of soil, groundwater, and water sources. Therefore, the adoption of biosurfactants with fungicidal effects proves to be a sustainable alternative for agricultural production.
Nalini and Parthasarathi [
96] demonstrated the action of rhamnolipids produced by
Serratia rubidaea SNAU02 as a biocontrol agent. In this study, the authors evaluated the use of mahua cake as a substrate for biosurfactant production via SSF. The rhamnolipid produced showed antifungal activity against
Fusarium oxysporum and
Colletotrichum gloeosporioides. The study suggested that this biosurfactant forms ion channels in the plasma membrane of the microorganism, creating pores in the membrane layer that affect the cell surface of plant pathogenic fungi.
The action of a rhamnolipid produced by
Pseudomonas aeruginosa Tr20 in combination with a compatible strain of
Trichoderma lixii TvR1 was also evaluated for the control of fusariosis and early blight in plants. Through these in vivo activities against the pathogens
Fusarium oxysporum and
Alternaria solani, a significant reduction in the incidence of fusariosis and early blight by 27.21% and 29.43%, respectively, was observed [
104].
Lipopeptides are also important bacterial biosurfactants with agricultural applications. According to Hoff et al. [
105], surfactin secreted by
Bacillus velenzis in contact with plant roots acted as a bioactive secondary metabolite (BSM), acting as a signal and/or antimicrobial and being responsible for optimizing biofilm formation, motility, and early root colonization and increasing plant immunity.
With the COVID-19 pandemic, studies on the antiviral potential of biosurfactants increased [
100,
106,
107,
108,
109]. In the agricultural sector, the antiviral effect of these molecules is of fundamental interest, since they can be used against phytopathogens such as Potyvirus (family
Potyviridae), Tospovirus (family
Bunyaviridae), Tobamovirus (family
Virgaviridae), Closteovirus (family
Closteoviridae), Begomovirus (
Geminiviridae), and Polerovirus (family
Luteoviridae), which affect crops of economic importance such as vegetables, soybeans, beans, sugar cane, potatoes, and citrus fruits [
110,
111,
112,
113,
114,
115]. In viruses, biosurfactants can act by disintegrating particles through interaction with lipid layers and denaturing proteins in structures such as the capsid and envelope [
107].
In addition to their effects against pathogens, biosurfactants are also responsible for the disruption of the waxy cuticles of some insects [
99]. The removal of these lipid structures can cause dehydration in the insect and also make it more susceptible to infection by biological control agents and the action of hydrolase enzymes, facilitating the death of the insect pest.
3. Phytohormones as Agricultural Bioinputs
3.1. Gibberellic Acids
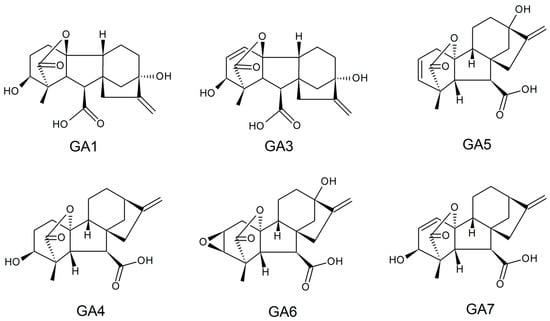
Gibberellins, or gibberellic acids (Gas), are a family of diterpenoid acid tetracyclics biosynthesized by plants, fungi, bacteria, and some algae genera that present hormonal effects in plant growth at low concentrations. These biomolecules exhibit structures with carboxylic acid, the presence or absence of unsaturated ring bonds, and different hydroxylation degrees; they have a molar mass of around 346.38 g/mol, are soluble in water (4.6 g/L in the room temperature), and have a melting point between 223 and 225 °C. Currently, 136 types of GA have been identified, and GA
1, GA
3, GA
4, GA
5, GA
6, and GA
7 are considered the most active. Their typical structures are represented in
Figure 3 [
116,
117].
Figure 3. Typical active structures reported for gibberellin.
GAs are important in plant physiology because they can promote stretching [
118], germination and seed dormancy [
119], flowering [
120], gender development [
121], leaf inhibition and senescence [
122], and fruit aging [
123]. Non-active forms of GA do not accumulate in plant tissues or cells but are rather transported in a root-to-shoot manner, or vice versa [
124]. On the other hand, bioactive forms of GA such as GA
4 accumulate in the sepals and petals during organ development [
125]. Moreover, one study showed that GA levels are higher in the root elongation zone compared with the meristematic zone, pointing towards the hypothesis that GAs are synthesized locally or even transported from the surrounding tissues to compensate for hormone dilution during cellular growth [
126,
127].
Currently, GAs are bioinputs that have expanded the market in recent years, having been mainly used in horticulture [
128,
129,
130,
131]. Recent studies also show that gibberellin has potential applications as a biostimulant related to the main agricultural products of developing countries like corn [
132,
133], soybeans [
134,
135], sugarcane [
136,
137], and cotton [
138]. Some studies have also shown that GA formulations (granular, pure, mixtures, and others) are used to protect seeds, flowers, and fruits against larvae and insects and that they act as herbicides [
139,
140].
According to the strategic importance of these bioinputs for achieving increasingly sustainable agriculture, the market trends show that production and profit are in ascendancy. The global market for GAs was valued at USD 500 million in 2017, and it is expected to reach revenues of 1167.8 million dollars in 2025, with an annual increase of 8.8% in CAGR [
141]. The countries with the largest GA-producing companies are China, USA, and New Zealand.
Although the use of GAs is still restricted to small- and medium-sized plantations, the adoption of these bioinputs for farmers in developing countries, considered as “world granaries”, could further boost their use. Therefore, it is necessary to achieve high-yield production processes that use sustainable technologies and renewable raw materials such as lignocellulosic biomasses.
Currently, the industrial production of GAs has been predominantly carried out by SmF. However, diluting the sample in the fermentation broth requires a later concentration step, which makes the process more expensive [
142]. It is also possible to produce GAs by chemical synthesis or by extraction from plants, but these methods are not economically viable. Thus, in recent years, the microbial production of GAs in SSF has presented itself as economically viable, whereby the main feedstock are agro-industrial byproducts [
143]. In addition, in GA production via SSF, there is no concern with catabolic repression owing to the fact that carbon sources are present in solid state, majorly as carbohydrates; therefore, the microorganisms can possibly have better control over the available nutrients that can be absorbed by the excretion of cellulolytic enzymes.
GAs are produced via SSF generally using filamentous fungi as fermenting agents, the most common of which being
Fusarium moniliforme and
Gibberella fujikuroi [
144]. In addition to filamentous fungi, bacteria can also produce GAs, especially those that promote plant growth and fulfill the role of nitrogen fixers, such as
Azotobacter chroococcum,
Bacillus licheniformis,
P. fluorescens and
Azospirillum lipoferum [
144,
145,
146]. The use of bacteria that promote plant growth in the production of GAs comes with the advantage of obtaining a product for multifunctional agricultural application, acting as a biofertilizer and stimulant/regulator of plant growth [
144].
Analogously, the nutritional elements and nature of the culture medium are also fundamentally important parameters for GA production. The main carbon sources used in GA production are known to be carbohydrates and polyols such as glucose, sucrose, galactose, mannitol, maltose, starch, and glycerol [
149,
150,
151,
152,
153]. These substrates are found in large quantities in lignocellulosic and starchy-derived materials such as corn stalks, citric peel, wheat bran, sugarcane bagasse, citric pulp, soy bran, sugarcane bagasse, soy husk, cassava bagasse, and coffee husk [
117].
Production of Gibberellic Acids via SSF
The use of agro-industry byproducts to obtain high-value-added products has been highlighted, and GAs could be alternatives to products in biological refineries.
N sources, mineral salts, and trace elements are also important in obtaining GAs. The use of agro-industrial byproducts with complex compositions dismisses supplementation with sources of N, mineral salts, vitamins, etc. If the addition of N and mineral salts is necessary, the use of liquid residual extracts such as corn steep liquor, plant meals, rice bran, or soy extract may be a viable and cheaper option than the addition of inorganic nitrogen salts solutions. It is interesting to use them as moisturizers, thus controlling the humidity of the process and supplementing it with nutrient sources. Another advantage of using organic N sources in fermentation is that during metabolism, the degradation of nitrogen compounds (hydrolysis reactions) will not drastically affect the pH. Due to these characteristics, complex organic nitrogen sources are favorable for the production of GAs.
However, even with the wide availability of N sources (e.g., organic and inorganic forms), it is not usual to vary nitrogen concentration while SSF occurs due to the difficulty of the operation, which makes it difficult to homogenize the system. Nonetheless, Panchal and Desai [
154] evaluated the effect of supplementary N sources (e.g., NH
4Cl, NH
4NO
3, (NH
4)
2SO
4, and urea) in commercial wheat bran (CWB) fermentation for the production of GA
3 under SSF with 70 mg of N (differing only in the nature) per 100 g CWB. The utilization of urea resulted in an enhancement in the productivity of the GA. Indeed, GA
3 production was higher (234, 489, and 532 µg/g) compared to the assay without N supplementation (95, 125, and 163 µg/g) at 120, 144, and 168 h, respectively.
Parameters such as pH and temperature are also important factors in SSF for GA production. Controlling the pH in SSF for GA production is difficult, but this problem can be solved by the addition of buffers or solutions with pH between 3.5 and 5.8. Satpute et al. [
143] tested SSF using pigeon pea at a pH range between 4.3 and 5.3, and it was shown that the optimal production of GA
3 occurred at pH 4.76, probably because of the growth of the fungus. On the contrary, Rodrigues et al. [
142] established an initial pH between 5.5 an 5.8, which corresponds to the natural pH of citric pulp used as a substrate/support for GA production. This strategy represented an interesting approach to lowering the cost of production of this bioinput.
3.2. Auxins
Auxins are compounds recognized as a family of aromatic molecules with a carboxylic acid group. They can be synthesized by plants and microorganisms and are responsible for controlling the plant growth response and development by means of cell differentiation, division, and expansion at low concentrations [
160,
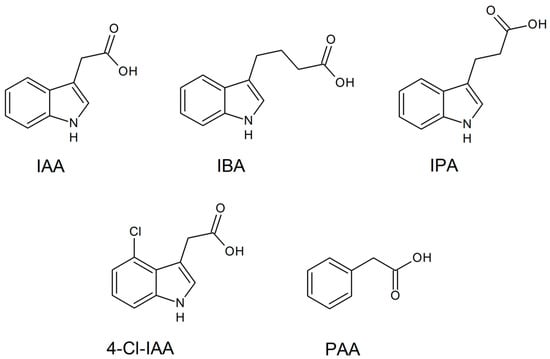
161]. Commonly, these biomolecules are found in natural forms, as exemplified by indole-3-acetic acid (IAA), 4-Cl-IAA, phenylacetic acid (PAA), indole-3-butyric acid (IBA), and indole-3-propionic acid (IPA), and as exhibited in
Figure 4 [
161,
162].
Figure 4. Main active auxin forms found in plants.
There is also the occurrence of other types of auxins such as auxin a (auxenotriolic acid), auxin b (auxenolonic acid), benzofuran-3-acetic acid (BFA), and phenyl-butyric acid (PBA) in plants or other organisms, which is still being debated by experts or not fully understood. Also, synthetic auxins exist as active 2,4-dichlorophenoxyacetic acid (2,4-D) and naphthalene acetic acid (NAA), as well as the inactive precursor 2,4-dichlorophenoxybutyric acid (2,4-DB) [
162]. In addition, auxins are hormones that partly regulate the processes of vascular tissue development, including tissue regeneration and vascular tissue connection, both of which are important for graft union [
163].
Over the years, many studies have shown that auxin-regulated cell expansion plays an important role in plant development, specifically in primary root growth [
164,
165], lateral root development [
166,
167], organ development [
168,
169], tropism [
170,
171], apical dominance [
172,
173], etc. Because of their multifunctionality, auxins can be used in strategic plantations as plant growth promoters, and this approach has shown promising results in sugarcane [
174], potato [
175], and tomato plantations [
176]. Auxins also help to obtain higher yields of good quality carrot seeds [
177], improve the root morphology and growth of grafted cucumber seedlings [
178], and can be used to alleviate abiotic stresses [
179,
180,
181].
The most well-known compound used to treat weeds using auxin-based products in plantations is 2,4-dichlorophenoxyacetic acid (2,4-D), which was discovered in the 1940s and has been used extensively for more than 70 years worldwide. Arylex™ (developed mainly for cereal plantations) and Rinskor™ (used in rice fields) are new types of auxin herbicides produced on the market which have proven to be an attractive alternative to auxin application [
182].
Due to studies carried out over the years showing the efficiency of using auxins in crops and improving their production, using this bioinput has been an important strategy for producers and their productions [
177,
178]. The production of auxins by chemical companies and laboratories started with the development and use of biostimulants with other phytohormones. Despite their great advantages, currently, synthetic auxins are largely employed rather than biosynthesized ones. Due to the environmental appeal and the commercial requirement for biodegradable products, auxin-producing microorganisms like fungi, bacteria, and algae are increasingly being researched and studied. The most well-known auxin of natural origin is indole-3-acetic acid (IAA), which has been widely studied in the literature. IAA is a molecule mainly produced by plants and microorganisms associated and not associated with plants. In the context of plant–microbe interactions, the presence of IAA plays a signaling role and helps to direct the growth and development of the plant [
183].
Within this context, it is important to investigate distinct routes and means to synthesize IAA that function independently of tryptophan, broadening the spectrum of alternatives to afford higher productivities regarding IAA and making its industrial implementation economically viable. Regarding the already identified pathways of auxin-producing microorganisms, it is still necessary to conduct research aimed at unraveling the parameters and alternative routes for the synthesis of auxins as well as understanding the metabolism of auxin regulation, seeking high productivity, preferably through using byproducts from renewable sources as raw materials.
Production of Auxins in SSF
Despite its immense advantages in the use of SSF, the production of auxin as IAA using this fermentative method has been underexplored. However, Prado et al. [
193] screened microorganism strains like
Aspergillus spp.,
Bacillus spp., and
Trichoderma spp. to investigate their capability to produce auxin (indole-3-acetic acid) and phytases through SSF in different substrates followed by the optimization of the process. The study revealed that all strains can produce auxin (comprising higher productivity with tryptophan supplementation). The highest indole derivative levels were observed in wheat bran as substrate by
B. subtilis D strain (158 μg/mL), but the same strain did not produce auxins in cassava bagasse. These findings led the authors to investigate the physico-chemical characteristics of each substrate to set a reliable framework of the reasons for that response.
Presumably, the high starch and lower protein content could hinder auxin synthesis due to the N-limiting condition. Additionally, due to the fact that cassava bagasse was the substrate with the highest lignin composition and as indicated by the results, lignin has a strong negative correlation with the production of IAA, observed in the
Bacillus and
Trichoderma strains. Indeed, lignin stands as a physical barrier hampering the microbial and enzymatic access to the carbohydrate fraction of the biomass. In this regard, the selective degradation of lignin to carbon dioxide can be carried out using white rot fungi. For instance, Giri and Sharma [
194] suggested pretreating wheat straw using
Phanerochaete chrysosporium, known for its ability to degrade lignin, as an alternative to accessing cellulose and converting it into easily fermentable sugars. Nevertheless, during secondary fermentation, these precursors may contribute to the synthesis of IAA via this fungus, along with the supplementation of tryptophan.
Similar to the addition of tryptophan as a precursor of IAA, it is important to consider other relevant factors and parameters in the process. Prado et al. [
195] carried out a factorial design 2
3 in SSF, varying the percentage of tryptophan (0.5, 1.0, and 1.5) and particle size (in mm; 0.5, 1.0, and >1.0) and adding water (in mL; 5, 10, and 15). In this experiment, using the
A. flavipes strain, it was observed that the amount of water and the use of tryptophan in the medium had a positive effect on the production of IAA in SSF. In addition, light and aeration influences have not been yet reported or described as parameters in the production of IAA in SSF.
4. Other Important Agricultural Bioinputs Obtained by SSF
In addition to the agricultural bioinputs obtained by SSF reported in this article, enzymes are also important products. Enzymes, along with inoculants and biological control agents, are commonly used in agriculture as a sustainable substitute for synthetic products. Agricultural enzymes help increase agricultural production, soil fertility, and food protection. They can also protect crops from various pests and diseases. The use of enzymes and inoculants increases the quality of commercial crops. Enzymes of agricultural importance, such as hydrolases, decompose plant residues and other organic matter that provide nutrients to plants and help in the initial stages of seed development, such as rooting and sprouting. Furthermore, the crop’s resistance to water stress and nutrient assimilation are improved. Thus, enzymes are essential for sustainable agricultural productivity and soil management. The most important enzymes used in plant growth and soil fertility include phosphatases, dehydrogenases, and ureases. Other enzymes used in agricultural applications include cellulases, proteases, phytases, sulfatases, and amylases [
200,
201,
202].
This entry is adapted from the peer-reviewed paper 10.3390/su16031076