
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adrián Hernández-Díazcouder | -- | 3855 | 2024-01-30 15:12:14 | | | |
| 2 | Jessie Wu | Meta information modification | 3855 | 2024-01-31 02:02:28 | | |
Video Upload Options
Asthma is one of the most common chronic non-communicable diseases worldwide, characterized by variable airflow limitation secondary to airway narrowing, airway wall thickening, and increased mucus resulting from chronic inflammation and airway remodeling. Current epidemiological studies reported that hypovitaminosis D is frequent in patients with asthma and is associated with worsening the disease and that supplementation with vitamin D3 improves asthma symptoms. However, despite several advances in the field, the molecular mechanisms of asthma have yet to be comprehensively understood. MicroRNAs play an important role in controlling several biological processes and their deregulation is implicated in diverse diseases, including asthma.
1. The Role of MicroRNA-21 in Asthma and Its Modulation by Vitamin D3
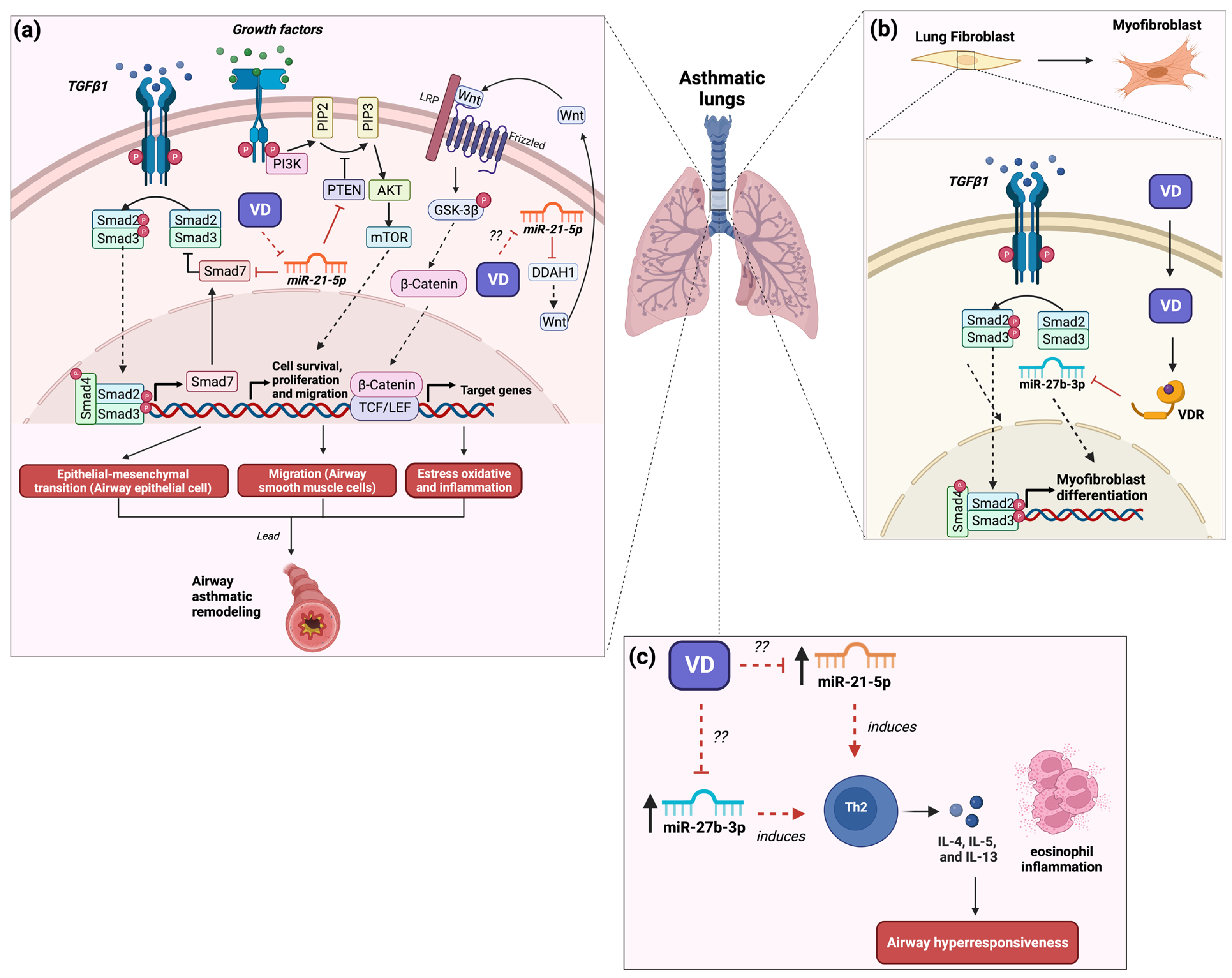
2. The Role of MicroRNA-27b in Asthma and Its Modulation by Vitamin D3
3. The Role of MicroRNA-145 in Asthma and Its Modulation by Vitamin D3
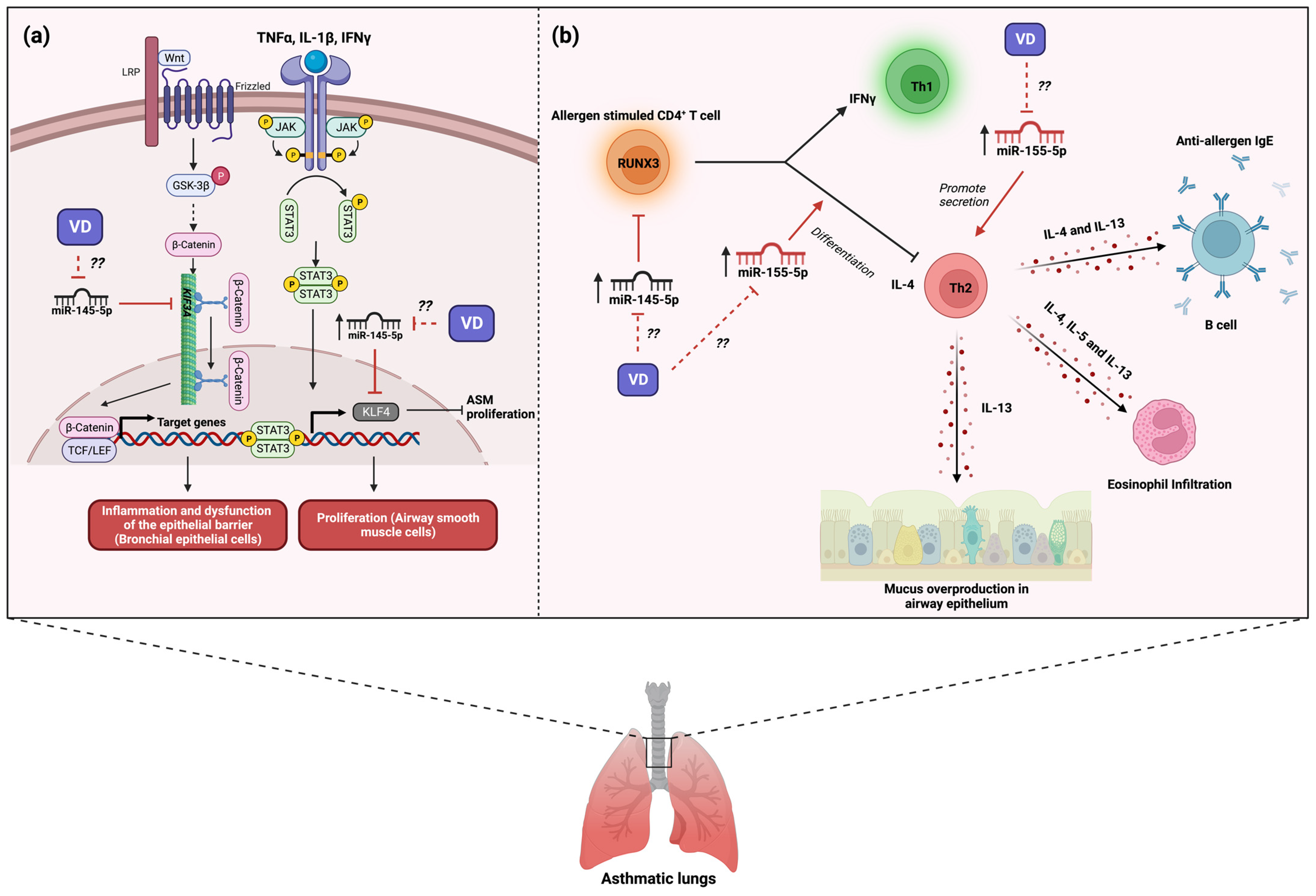
4. The Role of MicroRNA-155 in Asthma and Its Modulation by Vitamin D3
5. Conclusions
References
- Rostami Hir, S.; Alizadeh, Z.; Mazinani, M.; Mahlooji Rad, M.; Fazlollahi, M.R.; Kazemnejad, A.; Zavaran Hosseini, A.; Moin, M. Exosomal MicroRNAs as Biomarkers in Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 160–168.
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Saavedra-Nieves, P.; Arias, P.; González-Fernández, C.; Mosteiro-Añón, M.; Corbacho-Abelaira, M.D.; Blanco-Aparicio, M.; Méndez-Brea, P.; et al. Serum Exosome Inflamma-MiRs Are Surrogate Biomarkers for Asthma Phenotype and Severity. Allergy 2023, 78, 141–155.
- ElKashef, S.M.M.A.E.; Ahmad, S.E.A.; Soliman, Y.M.A.; Mostafa, M.S. Role of MicroRNA-21 and MicroRNA-155 as Biomarkers for Bronchial Asthma. Innate Immun. 2021, 27, 61.
- Hammad Mahmoud Hammad, R.; Hamed, D.H.E.D.; Eldosoky, M.A.E.R.; Ahmad, A.A.E.S.; Osman, H.M.; Abd Elgalil, H.M.; Mahmoud Hassan, M.M. Plasma MicroRNA-21, MicroRNA-146a and IL-13 Expression in Asthmatic Children. Innate Immun. 2018, 24, 171–179.
- Joo, H.; Park, S.Y.; Park, S.Y.; Park, S.Y.; Kim, S.H.; Cho, Y.S.; Yoo, K.H.; Jung, K.S.; Rhee, C.K. Phenotype of Asthma-COPD Overlap in COPD and Severe Asthma Cohorts. J. Korean Med. Sci. 2022, 37, e236.
- Li, X.; Yang, N.; Cheng, Q.; Zhang, H.; Liu, F.; Shang, Y. Mir-21-5p in Macrophage-Derived Exosomes Targets Smad7 to Promote Epithelial Mesenchymal Transition of Airway Epithelial Cells. J. Asthma Allergy 2021, 14, 513–524.
- Liu, Y.; Yang, K.; Shi, H.; Xu, J.; Zhang, D.; Wu, Y.; Zhou, S.; Sun, X. MiR-21 Modulates Human Airway Smooth Muscle Cell Proliferation and Migration in Asthma through Regulation of PTEN Expression. Exp. Lung Res. 2015, 41, 535–545.
- Lee, H.Y.; Hur, J.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. MicroRNA-21 Inhibition Suppresses Alveolar M2 Macrophages in an Ovalbumin-Induced Allergic Asthma Mice Model. Allergy Asthma Immunol. Res. 2021, 13, 312.
- Zou, Y.; Zhou, Q.; Zhang, Y. MicroRNA-21 Released from Mast Cells-Derived Extracellular Vesicles Drives Asthma in Mice by Potentiating Airway Inflammation and Oxidative Stress. Am. J. Transl. Res. 2021, 13, 7475.
- Sheane, B.; Smyth, P.; Scott, K.; Aziz, R.; Buckley, M.; Lodge, E.; Kiely, N.; Kingston, M.; McGovern, E.; Healy, M.; et al. An Association between MicroRNA-21 Expression and Vitamin D Deficiency in Coronary Artery Disease. Microrna 2015, 4, 57–63.
- Grieco, G.E.; Cataldo, D.; Ceccarelli, E.; Nigi, L.; Catalano, G.; Brusco, N.; Mancarella, F.; Ventriglia, G.; Fondelli, C.; Guarino, E.; et al. Serum Levels of MiR-148a and MiR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Non-Coding RNA 2018, 4, 37.
- Xu, Y.; Qian, J.; Yu, Z. Budesonide Up-Regulates Vitamin D Receptor Expression in Human Bronchial Fibroblasts and Enhances the Inhibitory Effect of Calcitriol on Airway Remodeling. Allergol. Immunopathol. 2019, 47, 585–590.
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. MiR-21 Mediates Fibrogenic Activation of Pulmonary Fibroblasts and Lung Fibrosis. J. Exp. Med. 2010, 207, 1589–1597.
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b Attenuates Mitochondrial Oxidative Stress and Inflammation in Endothelial Cells. Redox Biol. 2023, 62, 102681.
- Tang, Y.; Yang, L.J.; Liu, H.; Song, Y.J.; Yang, Q.Q.; Liu, Y.; Qian, S.W.; Tang, Q.Q. Exosomal MiR-27b-3p Secreted by Visceral Adipocytes Contributes to Endothelial Inflammation and Atherogenesis. Cell Rep. 2023, 42, 111948.
- Kho, A.T.; Sharma, S.; Davis, J.S.; Spina, J.; Howard, D.; McEnroy, K.; Moore, K.; Sylvia, J.; Qiu, W.; Weiss, S.T.; et al. Circulating MicroRNAs: Association with Lung Function in Asthma. PLoS ONE 2016, 11, e0157998.
- Dong, X.; Zhong, N.; Fang, Y.; Cai, Q.; Lu, M.; Lu, Q. MicroRNA 27b-3p Modulates SYK in Pediatric Asthma Induced by Dust Mites. Front. Pediatr. 2018, 6, 301.
- Macglashan, D.; Moore, G.; Muchhal, U. Regulation of IgE-Mediated Signalling in Human Basophils by CD32b and Its Role in Syk down-Regulation: Basic Mechanisms in Allergic Disease. Clin. Exp. Allergy 2014, 44, 713–723.
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between Vitamin D Status and Asthma Control: A Meta-Analysis of Randomized Trials. Respir. Med. 2019, 150, 85–94.
- Coskunpinar, E.; Akcesme, B.; Tas, S.K.; Aynaci, A. Investigation of MiRNAs That Are Effective in the Pathogenesis of Asthma. J. Asthma 2023, 60, 2145–2152.
- Alexandrova, E.; Miglino, N.; Hashim, A.; Nassa, G.; Stellato, C.; Tamm, M.; Baty, F.; Brutsche, M.; Weisz, A.; Borger, P. Small RNA Profiling Reveals Deregulated Phosphatase and Tensin Homolog (PTEN)/Phosphoinositide 3-Kinase (PI3K)/Akt Pathway in Bronchial Smooth Muscle Cells from Asthmatic Patients. J. Allergy Clin. Immunol. 2016, 137, 58–67.
- Kılıç, A.; Santolini, M.; Nakano, T.; Schiller, M.; Teranishi, M.; Gellert, P.; Ponomareva, Y.; Braun, T.; Uchida, S.; Weiss, S.T.; et al. A Systems Immunology Approach Identifies the Collective Impact of 5 MiRs in Th2 Inflammation. JCI Insight 2018, 3, e97503.
- Li, F.; Zhang, A.; Shi, Y.; Ma, Y.; Du, Y. 1a,25-DihydroxyVitamin D3 Prevents the Differentiation of Human Lung Fibroblasts via MicroRNA-27b Targeting the Vitamin D Receptor. Int. J. Mol. Med. 2015, 36, 967–974.
- Kordkhayli, M.M.; Mansouri, F.; Talebi, F.; Noorbakhsh, F.; Saboor-Yaraghi, A.A. Influence of Vitamins A and D on the Expression of MicroRNA27-3p Isoforms and GATA3 in Experimental Autoimmune Encephalomyelitis. Iran. J. Allergy Asthma Immunol. 2022, 21, 429–440.
- Ge, X.; Yuan, L.; Wei, J.; Nguyen, T.; Tang, C.; Liao, W.; Li, R.; Yang, F.; Zhang, F.; Zhao, B.; et al. Vitamin D/VDR Signaling Induces MiR-27a/b Expression in Oral Lichen Planus. Sci. Rep. 2020, 10, 301.
- Quintanilha, B.J.; Reis, B.Z.; Corrêa, T.A.F.; Duarte, G.B.D.S.; Rogero, M.M. MicroRNAs and Inflammation Biomarkers in Obesity. In Precision Medicine for Investigators, Practitioners and Providers; Academic Press: Cambridge, MA, USA, 2020; pp. 179–185.
- Kadkhoda, S.; Ghafouri-Fard, S. Function of MiRNA-145–5p in the Pathogenesis of Human Disorders. Pathol. Res. Pract. 2022, 231, 153780.
- Panganiban, R.P.; Wang, Y.; Howrylak, J.; Chinchilli, V.M.; Craig, T.J.; August, A.; Ishmael, F.T. Circulating MicroRNAs as Biomarkers in Patients with Allergic Rhinitis and Asthma. J. Allergy Clin. Immunol. 2016, 137, 1423–1432.
- Lacedonia, D.; Palladino, G.P.; Foschino-Barbaro, M.P.; Scioscia, G.; Elisiana, G. Carpagnano Expression Profiling of MiRNA-145 and MiRNA-338 in Serum and Sputum of Patients with COPD, Asthma, and Asthma–COPD Overlap Syndrome Phenotype. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 1811.
- Mendes, F.C.; Paciência, I.; Ferreira, A.C.; Martins, C.; Rufo, J.C.; Silva, D.; Cunha, P.; Farraia, M.; Moreira, P.; Delgado, L.; et al. Development and Validation of Exhaled Breath Condensate MicroRNAs to Identify and Endotype Asthma in Children. PLoS ONE 2019, 14, e0224983.
- Tiwari, A.; Li, J.; Kho, A.T.; Sun, M.; Lu, Q.; Weiss, S.T.; Tantisira, K.G.; McGeachie, M.J. COPD-Associated MiR-145-5p Is Downregulated in Early-Decline FEV1 Trajectories in Childhood Asthma. J. Allergy Clin. Immunol. 2021, 147, 2181–2190.
- Collison, A.; Mattes, J.; Plank, M.; Foster, P.S. Inhibition of House Dust Mite-Induced Allergic Airways Disease by Antagonism of MicroRNA-145 Is Comparable to Glucocorticoid Treatment. J. Allergy Clin. Immunol. 2011, 128, 160–167.e4.
- Xiong, T.; Du, Y.; Fu, Z.; Geng, G. MicroRNA-145-5p Promotes Asthma Pathogenesis by Inhibiting Kinesin Family Member 3A Expression in Mouse Airway Epithelial Cells. J. Int. Med. Res. 2019, 47, 3307.
- Cheng, Z.; Dai, L.L.; Wang, X.; Jia, L.Q.; Jing, X.G.; Li, P.F.; Liu, M.; Wang, H.; An, L. MicroRNA-145 down-Regulates Mucin 5AC to Alleviate Airway Remodeling and Targets EGFR to Inhibit Cytokine Expression. Oncotarget 2017, 8, 46312.
- Liu, Y.; Sun, X.; Wu, Y.; Fang, P.; Shi, H.; Xu, J.; Li, M. Effects of MiRNA-145 on Airway Smooth Muscle Cells Function. Mol. Cell Biochem. 2015, 409, 135–143.
- Qiu, Y.Y.; Zhang, Y.W.; Qian, X.F.; Bian, T. MiR-371, MiR-138, MiR-544, MiR-145, and MiR-214 Could Modulate Th1/Th2 Balance in Asthma through the Combinatorial Regulation of Runx3. Am. J. Transl. Res. 2017, 9, 3184.
- Fan, L.; Wang, X.; Fan, L.; Chen, Q.; Zhang, H.; Pan, H.; Xu, A.; Wang, H.; Yu, Y. MicroRNA-145 Influences the Balance of Th1/Th2 via Regulating RUNX3 in Asthma Patients. Exp. Lung Res. 2016, 42, 417–424.
- Aladel, A.; Khatoon, F.; Khan, M.I.; Alsheweir, A.; Almutairi, M.G.; Almutairi, S.O.; Almutairi, F.K.; Osmonaliev, K.; Beg, M.M.A. Evaluation of MiRNA-143 and MiRNA-145 Expression and Their Association with Vitamin-D Status Among Obese and Non-Obese Type-2 Diabetic Patients. J. Multidiscip. Healthc. 2022, 15, 2979.
- Chang, S.; Gao, L.; Yang, Y.; Tong, D.; Guo, B.; Liu, L.; Li, Z.; Song, T.; Huang, C. MiR-145 Mediates the Antiproliferative and Gene Regulatory Effects of Vitamin D3 by Directly Targeting E2F3 in Gastric Cancer Cells. Oncotarget 2015, 6, 7675–7685.
- Carrillo-lópez, N.; Panizo, S.; Arcidiacono, M.V.; de la Fuente, S.; Martínez-arias, L.; Ottaviano, E.; Ulloa, C.; Ruiz-torres, M.P.; Rodríguez, I.; Cannata-Andía, J.B.; et al. Vitamin D Treatment Prevents Uremia-Induced Reductions in Aortic MicroRNA-145 Attenuating Osteogenic Differentiation despite Hyperphosphatemia. Nutrients 2022, 14, 2589.
- Liu, Q.; Wang, W.; Jing, W. Indoor Air Pollution Aggravates Asthma in Chinese Children and Induces the Changes in Serum Level of MiR-155. Int. J. Environ. Health Res. 2018, 29, 22–30.
- Karam, R.A.; Abd Elrahman, D.M. Differential Expression of MiR-155 and Let-7a in the Plasma of Childhood Asthma: Potential Biomarkers for Diagnosis and Severity. Clin. Biochem. 2019, 68, 30–36.
- Chen, H.; Xu, X.; Cheng, S.; Xu, Y.; Xuefei, Q.; Cao, Y.; Xie, J.; Wang, C.Y.; Xu, Y.; Xiong, W. Small Interfering RNA Directed against MicroRNA-155 Delivered by a Lentiviral Vector Attenuates Asthmatic Features in a Mouse Model of Allergic Asthma. Exp. Ther. Med. 2017, 14, 4391.
- Malmhäll, C.; Alawieh, S.; Lu, Y.; Sjöstrand, M.; Bossios, A.; Eldh, M.; Rådinger, M. MicroRNA-155 Is Essential for TH2-Mediated Allergen-Induced Eosinophilic Inflammation in the Lung. J. Allergy Clin. Immunol. 2014, 133, 1429–1438.
- Qiu, L.; Zhang, Y.; Do, D.C.; Ke, X.; Zhang, S.; Lambert, K.; Kumar, S.; Hu, C.; Zhou, Y.; Ishmael, F.T.; et al. MiR-155 Modulates Cockroach Allergen and Oxidative Stress–Induced Cyclooxygenase-2 in Asthma. J. Immunol. 2018, 201, 916–929.
- Chia, N.; Kumar, R.K.; Foster, P.S.; Herbert, C. Enhanced Pro-Inflammatory Response of Macrophages to Interleukin-33 in an Allergic Environment. Int. Arch. Allergy Immunol. 2018, 176, 74–82.
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D Promotes Negative Feedback Regulation of TLR Signaling via Targeting MicroRNA-155–SOCS1 in Macrophages. J. Immunol. 2013, 190, 3687–3695.
- Arboleda, J.F.; Fernandez, G.J.; Urcuqui-Inchima, S. Vitamin D-Mediated Attenuation of MiR-155 in Human Macrophages Infected with Dengue Virus: Implications for the Cytokine Response. Infect. Genet. Evol. 2019, 69, 12–21.
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.F. Vitamin D Limits Inflammation-Linked MicroRNA Expression in Adipocytes in Vitro and in Vivo: A New Mechanism for the Regulation of Inflammation by Vitamin D. Epigenetics 2018, 13, 156–162.




