Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergio Terracina | -- | 4584 | 2024-01-30 15:02:53 | | | |
| 2 | Sirius Huang | Meta information modification | 4584 | 2024-01-31 01:54:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferraguti, G.; Terracina, S.; Tarani, L.; Fanfarillo, F.; Allushi, S.; Caronti, B.; Tirassa, P.; Polimeni, A.; Lucarelli, M.; Cavalcanti, L.; et al. Role of NGF in Inflammation and Tumor Growth. Encyclopedia. Available online: https://encyclopedia.pub/entry/54534 (accessed on 13 January 2026).
Ferraguti G, Terracina S, Tarani L, Fanfarillo F, Allushi S, Caronti B, et al. Role of NGF in Inflammation and Tumor Growth. Encyclopedia. Available at: https://encyclopedia.pub/entry/54534. Accessed January 13, 2026.
Ferraguti, Giampiero, Sergio Terracina, Luigi Tarani, Francesca Fanfarillo, Sara Allushi, Brunella Caronti, Paola Tirassa, Antonella Polimeni, Marco Lucarelli, Luca Cavalcanti, et al. "Role of NGF in Inflammation and Tumor Growth" Encyclopedia, https://encyclopedia.pub/entry/54534 (accessed January 13, 2026).
Ferraguti, G., Terracina, S., Tarani, L., Fanfarillo, F., Allushi, S., Caronti, B., Tirassa, P., Polimeni, A., Lucarelli, M., Cavalcanti, L., Greco, A., & Fiore, M. (2024, January 30). Role of NGF in Inflammation and Tumor Growth. In Encyclopedia. https://encyclopedia.pub/entry/54534
Ferraguti, Giampiero, et al. "Role of NGF in Inflammation and Tumor Growth." Encyclopedia. Web. 30 January, 2024.
Copy Citation
Nerve growth factor (NGF) plays a dual role both in inflammatory states and cancer, acting both as a pro-inflammatory and oncogenic factor and as an anti-inflammatory and pro-apoptotic mediator in a context-dependent way based on the signaling networks and its interaction with diverse cellular components within the microenvironment.
apoptosis
cancer stem cells
metastasis
epigenetic
inflammation
microenvironment
neurotrophins
NGF
tumor
1. Introduction
Inflammatory processes play a multifaceted role in tumor development and progression [1][2][3][4][5][6][7]. While the immune system’s inflammatory response is typically a defense mechanism against infections and tissue damage, chronic or persistent inflammation can contribute to the initiation, growth, and spread of certain types of tumors. Thus, the understanding of the relationship between inflammation and cancer could better focus on anti-inflammatory therapies and drugs that target inflammatory pathways as potential strategies for cancer prevention and treatment [8]. In recent years, a growing body of evidence has emphasized the involvement of neurotrophins (NTs) in the complex landscape of both inflammation and cancer biology, showing their significant role in determining tumor cell growth and survival, particularly in certain types of cancers expressing NT receptors on their cell surfaces [9][10]. One of the most well-studied NTs is the nerve growth factor (NGF), which binds to its specific receptor, tropomyosin-related kinase A (TrkA), expressed on various types of cancer cells, including those derived from the brain, prostate, breast, and pancreas, among others [11][12][13]. NGF contributes to inflammation by acting as a signaling molecule that stimulates immune cells to release cytokines and enhances the sensitivity of sensory nerves, contributing to the perception of pain and hypersensitivity in inflamed tissues [14]. NTs promote tumor growth by stimulating cancer cell proliferation and suppressing apoptosis, which physiologically helps eliminate damaged or unwanted cells from the body [15]. Furthermore, NTs can stimulate the production of pro-angiogenic factors, modulate the tumor microenvironment (TME), and induce the epithelial–mesenchymal transition (EMT), leading to increased cell mobility and invasiveness [16].
Overall, the activation of NGF signaling pathways in cancer cells can contribute to aggressive tumor growth, metastasis, and therapy resistance. Originally identified for its pivotal role in neuronal development and function, NGF has emerged as a critical player in the growth and survival of various tumor types. The intriguing aspect of NGF’s contribution to cancer lies in its dual role, acting as both an oncogenic factor, fueling tumor cell growth, and as a pro-apoptotic mediator, promoting tumor cell death under certain circumstances [17][18][19]. The context-dependent actions of NGF in cancer underscore the complexity of the signaling networks in which it operates and its interaction with diverse cellular components within the TME. Overall, NGF’s involvement in both inflammation and tumors further highlights its complex role in regulating physiological processes and disease states. Understanding the intricate interactions of NGF with various cell types and pathways is crucial for developing targeted therapies that can modulate its effects in different disease contexts, offering a potential therapeutic strategy to inhibit tumor progression and improve patient outcomes. However, further research is needed to fully understand the complexities of NGF signaling in different cancer types and to develop effective and safe targeted therapies.
2. NGF and Inflammation and Tumor Growth
2.1. Nerve Growth Factor and Neurotrophins
NGF is a crucial neurotrophic factor responsible for the growth, survival, and maintenance of neuronal and non-neuronal cells [20][21]. Discovered in the 1950s, it was one of the first NTs identified and extensively studied for its role in the development and function of the nervous system [22][23]. NGF belongs to the family of NTs, which includes other proteins like Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), and Neurotrophin-4/5 (NT-4/5) [24][25]. NGF is synthesized as a precursor, called pro-NGF, which after processing generates the mature NGF molecule. NGF is primarily produced by various cell types, including immune cells, endothelial cells, and tissues within the nervous system [14][26][27][28].
Pro-NGF has a physiological role that goes beyond that of a simple precursor, especially in the nervous system, where it may possess pro-apoptotic activity [29]. Indeed, pro-NGF regulates apoptosis and inflammation and is associated with several neurodegenerative diseases, myocardial infarction, and diabetes [30][31][32][33][34]. The actions of NGF are mediated through its interaction with specific receptors. There are two primary receptors associated with NGF:
- (1)
-
TrkA is the high-affinity receptor for NGF; its activation triggers a cascade of intracellular signaling events, including the MAPK/ERK pathway and the PI3K/Akt pathway [35][36]. These pathways play essential roles in cell growth, differentiation, and survival. TrkA is expressed on the surface of neurons and other cell types, enabling NGF to exert its neurotrophic effects;
- (2)
-
p75 neurotrophin receptor (p75NTR) has a lower affinity for NGF but plays a modulatory role in NGF signaling [20][37]. It can interact with TrkA and enhance its binding affinity for NGF, influencing the cellular responses elicited by NGF. p75NTR is mostly involved in processes like cell death, survival decisions, and axonal growth.
Furthermore, NGF may attach to the membrane receptor sortilin, which has been demonstrated to participate in cancer growth [38], and also to neuropilin-1 (NRP1), a nociceptor-enriched co-receptor for NGF that is necessary for the TrkA signaling of pain [39][40][41].
Indeed, modifications in NGF levels in the serum and plasma have been shown during the beginning and evolution of many health conditions, including the post-partum period, stressful events, cardiometabolic disruptions, aging, alcohol addiction, and other pathophysiological conditions, such as psychiatric, neurological, and immune disorders [42][43][44][45][46][47][48][49][50][51][52]. Thus, the understanding of the interplay between NGF and its receptors is crucial not only for comprehending the complexities of neuronal development and function but also for exploring potential therapeutic interventions in various diseases, including neurodegenerative disorders and certain types of cancers where NGF signaling may be implicated [51][53][54][55].
2.2. Inflammation
Inflammation is a natural response by the body’s immune system to protect against harmful stimuli like pathogens, irritants, or damaged cells. It is a complex biological process that aims to eliminate the initial cause of cell injury, clear out damaged cells, and initiate tissue repair [56]. Actually, in medicine, inflammation, a term coined by the Romans, lacks a precise, universally accepted definition, varying in interpretation based on context and individual perspectives [57]. It often carries a negative connotation as an uncontrolled reaction likened to a destructive wildfire, requiring immediate containment.
However, overshadowed in this view is the fundamental role that inflammation plays in both maintaining health and ensuring survival. Inflammation involves a complex interplay of cells, chemicals, and molecular signals including blood vessel dilatation (causing redness and heat) and increased permeability, allowing immune cells and fluids to move from the bloodstream into the tissues (leading to swelling); the cellular release of chemicals such as histamine, cytokines, and prostaglandins, which help to trigger the immune response and promote healing; and finally the activation of immune cells which migrate to the affected area to destroy pathogens or damaged cells [58].
Cytokines play a pivotal role in orchestrating or modulating inflammation, confirming not only the presence and magnitude of inflammation but also guiding treatment decisions. Inflammation can be distinguished as acute and chronic. Acute inflammation is the body’s immediate and short-term response to an injury or infection [59]. It is characterized by symptoms like redness, swelling, heat, pain, and sometimes loss of function [60][61]. Chronic inflammation, on the other hand, is long-term and can last for weeks, months, or even years [62]. It occurs when the immune system’s response persists, often due to underlying health conditions, such as autoimmune disorders, ongoing infections, obesity, or prolonged exposure to irritants like smoke.
Lifestyle factors like stress, poor diet, lack of exercise, and environmental toxins can contribute to chronic inflammation [63]. While inflammation is a vital part of the body’s defense mechanism, chronic inflammation can be a problematic event leading to tissue damage and various diseases including diabetes, cancer, cardiovascular diseases, eye disorders, arthritis, obesity, autoimmune diseases, and inflammatory bowel disease [64].
Interestingly, inflammation is at the base of various conditions and a plethora of etiopathogenetic events; for example, hepatitis (liver inflammation) has been associated with viral infections, excessive alcohol consumption, certain medications, or autoimmune responses [62][65][66][67][68]. Long-term inflammation of the liver can lead to cirrhosis and liver cancer [69][70]. Inflammatory states and diseases cover a wide range of conditions affecting various parts of the body, varying in severity and requiring different treatments, including medications, lifestyle changes, and sometimes, in more severe cases, surgical interventions or specialized therapies.
The widespread use of anti-inflammatory medications assumed to counteract all inflammatory responses potentially may hinder the body’s ability to fully recover [71][72]. Indeed, not all situations warrant an inflammatory response (such as blunt trauma and exposure to toxins), but since inflammation affects both unhealthy and healthy tissues without discrimination, it should be treated when it has the potential to persist or spread uncontrollably, causing prolonged damage. Effective management often entangles a multidisciplinary approach that requires the support of specialized healthcare professionals in this specific condition. Actually, it has been suggested that an effective way to guide therapy for inflammation is to assess a combination of markers associated with inflammation and fibrosis, such as C-reactive protein, ferritin, serum amyloid A (SAA), pro-calcitonin, and transforming growth factor-β (TGF-β, a significant contributor to fibrosis), alongside cytokine profiling [73].
2.3. Role of NGF in Carcinogenesis
NGF plays a significant role in various aspects of human health, including its involvement in tumors. Overall, evidence indicates that NGF is unable to generate cell carcinogenesis alone, both in normal neuronal and non-neuronal cells/tissues; however, it could be a major determinant in the case of co-expression with pro-carcinogenic molecules [74]. Quite intriguingly, NGF was initially discovered by R. Levi-Montalcini nearly 60 years ago in the context of a transplantation experiment involving a malignant mouse sarcoma [75][76].
NGF as a Tumor Growth Facilitator or Suppressor
Depending on the tumor’s origin, pro-survival signaling can be facilitated through TrkA and/or p75NTR receptors [77]. In breast cancer, NGF plays a crucial role in stimulating proliferative signaling via TrkA and pro-survival signaling through p75NTR [35]. Furthermore, the activation of p75NTR in breast cancer promotes increased resistance to cell death induced by chemotherapeutic treatments. On the other hand, the role of p75NTR in prostate cells is distinct since p75NTR mediates cell death and acts as a tumor suppressor in the case of normal prostate cells [78]. In prostate cancer, the expression of p75NTR is lost, contributing to tumor progression, death evasion, uncontrolled proliferation, and metastasis to distant sites [79]. Interestingly, other mechanisms were found in recent studies. For example, NGF plays a significant role in liver cancer progression and metastasis, exerting wide influences on liver cancer cell polarity and motility by regulating signaling pathways involved in cell movement, cytoskeletal organization, and cellular polarity [80]. Heightened NGF disrupts cell polarity, boosts cell movement, triggers changes related to cell transition, rearranges the cell’s structural framework, and protects cells from apoptosis and detachment-induced cell death [80]. Table 1 reports the main role of NGF and its receptors in various cancers.
Table 1. Detailed overview of the role of NGF and its receptors in various types of tumors. While NGF’s primary function is related to neural development and function, its relationship with tumors is complex and multifaceted. The involvement of NGF in tumors is not as straightforward as in normal nerve growth, and its effects on different types of tumors can vary. Acetylcholine, Ach; A disintegrin and metalloprotease 17, ADAM17; protein kinase B, Akt; extracellular signal-regulated kinase, ERK; F-box-only protein 22, FBOXO22; hypoxia-inducible factor 1 subunit alpha, HIF1α; nerve growth factor, NGF; non-small-cell lung cancer, NSCLC; neurotrophic tyrosine receptor kinase, NTRK; nuclear factor kB, NF-kB; p75 pan-neurotrophin receptor, p75NTR; programmed death-ligand 1, PD-L1; rearranged during transfection, RET; small nuclear ribonucleoprotein polypeptide A, SNRPA; tissue inhibitor of metalloproteinases, TIMP; tropomyosin receptor kinase, Trk; vascular endothelial growth factor, VEGF. (*) MYCN is amplified in 20% of neuroblastomas and correlates with aggressive phenotype and poor prognosis. (**) Perineural invasion driven by the TME has been identified as a key pattern of several malignancies including breast, pancreatic, and prostate cancers.
| Kind of Tumor | Role of NGF | References |
|---|---|---|
| Brain tumors |
|
[81][82][83][84][85][86][87][88][89] |
| Breast Cancer |
|
[26][90][91][92][93][94][95][96][97][98][99] |
| Colorectal Cancer |
|
[17][47][100][101][102][103] |
| Gastric Cancer |
|
[104][105][106][107][108] |
| Head and Neck Cancer |
|
[10][44][109][110][111][112][113][114][115][116][117] |
| Leukemias |
|
[118][119][120][121] |
| Liver Cancer |
|
[80][122][123][124][125][126][127][128][129][130][131][132][133] |
| Lung Cancer |
|
[83][134][135][136][137][138][139] |
| Ovarian Cancer |
|
[140][141][142][143][144][145][146][147][148][149] |
| Neuroblastoma |
|
[24][150][151][152][153][154][155][156] |
| Pancreatic Cancer |
|
[13][15][16][157][158][159][160][161][162][163][164][165][166][167] |
| Pediatric tumors |
|
[28][36][82][168][169][170] |
| Prostate Cancer |
|
[77][171][172][173][174][175][176] |
| Skin tumors |
|
[54][177][178][179][180][181][182][183][184][185][186] |
While the primary role of NGF is related to the development and function of nerve cells, it also plays a main role in inflammation. Inflammation, as stated before, is a complex biological response triggered by the body’s immune system to protect against harmful stimuli, such as tissue damage and pathogens. In this scenario, NGF can regulate the innervation and neuronal activity of peripheral neurons, inducing the release of immune-active cytokines, neuropeptides, and neurotransmitters [14][187][188][189]. Furthermore, NGF can also directly influence innate and adaptive immune responses through its interaction with various cells involved in the immune response, including mast cells, lymphocytes, and macrophages [28][190]. Actually, NGF has a variety of effects that can be either pro-inflammatory or anti-inflammatory depending on the expression of its receptors, which are dynamically regulated in immune cells depending on their state of differentiation and functional activity [191][192]. The seeming ambiguity is mainly due to the role of NGF as an endogenous molecule capable of triggering immune responses while also initiating pathways that control inflammation and prevent excessive tissue damage so that altered expression of its receptors could hinder NGF’s ability to engage the regulatory feedback processes for finally sustaining the perpetuation of inflammation in conditions such as chronic inflammatory diseases or autoimmune disorders [14]. Additionally, as NGF can contribute to the sensitivity and pain associated with the neurogenic inflammation of tissues, a potential role of NTs has been suggested as novel treatment strategies in chronic inflammatory diseases [193][194].
More specifically, NGF has intricate connections with neuroinflammation, which involves complex interactions between immune cells, glial cells, and various signaling molecules. Microglia act as the resident immune cells of the central nervous system (CNS) and can become activated in response to injury or inflammation [195]. NGF can modulate the activation and function of glial cells, particularly microglia and astrocytes, influencing their release of inflammatory mediators [196]. It can both promote and dampen the release of various cytokines depending on the context, contributing to the fine-tuning of the inflammatory response in the CNS [157][197][198] (see Figure 1).
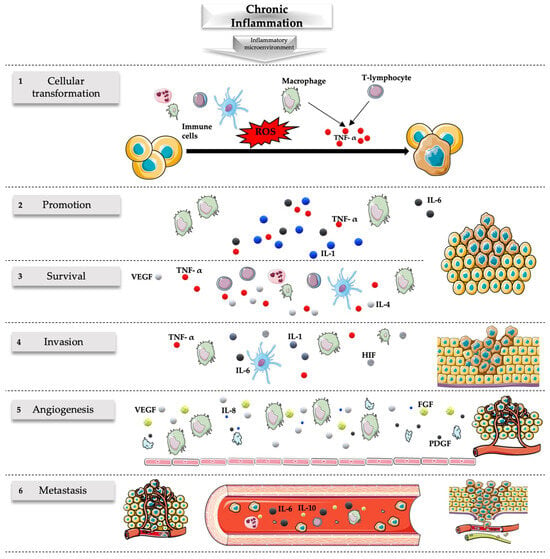
Figure 1. Role of chronic inflammation in cancer. Chronic inflammation originating from persistent stimuli has been associated with many steps of the carcinogenesis process including transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis. (1) Cellular transformation is favored by the mutagenic action of ROS released by the immune cells and the action of TNF-α released by macrophages and T lymphocytes. (2) Carcinogenesis is promoted by various cytokines released during the chronic inflammatory process, including IL-1, IL-6, and TNF-α released by macrophages. (3) The survival of the tumor is associated with an ineffective response of the immune system associated with the defective action of inflammatory cells (like the release of IL-4 and IL-5 by T cells associated with T-helper 2 but not T-helper 1 responses) related to the release of various molecules like TNF-α, VEGF, Fas ligand, and transforming growth factor-β. (4) The invasion is favored by numerous molecules during inflammatory states; some of the most important are those associated with hypoxia including HIF, TNF-α, IL-1, and IL-6. (5) Inflammatory cells, especially macrophages, but also endothelial cells and platelets stimulate vascular growth through the release of many angiogenic factors (e.g., VEGF, IL-8, FGF, PDGF), sustaining the nutritional needs of the tumor and favoring its survival and migration. (6) Numerous cytokines and factors released during inflammation can lead to metastatic events of tumors including IL-6 and IL-10. The inflammatory microenvironment has a major role in this setting with implications for the prevention and treatment of cancer. FGF, fibroblast growth factor; HIF, hypoxia-inducible factor; IL, interleukin; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/4.0/, accessed on 21 January 2024).
On the other hand, NGF exhibits neuroprotective effects by mitigating the harmful consequences of neuroinflammation and supporting neuronal survival and function, potentially counteracting the detrimental effects of excessive inflammation on neurons [199][200]. Furthermore, dysregulation of NGF signaling has been associated with various neurological disorders characterized by neuroinflammation, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and neuropathic pain conditions [201][202]. In these disorders, altered NGF levels or signaling pathways contribute to the progression of neuroinflammation and neuronal damage, so it has been suggested that modulating NGF levels or its interactions within the CNS may offer potential avenues for managing neuroinflammatory conditions and related neurological disorders [203][204].
Unfortunately, inflammation plays a multifaceted role in tumor development and progression. While the immune system’s inflammatory response is typically a defense mechanism against infections and tissue damage, chronic or persistent inflammation can contribute to the initiation, growth, and spread of certain types of tumors in several ways [69]. Cycles of tissue damage and subsequent repair processes can create an environment conducive to genetic mutations and abnormalities, increasing the likelihood of cancerous changes in cells [205]. Inflammation can indeed sustain tissue damage and attract immune cells to the site of tissue damage.
Hallmarks of cancer-associated inflammation include the presence of infiltrating leukocytes, cytokines, chemokines, growth factors, lipid messengers, and matrix-degrading enzymes [206].
Some of these immune cells, like certain types of macrophages and lymphocytes, can produce factors that support tumor growth and suppress the immune system’s ability to eliminate cancer cells [207]. Inflammatory signals can stimulate angiogenesis for oxygen provision and nutrients to the tumor cells, aiding their proliferation and survival [10][208]. Managing chronic inflammation, either through lifestyle changes or medication, may play a role in reducing the risk of certain cancers or improving treatment outcomes [209].
References
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940.
- Mir, S.A.; Dar, A.; Hamid, L.; Nisar, N.; Malik, J.A.; Ali, T.; Nabi Bader, G. Flavonoids as promising molecules in the cancer therapy: An insight. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100167.
- Thiery, J.; Fahrner, M. Integration of proteomics in the molecular tumor board. Proteomics 2023, e2300002.
- Verma, C.; Pawar, V.A.; Srivastava, S.; Tyagi, A.; Kaushik, G.; Shukla, S.K.; Kumar, V. Cancer Vaccines in the Immunotherapy Era: Promise and Potential. Vaccines 2023, 11, 1783.
- De Raffele, D.; Ilie, I.M. Unlocking novel therapies: Cyclic peptide design for amyloidogenic targets through synergies of experiments, simulations, and machine learning. Chem. Commun. 2023, 60, 632–645.
- Chen, Y.; Wang, X.; Ye, Y.; Ren, Q. Gut microbiota in cancer: Insights on microbial metabolites and therapeutic strategies. Med. Oncol. 2023, 41, 25.
- Liao, C.P.; Booker, R.C.; Brosseau, J.P.; Chen, Z.; Mo, J.; Tchegnon, E.; Wang, Y.; Wade Clapp, D.; Le, L.Q. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J. Clin. Investig. 2018, 128, 2848–2861.
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target Ther. 2021, 6, 263.
- Triaca, V.; Carito, V.; Fico, E.; Rosso, P.; Fiore, M.; Ralli, M.; Lambiase, A.; Greco, A.; Tirassa, P. Cancer stem cells-driven tumor growth and immune escape: The Janus face of neurotrophins. Aging 2019, 11, 11770–11792.
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, M.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145.
- Aygun, N. Biological and Genetic Features of Neuroblastoma and Their Clinical Importance. Curr. Pediatr. Rev. 2018, 14, 73–90.
- Singh, R.; Karri, D.; Shen, H.; Shao, J.; Dasgupta, S.; Huang, S.; Edwards, D.E.; Ittmann, M.M.; O’Malley, B.W.; Yi, P. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J. Clin. Investig. 2018, 128, 3129–3143.
- Jiang, J.; Bai, J.; Qin, T.; Wang, Z.; Han, L. NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. J. Cell Mol. Med. 2020, 24, 5901–5910.
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 2017, 18, 1028.
- Peng, T.; Guo, Y.; Gan, Z.; Ling, Y.; Xiong, J.; Liang, X.; Cui, J. Nerve Growth Factor (NGF) Encourages the Neuroinvasive Potential of Pancreatic Cancer Cells by Activating the Warburg Effect and Promoting Tumor Derived Exosomal miRNA-21 Expression. Oxid. Med. Cell Longev. 2022, 2022, 8445093.
- Zuo, J.; Yi, C.; Chen, Z.; Zhou, B.; Yang, T.; Lin, J. A novel refined pyroptosis and inflammasome-related genes signature for predicting prognosis and immune microenvironment in pancreatic ductal adenocarcinoma. Sci. Rep. 2022, 12, 18384.
- Anagnostopoulou, V.; Pediaditakis, I.; Alkahtani, S.; Alarifi, S.A.; Schmidt, E.M.; Lang, F.; Gravanis, A.; Charalampopoulos, I.; Stournaras, C. Differential effects of dehydroepiandrosterone and testosterone in prostate and colon cancer cell apoptosis: The role of nerve growth factor (NGF) receptors. Endocrinology 2013, 154, 2446–2456.
- Bono, F.; Lamarche, I.; Bornia, J.; Savi, P.; Della Valle, G.; Herbert, J.M. Nerve growth factor (NGF) exerts its pro-apoptotic effect via the P75(NTR) receptor in a cell cycle-dependent manner. FEBS Lett. 1999, 457, 93–97.
- Molloy, N.H.; Read, D.E.; Gorman, A.M. Nerve growth factor in cancer cell death and survival. Cancers 2011, 3, 510–530.
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009, 20, 133–145.
- Lykissas, M.G.; Batistatou, A.K.; Charalabopoulos, K.A.; Beris, A.E. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 2007, 4, 143–151.
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465.
- Shooter, E.M. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 2001, 24, 601–629.
- Schramm, A.; Schulte, J.H.; Astrahantseff, K.; Apostolov, O.; Van Limpt, V.; Sieverts, H.; Kuhfittig-Kulle, S.; Pfeiffer, P.; Versteeg, R.; Eggert, A. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005, 228, 143–153.
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642.
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27.
- Baldassarro, V.A.; Cescatti, M.; Rocco, M.L.; Aloe, L.; Lorenzini, L.; Giardino, L.; Calzà, L. Nerve growth factor promotes differentiation and protects the oligodendrocyte precursor cells from in vitro hypoxia/ischemia. Front. Neurosci. 2023, 17, 1111170.
- Ferraguti, G.; Terracina, S.; Micangeli, G.; Lucarelli, M.; Tarani, L.; Ceccanti, M.; Spaziani, M.; D’Orazi, V.; Petrella, C.; Fiore, M. NGF and BDNF in pediatrics syndromes. Neurosci. Biobehav. Rev. 2023, 145, 105015.
- Fahnestock, M.; Yu, G.; Coughlin, M.D. ProNGF: A neurotrophic or an apoptotic molecule? Prog. Brain Res. 2004, 146, 101–110.
- Mohamed, R.; Coucha, M.; Elshaer, S.L.; Artham, S.; Lemtalsi, T.; El-Remessy, A.B. Inducible overexpression of endothelial proNGF as a mouse model to study microvascular dysfunction. Biochim. Biophys. Acta—Mol. Basis Dis. 2018, 1864, 746–757.
- Song, L.; Xu, L.F.; Pu, Z.X.; Wang, H.H. HHIL-10 inhibits apoptosis in brain tissue around the hematoma after ICH by inhibiting proNGF. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3005–3011.
- Minnone, G.; Soligo, M.; Caiello, I.; Prencipe, G.; Manni, L.; Marafon, D.P.; Magni-Manzoni, S.; Manzo, A.; De Benedetti, F.; Gracci-Laudiero, L. ProNGF-p75NTR axis plays a proinflammatory role in inflamed joints: A novel pathogenic mechanism in chronic arthritis. RMD Open 2017, 3, e000441.
- Barcelona, P.F.; Sitaras, N.; Galan, A.; Esquiva, G.; Jmaeff, S.; Jian, Y.; Sarunic, M.V.; Cuenca, N.; Sapieha, P.; Saragovi, H.U. p75NTR and its ligand ProNGF activate paracrine mechanisms etiological to the vascular, inflammatory, and neurodegenerative pathologies of diabetic retinopathy. J. Neurosci. 2016, 36, 8826–8841.
- Capsoni, S.; Brandi, R.; Arisi, I.; D’Onofrio, M.; Cattaneo, A. A Dual Mechanism Linking NGF/proNGF Imbalance and Early Inflammation to Alzheimer’s Disease Neurodegeneration in the AD11 Anti-NGF Mouse Model. CNS Neurol. Disord—Drug Targets 2012, 10, 635–647.
- Descamps, S.; Toillon, R.A.; Adriaenssens, E.; Pawlowski, V.; Cool, S.M.; Nurcombe, V.; Le Bourhis, X.; Boilly, B.; Peyrat, J.P.; Hondermarck, H. Nerve Growth Factor Stimulates Proliferation and Survival of Human Breast Cancer Cells through Two Distinct Signaling Pathways. J. Biol. Chem. 2001, 276, 17864–17870.
- Meco, D.; Di Francesco, A.M.; Melotti, L.; Ruggiero, A.; Riccardi, R. Ectopic nerve growth factor prevents proliferation in glioma cells by senescence induction. J. Cell Physiol. 2019, 234, 6820–6830.
- Chopin, V.; Lagadec, C.; Toillon, R.A.; Le Bourhis, X. Neurotrophin signaling in cancer stem cells. Cell Mol. Life Sci. 2016, 73, 1859–1870.
- Marsland, M.; Dowdell, A.; Jiang, C.C.; Wilmott, J.S.; Scolyer, R.A.; Zhang, X.D.; Hondermarck, H.; Faulkner, S. Expression of NGF/proNGF and Their Receptors TrkA, p75NTR and Sortilin in Melanoma. Int. J. Mol. Sci. 2022, 23, 4260.
- Peach, C.J.; Tonello, R.; Gomez, K.; Calderon-Rivera, A.; Sánchez, M.R.; Maile, L.; Perez-Miller, S.; Manu, A.M.; Hahn, H.; Thomsen, A.R.B.; et al. Neuropilin-1 is a co-receptor for NGF and TrkA-evoked pain. BioRxiv 2023.
- Pond, A.; Roche, F.K.; Letourneau, P.C. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J. Neurobiol. 2002, 51, 43–53.
- Reza, J.N.; Gavazzi, I.; Cohen, J. Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol. Cell Neurosci. 1999, 14, 317–326.
- Claudio Cuello, A.; Pentz, R.; Hall, H. The brain NGF metabolic pathway in health and in Alzheimer’s pathology. Front. Neurosci. 2019, 13, 62.
- Hristova, M.; Aloe, L. Metabolic syndrome—Neurotrophic hypothesis. Med. Hypotheses 2006, 66, 545–549.
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral Pathol. Med. 2020, 49, 227–234.
- Smith, M.A. Hippocampal vulnerability to stress and aging: Possible role of neurotrophic factors. Behav. Brain Res. 1996, 78, 25–36.
- Miller, R.; King, M.A.; Heaton, M.B.; Walker, D.W. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002, 950, 137–147.
- Lin, K.; Huang, J.; Luo, H.; Luo, C.; Zhu, X.; Bu, F.; Xiao, H.; Xiao, L.; Zhu, Z. Development of a prognostic index and screening of potential biomarkers based on immunogenomic landscape analysis of colorectal cancer. Aging 2020, 12, 5832–5857.
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Vitali, M.; Ceccanti, M.; Chaldakov, G.N.; et al. Nerve Growth Factor, Stress and Diseases. Curr. Med. Chem. 2020, 28, 2943–2959.
- D’Angelo, A.; Ceccanti, M.; Petrella, C.; Greco, A.; Tirassa, P.; Rosso, P.; Ralli, M.; Ferraguti, G.; Fiore, M.; Messina, M.P. Role of neurotrophins in pregnancy, delivery and postpartum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 32–41.
- Fiore, M.; Korf, J.; Antonelli, A.; Talamini, L.; Aloe, L. Long-lasting effects of prenatal MAM treatment on water maze performance in rats: Associations with altered brain development and neurotrophin levels. Neurotoxicol. Teratol. 2002, 24, 179–191.
- Ciafrè, S.; Ferraguti, G.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Greco, A.; Chaldakov, G.N.; Rosso, P.; Fico, E.; Messina, M.P.; et al. Nerve growth factor in the psychiatric brain. Riv. Psichiatr. 2020, 55, 4–15.
- Terracina, S.; Ferraguti, G.; Tarani, L.; Fanfarillo, F.; Tirassa, P.; Ralli, M.; Iannella, G.; Polimeni, A.; Lucarelli, M.; Greco, A.; et al. Nerve Growth Factor and Autoimmune Diseases. Curr. Issues Mol. Biol. 2023, 45, 8950–8973.
- Lebedev, T.; Vagapova, E.; Spirin, P.; Rubtsov, P.; Astashkova, O.; Mikheeva, A.; Sorokin, M.; Vladimirova, U.; Suntsova, M.; Konovalov, D.; et al. Growth factor signaling predicts therapy resistance mechanisms and defines neuroblastoma subtypes. Oncogene 2021, 40, 6258–6272.
- Faulkner, S.; Griffin, N.; Rowe, C.W.; Jobling, P.; Lombard, J.M.; Oliveira, S.M.; Walker, M.M.; Hondermarck, H. Nerve growth factor and its receptor tyrosine kinase TrkA are overexpressed in cervical squamous cell carcinoma. FASEB BioAdv. 2020, 2, 398–408.
- Aloe, L.; Simone, M.D.; Properzi, F. Nerve growth factor: A neurotrophin with activity on cells of the immune system. Microsc. Res. Tech. 1999, 45, 285–291.
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970.
- Oronsky, B.; Caroen, S.; Reid, T. What Exactly Is Inflammation (and What Is It Not?). Int. J. Mol. Sci. 2022, 23, 14905.
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151.
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127.
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299.
- Tracy, R.P. The five cardinal signs of inflammation: Calor, dolor, rubor, tumor... and penuria (apologies to Aulus Cornelius Celsus, de medicina, c. A.D. 25). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1051–1052.
- Dandri, M.; Bertoletti, A.; Lütgehetmann, M. Innate immunity in hepatitis B and D virus infection: Consequences for viral persistence, inflammation, and T cell recognition. Semin. Immunopathol. 2021, 43, 535–548.
- Cavaillon, J.M. Once upon a time, inflammation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200147.
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 1–15.
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020, 19, 45–60.
- Muratori, L.; Lohse, A.W.; Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ 2023, 380, e070201.
- Sun, B.; Karin, M. Inflammation and liver tumorigenesis. Front. Med. 2013, 7, 242–254.
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668.
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126.
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553.
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011, 3, 39–48.
- Curtis, J.R.; Olivieri, J.; Allison, J.J.; Gaffo, A.; Juarez, L.; Kovac, S.H.; Person, S.; Saag, K.G. A group randomized trial to improve safe use of nonsteroidal anti-inflammatory drugs. Am. J. Manag. Care 2005, 11, 537–543.
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016, 12, e1005973.
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve growth factor: Role in growth, differentiation and controlling cancer cell development. J. Exp. Clin. Cancer Res. 2016, 35, 1–7.
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A Nerve Growth-Stimulating Factor Isolated from Sarcom As 37 and 180. Proc. Natl. Acad. Sci. USA 1954, 40, 1014–1018.
- Zeliadt, N. Rita Levi-Montalcini: NGF, the prototypical growth factor. Proc. Natl. Acad. Sci. USA 2013, 110, 4873–4876.
- Goda, M.; Atagi, S.; Amitani, K.; Hobara, N.; Kitamura, Y.; Kawasaki, H. Nerve growth factor suppresses prostate tumor growth. J. Pharmacol. Sci. 2010, 112, 463–466.
- Krygier, S.; Djakiew, D. Neurotrophin receptor p75NTR suppresses growth and nerve growth factor-mediated metastasis of human prostate cancer cells. Int. J. Cancer 2002, 98, 1–7.
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Colleen Karlo, J.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009, 4, e8283.
- Lin, H.; Huang, H.; Yu, Y.; Chen, W.; Zhang, S.; Zhang, Y. Nerve growth factor regulates liver cancer cell polarity and motility. Mol. Med. Rep. 2021, 23, 288.
- Tarani, L.; Ceci, F.M.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Minni, A.; Spaziani, M.; Isidori, A.M.; et al. Neuroimmune Dysregulation in Prepubertal and Adolescent Individuals Affected by Klinefelter Syndrome. Endocr. Metab Immune Disord. Drug Targets 2022, 22, 105–114.
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; de Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 854–868.
- Tarani, L.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Messina, M.P.; Rasio, D.; De Luca, E.; Putotto, C.; et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with down Syndrome. J. Immunol. Res. 2020, 2020, 6937154.
- Kritas, S.K.; Saggini, A.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Frydas, S.; Rosati, M.; Tei, M.; Speziali, A.; et al. Neuropeptide NGF mediates neuro-immune response and inflammation through mast cell activation. J. Biol. Regul. Homeost Agents 2014, 28, 177–181.
- Bracci-Laudiero, L.; De Stefano, M.E. NGF in early embryogenesis, differentiation, and pathology in the nervous and immune systems. Curr. Top. Behav. Neurosci. 2016, 29, 125–152.
- Vega, J.A.; García-Suárez, O.; Hannestad, J.; Pérez-Pérez, M.; Germanà, A. Neurotrophins and the immune system. J. Anat. 2003, 203, 1–19.
- Seidel, M.F.; Hügle, T.; Morlion, B.; Koltzenburg, M.; Chapman, V.; Maassen Van Den Brink, A.; Lane, N.E.; Perrot, S.; Zieglgänsberger, W. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp. Neurol. 2022, 356, 114108.
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2023, 22, 6–14.
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402.
- Rizzi, C.; Tiberi, A.; Giustizieri, M.; Marrone, M.C.; Gobbo, F.; Carucci, N.M.; Meli, G.; Arisi, I.; D’Onofrio, M.; Marinelli, S.; et al. NGF steers microglia toward a neuroprotective phenotype. Glia 2018, 66, 1395–1416.
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983.
- Saloman, J.L.; Singhi, A.D.; Hartman, D.J.; Normolle, D.P.; Albers, K.M.; Davis, B.M. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 856–863.
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, N.G.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197.
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281.
- Tafreshi, A.P. Nerve growth factor prevents demyelination, cell death and progression of the disease in experimental allergic encephalomyelitis. Iran. J. Allergy Asthma Immunol. 2006, 5, 177–181.
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175.
- Castrén, E.; Tanila, H. Neurotrophins and Dementia-Keeping in Touch. Neuron 2006, 51, 1–3.
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348.
- Chao, M.Y.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173.
- Mierke, C.T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep. Prog. Phys. 2014, 77, 076602.
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10.
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015, 25, 214–220.
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616.
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905.
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925.
- Li, X.; Zhao, S.; Bian, X.; Zhang, L.; Lu, L.; Pei, S.; Dong, L.; Shi, W.; Huang, L.; Zhang, X.; et al. Signatures of EMT, immunosuppression, and inflammation in primary and recurrent human cutaneous squamous cell carcinoma at single-cell resolution. Theranostics 2022, 12, 7532–7549.
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681.
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11.
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996, 19, 514–520.
- Romon, R.; Adriaenssens, E.; Lagadec, C.; Germain, E.; Hondermarck, H.; Le Bourhis, X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol. Cancer 2010, 9, 157.
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cross talk between the cardiovascular and nervous systems: Neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr. Pharm. Des. 2006, 12, 2609–2622.
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 82.
- Lambert, C.; Mathy-Hartert, M.; Dubuc, J.E.; Montell, E.; Vergés, J.; Munaut, C.; Noel, A.; Henrotin, Y. Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis Res. Ther. 2012, 14, R58.
- Zhang, L.; Zhang, W.; Zhang, X.; Yihe, M.I.N.; Zhao, Y.; Wang, B.; Wei, L.; Mao, S.; Min, W. High-glucose microenvironment promotes perineural invasion of pancreatic cancer via activation of hypoxia inducible factor 1α. Oncol. Rep. 2022, 47, 64.
- Han, S.; Wang, D.; Huang, Y.; Zeng, Z.; Xu, P.; Xiong, H.; Ke, Z.; Zhang, Y.; Hu, Y.; Wang, F.; et al. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J. Exp. Clin. Cancer Res. 2022, 41, 348.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912.
- Jung, H.H.; Kim, J.Y.; Cho, E.Y.; Oh, J.M.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; et al. Elevated level of nerve growth factor (Ngf) in serum-derived exosomes predicts poor survival in patients with breast cancer undergoing neoadjuvant chemotherapy. Cancers 2021, 13, 5260.
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8, 601738.
- Sun, L.; Chen, S.; Chen, M. Schwann Cells in the Tumor Microenvironment: Need More Attention. J. Oncol. 2022, 2022, 1–10.
- Tetri, L.H.; Kolla, V.; Golden, R.L.; Iyer, R.; Croucher, J.L.; Choi, J.H.; Macfarland, S.P.; Naparaju, K.; Guan, P.; Nguyen, F.; et al. RET receptor expression and interaction with TRK receptors in neuroblastomas. Oncol. Rep. 2020, 44, 263–272.
- Park, S.Y.; Yang, H.J.; Ye, M.; Liu, X.; Shim, I.; Chang, Y.T.; Bae, H. Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice. J. Neuroinflamm. 2022, 19, 143.
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742.
- Shan, Q.; Takabatake, K.; Kawai, H.; Oo, M.W.; Sukegawa, S.; Fujii, M.; Nakano, K.; Nagatsuka, H. Crosstalk between cancer and different cancer stroma subtypes promotes the infiltration of tumor-associated macrophages into the tumor microenvironment of oral squamous cell carcinoma. Int. J. Oncol. 2022, 60, 78.
- Zhang, Y.; Lin, C.; Liu, Z.; Sun, Y.; Chen, M.; Guo, Y.; Liu, W.; Zhang, C.; Chen, W.; Sun, J.; et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 2022, 34, 1999–2017.e10.
- Urzua, U.; Tapia, V.; Geraldo, M.P.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor stimulates cellular proliferation of human epithelial ovarian cancer. Horm. Metab. Res. 2012, 44, 656–661.
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Wang, X.; Dong, Z.; Chen, F.; Cui, H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8.
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487.
- Wu, Y.; Zhou, B.P. TNF-α/NFκ-B/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644.
- Scalia, P.; Williams, S.J.; Fujita-Yamaguchi, Y.; Giordano, A. Cell cycle control by the insulin-like growth factor signal: At the crossroad between cell growth and mitotic regulation. Cell Cycle 2023, 22, 1–37.
- Tomellini, E.; Touil, Y.; Lagadec, C.; Julien, S.; Ostyn, P.; Ziental-Gelus, N.; Meigna, S.; Lengrand, J.; Adrianseen, E.; Palokowska, R.; et al. Nerve growth factor and prongf simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells 2015, 33, 342–353.
- Di Donato, M.; Cernera, G.; Migliaccio, A.; Castoria, G. Nerve growth factor induces proliferation and aggressiveness in prostate cancer cells. Cancers 2019, 11, 784.
- Marsland, M.; Dowdell, A.; Faulkner, S.; Jobling, P.; Rush, R.A.; Gedye, C.; Lynam, J.; Griffin, C.P.; Baker, M.; Marsland, J.; et al. ProNGF Expression and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2023, 24, 1616.
- Jiang, T.; Wang, G.; Liu, Y.; Feng, L.; Wang, M.; Liu, J.; Chen, Y.; Ouyang, L. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 2021, 11, 355–372.
- Oelmann, E.; Sreter, L.; Schuller, I.; Serve, H.; Koenigsmann, M.; Wiedenmann, B.; Oderberg, D.; Reufi, B.; Thiel, E.; Berdel, W.E. Nerve Growth Factor Stimulates Clonal Growth of Human Lung Cancer Cell Lines and a Human Glioblastoma Cell Line Expressing High-Affinity Nerve Growth Factor Binding Sites Involving Tyrosine Kinase Signaling. Cancer Res. 1995, 55, 2212–2219.
- Singer, H.S.; Hansen, B.; Martinie, D.; Karp, C.L. Mitogenesis in glioblastoma multiforme cell lines: A role for NGF and its TrkA receptors. J. Neurooncol. 1999, 45, 1–8.
- Forsyth, P.A.; Krishna, N.; Lawn, S.; Valadez, J.G.; Qu, X.; Fenstermacher, D.A.; Fournier, M.; Potthast, L.; Chinnaiyan, P.; Gibney, G.T.; et al. P75 Neurotrophin Receptor Cleavage By A- and Γ-Secretases Is Required for Neurotrophin-Mediated Proliferation of Brain Tumor-Initiating Cells. J. Biol. Chem. 2014, 289, 8067–8085.
- Zhang, Z.; Yang, Y.; Gong, A.; Wang, C.; Liang, Y.; Chen, Y. Localization of NGF and TrkA at mitotic apparatus in human glioma cell line U251. Biochem. Biophys. Res. Commun. 2005, 337, 68–74.
- Antonelli, A.; Chiaretti, A.; Piastra, M.; Vigneti, E.; Aloe, L. In vitro human ependymoblastoma cells differentiate after exposure to nerve growth factor. J. Neurocytol. 2004, 33, 503–515.
- Engebraaten, O.; Bjerkvig, R.; Pedersen, P.-H.; Laerum, O.D. Effects of EGF, BFGF, NGF and PDGF(bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies In Vitro. Int. J. Cancer 1993, 53, 209–214.
- Fanfarillo, F.; Ferraguti, G.; Lucarelli, M.; Francati, S.; Barbato, C.; Minni, A.; Ceccanti, M.; Tarani, L.; Petrella, C.; Fiore, M. The Impact of ROS and NGF in the Gliomagenesis and their Emerging Implications in the Glioma Treatment. CNS Neurol. Disord. Drug Targets 2023, 23, 449–462.
- Chang, A.; Botteri, E.; Gillis, R.D.; Löfling, L.; Le, C.P.; Ziegler, A.I.; Chung, N.C.; Rowe, M.C.; Fabb, S.A.; Hartley, B.J.; et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1147.
- Descamp, S.; Pawlowski, V.; Révillion, F.; Hornez, L.; Hebbar, M.; Boilly, B.; Hondermarck, H.; Peyrat, J.P. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001, 61, 4337–4340.
- Jin, M.; Wang, Y.; Zhou, T.; Li, W.; Wen, Q. Norepinephrine/β2-Adrenergic Receptor Pathway Promotes the Cell Proliferation and Nerve Growth Factor Production in Triple-Negative Breast Cancer. J. Breast Cancer 2023, 26, 268–285.
- Tian, Y.; Qiu, X.; Qi, X.; Dong, Z.; Zhao, J.; Huang, J.; Xiang, J. Electroacupuncture promotes apoptosis and inhibits axonogenesis by activating p75 neurotrophin receptor for triple-negative breast xenograft in mice. J. Chem. Neuroanat. 2022, 124, 102133.
- Bruno, F.; Arcuri, D.; Vozzo, F.; Malvaso, A.; Montesanto, A.; Maletta, R. Expression and Signaling Pathways of Nerve Growth Factor (NGF) and Pro-NGF in Breast Cancer: A Systematic Review. Curr. Oncol. 2022, 29, 8103–8120.
- Dollé, L.; El Yazidi-Belkoura, I.; Adriaenssens, E.; Nurcombe, V.; Hondermarck, H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 2003, 22, 5592–5601.
- Lévêque, R.; Corbet, C.; Aubert, L.; Guilbert, M.; Lagadec, C.; Adriaenssens, E.; Duval, J.; Finetti, P.; Birnbaum, D.; Magné, N.; et al. ProNGF increases breast tumor aggressiveness through functional association of TrkA with EphA2. Cancer Lett. 2019, 449, 196–206.
- Mazaki, A.; Yamauchi, K.; Orita, S.; Inage, K.; Suzuki, M.; Fujimoto, K.; Shiga, Y.; Abe, K.; Inoue, M.; Norimoto, M.; et al. Nerve Growth Factor in Breast Cancer Cells Promotes Axonal Growth and Expression of Calcitonin Gene-related Peptide in a Rat Model of Spinal Metastasis. Anticancer Res. 2022, 42, 581–587.
- Gao, X.; Yang, Z.; Xu, C.; Yu, Q.; Wang, M.; Song, J.; Wu, C.; Chen, M. GeneChip expression profiling identified OLFML2A as a potential therapeutic target in TNBC cells. Ann. Transl. Med. 2022, 10, 274.
- Di Donato, M.; Galasso, G.; Giovannelli, P.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Targeting the Nerve Growth Factor Signaling Impairs the Proliferative and Migratory Phenotype of Triple-Negative Breast Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 676568.
- Johansson, M.; Jonsson, M.; Norrgard, O.; Forsgren, S. New aspects concerning ulcerative colitis and colonic carcinoma: Analysis of levels of neuropeptides, neurotrophins, and TNFalpha/TNF receptor in plasma and mucosa in parallel with histological evaluation of the intestine. Inflamm. Bowel. Dis. 2008, 14, 1331–1340.
- De Farias, C.; Stertz, L.; Lima, R.; Kapczinski, F.; Schwartsmann, G.; Roesler, R. Reduced NGF Secretion by HT-29 Human Colon Cancer Cells Treated with a GRPR Antagonist. Protein Pept. Lett. 2009, 16, 650–652.
- Liebl, F.; Demir, I.E.; Rosenberg, R.; Boldis, A.; Yildiz, E.; Kujundzic, K.; Kehl, T.; Dischl, D.; Schuster, T.; Maak, M.; et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res. 2013, 19, 50–61.
- Jin, H.; Pan, Y.; He, L.; Zhai, H.; Li, X.; Zhao, L.; Sun, L.; Liu, J.; Hong, L.; Song, J.; et al. P75 Neurotrophin Receptor Inhibits Invasion and Metastasis of Gastric Cancer. Mol. Cancer Res. 2007, 5, 423–433.
- Du, J.J.; Dou, K.F.; Peng, S.Y.; Qian, B.Z.; Xiao, H.S.; Liu, F.; Wang, W.Z.; Guan, W.X.; Gao, Z.Q.; Liu, Y.B.; et al. Expression of NGF family and their receptors in gastric carcinoma: A cDNA microarray study. World J. Gastroenterol. 2003, 9, 1431–1434.
- Dou, N.; Yang, D.; Yu, S.; Wu, B.; Gao, Y.; Li, Y. SNRPA enhances tumour cell growth in gastric cancer through modulating NGF expression. Cell Prolif. 2018, 51, e12484.
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Taylor, Y.; Macchini, M.; Middelholff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34.
- Noh, S.J.; Kim, K.M.; Jang, K.Y. Individual and co-expression patterns of nerve growth factor and heme oxygenase-1 predict shorter survival of gastric carcinoma patients. Diagn. Pathol. 2017, 12, 48.
- Dudás, J.; Dietl, W.; Romani, A.; Reinold, S.; Glueckert, R.; Schrott-Fischer, A.; Dejaco, D.; Chacko, L.J.; Tuertscher, R.; Schartinger, V.H.; et al. Nerve growth factor (NGF)—Receptor survival axis in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 1771.
- Kolokythas, A.; Cox, D.P.; Dekker, N.; Schmidt, B.L. Nerve Growth Factor and Tyrosine Kinase A Receptor in Oral Squamous Cell Carcinoma: Is There an Association with Perineural Invasion? J. Oral Maxillofac. Surg. 2010, 68, 1290–1295.
- Wang, L.; Sun, M.; Jiang, Y.; Yang, L.; Lei, D.; Lu, C.; Zhao, Y.; Zhang, P.; Yang, Y.; Li, J. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: Expression patterns and effects on in vitro invasive behavior. J. Oral. Maxillofac. Surg. 2006, 64, 636–641.
- Assimakopoulou, M.; Zolota, V.; Chondrogianni, C.; Gatzounis, G.; Varakis, J. p75NTR and TrkC neurotrophin receptors demonstrate a different immunoreactivity profile in comparison to TrkA and TrkB receptors in human normal pituitary gland and adenomas. Neuroendocrinology 2008, 88, 127–134.
- Faulkner, S.; Roselli, S.; Demont, Y.; Pundavela, J.; Choquet, G.; Leissner, P.; Oldmeadow, C.; Attia, J.; Walker, M.M.; Hondermarck, H. ProNGF is a potential diagnostic biomarker for thyroid cancer. Oncotarget 2016, 7, 28488–28497.
- Griffin, N.; Gao, F.; Jobling, P.; Oldmeadow, C.; Wills, V.; Walker, M.M.; Faulkner, S.; Hondermarck, H. The neurotrophic tyrosine kinase receptor 1 (TrkA) is overexpressed in oesophageal squamous cell carcinoma. Pathology 2021, 53, 470–477.
- Ye, Y.; Dang, D.; Zhang, J.; Viet, C.T.; Lam, D.K.; Dolan, J.C.; Gibbs, J.L.; Schmidt, B.L. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Cancer Ther. 2011, 10, 1667–1676.
- Søland, T.M.; Brusevold, I.J.; Koppang, H.S.; Schenck, K.; Bryne, M. Nerve growth factor receptor (p75NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology 2008, 53, 62–72.
- Alzawi, A.; Iftikhar, A.; Shalgm, B.; Jones, S.; Ellis, I.; Islam, M. Receptor, Signal, Nucleus, Action: Signals That Pass through Akt on the Road to Head and Neck Cancer Cell Migration. Cancers 2022, 14, 2606.
- Herbrich, S.M.; Kannan, S.; Nolo, R.M.; Hornbaker, M.; Chandra, J.; Zweidler-McKay, P.A. Characterization of TRKA signaling in acute myeloid leukemia. Oncotarget 2018, 9, 30092–30105.
- Lebedev, T.D.; Vagapova, E.R.; Popenko, V.I.; Leonova, O.G.; Spirin, P.V.; Prassolov, V.S. Two receptors, two isoforms, two cancers: Comprehensive analysis of kit and trka expression in neuroblastoma and acute myeloid leukemia. Front. Oncol. 2019, 9, 1046.
- Hillis, J.; O’Dwyer, M.; Gorman, A.M. Neurotrophins and B-cell malignancies. Cell Mol. Life Sci. 2016, 73, 41–56.
- Franzese, O.; Di Francesco, A.M.; Meco, D.; Graziani, G.; Cusano, G.; Levati, L.; Riccardi, R.; Ruggiero, A. hTERT transduction extends the lifespan of primary pediatric low-grade glioma cells while preserving the biological response to NGF. Pathol. Oncol. Res. 2021, 27, 612375.
- Damm, R.; Pech, M.; Cavalli, P.; Haag, F.; Gylstorff, S.; Omari, J.; Thormann, M.; Seidensticker, R.; Ricke, J.; Seidensticker, M.; et al. Correlation of chemokines and growth factors with radiation-induced liver injury after interstitial high dose rate (HDR) brachytherapy of liver metastases. J. Cancer Res. Clin. Oncol. 2022, 148, 2815–2826.
- Kishibe, K.; Yamada, Y.; Ogawa, K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology 2002, 122, 1978–1986.
- Koizumi, H.; Morita, M.; Mikami, S.; Shibayama, E.; Uchikoshi, T. Immunohistochemical analysis of TrkA neurotrophin receptor expression in human non-neuronal carcinomas. Pathol. Int. 1998, 48, 93–101.
- Trim, N.; Morgan, S.; Evans, M.; Issa, R.; Fine, D.; Afford, S.; Winkins, B.; Iredale, J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am. J. Pathol. 2000, 156, 1235–1243.
- Rasi, G.; Serafino, A.; Bellis, L.; Lonardo, M.T.; Andreola, F.; Zonfrillo, M.; Vennarecci, G.; Pierimarchi, P.; Sinibaldi Vallebona, P.; Ettorre, G.M.; et al. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 4986–4995.
- Malaguarnera, G.; Giordano, M.; Paladina, I.; Berretta, M.; Cappellani, A.; Malaguarnera, M. Serum markers of hepatocellular carcinoma. Dig. Dis. Sci. 2010, 55, 2744–2755.
- Luo, T.; Zhang, S.G.; Zhu, L.F.; Zhang, F.X.; Li, W.; Zhao, K.; Wen, X.X.; Yu, M.; Zhan, Y.K.; Chen, H.; et al. A selective c-Met and Trks inhibitor Indo5 suppresses hepatocellular carcinoma growth. J. Exp. Clin. Cancer Res. 2019, 38, 130.
- Natri, H.M.; Wilson, M.A.; Buetow, K.H. Distinct molecular etiologies of male and female hepatocellular carcinoma. BMC Cancer 2019, 19, 951.
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93.
- Thompson, S.M.; Jondal, D.E.; Butters, K.A.; Knudsen, B.E.; Anderson, J.L.; Roberts, L.R.; Callstrom, M.R.; Woodrum, D.A. Heat stress and thermal ablation induce local expression of nerve growth factor inducible (VGF) in hepatocytes and hepatocellular carcinoma: Preclinical and clinical studies. Gene Expr. 2018, 19, 37–47.
- Tokusashi, Y.; Asai, K.; Tamakawa, S.; Yamamoto, M.; Yoshie, M.; Yaginuma, Y.; Miyokawa, N.; Aoki, T.; Kino, S.; Kasai, S.; et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int. J. Cancer 2005, 114, 39–45.
- Owusu Sekyere, S.; Port, K.; Deterding, K.; Cornberg, M.; Wedemeyer, H. Inflammatory patterns in plasma associate with hepatocellular carcinoma development in cured hepatitis C cirrhotic patients. United Eur. Gastroenterol. J. 2021, 9, 486–496.
- Ye, L.; Wu, Y.; Zhou, J.; Xie, M.; Zhang, Z.; Su, C. Influence of Exosomes on Astrocytes in the Pre-Metastatic Niche of Lung Cancer Brain Metastases. Biol. Proced. Online 2023, 25, 5.
- Ricci, A.; Salvucci, C.; Castelli, S.; Carraturo, A.; de Vitis, C.; D’Ascanio, M. Adenocarcinomas of the Lung and Neurotrophin System: A Review. Biomedicines 2022, 10, 2531.
- Gao, F.; Griffin, N.; Faulkner, S.; Rowe, C.W.; Williams, L.; Roselli, S.; Thorne, R.F.; Ferdoshi, A.; Jobling, P.; Walker, M.M.; et al. The neurotrophic tyrosine kinase receptor TrkA and its ligand NGF are increased in squamous cell carcinomas of the lung. Sci. Rep. 2018, 8, 8135.
- Vaishnavi, A.; Capelletti, M.; Le, A.T.; Kako, S.; Butaney, M.; Ercan, D.; Mahale, S.; Davies, K.D.; Aisner, D.L.; Pilling, A.B.; et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 2013, 19, 1469–1472.
- Jiang, Q.; Li, M.; Li, H.; Chen, L. Entrectinib, a new multi-target inhibitor for cancer therapy. Biomed. Pharmacother. 2022, 150, 112974.
- Ricci, A.; Greco, S.; Mariotta, S.; Felici, L.; Bronzetti, E.; Cavazzana, A.; Cardillo, G.; Amenta, F.; Bisetti, A.; Barbolini, G. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respir. Cell Mol. Biol. 2001, 25, 439–446.
- Retamales-Ortega, R.; Oróstica, L.; Vera, C.; Cuevas, P.; Hernández, A.; Hurtado, I.; Vega, M.; Romero, C. Role of nerve growth factor (NGF) and miRNAs in epithelial ovarian cancer. Int. J. Mol. Sci. 2017, 18, 507.
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175.
- Vera, D.B.; Fredes, A.N.; Garrido, M.P.; Romero, C. Role of mitochondria in interplay between ngf/trka, mir-145 and possible therapeutic strategies for epithelial ovarian cancer. Life 2022, 12, 8.
- Garrido, M.P.; Salvatierra, R.; Valenzuela-Valderrama, M.; Vallejos, C.; Bruneau, N.; Hernández, A.; Vega, M.; Selman, A.; Quest, A.F.G.; Romero, C. Metformin reduces ngf-induced tumour promoter effects in epithelial ovarian cancer cells. Pharmaceuticals 2020, 13, 315.
- Garrido, M.P.; Torres, I.; Avila, A.; Chnaiderman, J.; Valenzuela-Valderrama, M.; Aramburo, J.; Orostica, L.; Duran-Jara, E.; Lobos-Gonzalez, L.; Romero, C. Ngf/trka decrease mir-145-5p levels in epithelial ovarian cancer cells. Int. J. Mol. Sci. 2020, 21, 7657.
- Wijaya, L.K.; Stumbles, P.A.; Drummond, P.D. A positive feedback loop between alpha1-adrenoceptors and inflammatory cytokines in keratinocytes. Exp. Cell Res. 2020, 391, 112008.
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (trkA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23.
- Yu, X.; Liu, Z.; Hou, R.; Nie, Y.; Chen, R. Nerve growth factor and its receptors on onset and diagnosis of ovarian cancer. Oncol. Lett. 2017, 14, 2864–2868.
- Brodeur, G.M.; Minturn, J.E.; Ho, R.; Simpson, A.M.; Iyer, R.; Varela, C.R.; Light, J.E.; Kolla, V.; Evans, A.E. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 2009, 15, 3244–3250.
- Hoehner, J.C.; Olsen, L.; Sandstedt, B.; Kaplan, D.R.; Pahlman, S. Association of neurotrophin receptor expression and differentiation in human neuroblastoma. Am. J. Pathol. 1995, 147, 102–113.
- Nakagawara, A.; Arima, M.; Azar, C.G.; Scavarda, N.J.; Brodeur, G.M. Inverse Relationship between trk Expression and N-myc Amplification in Human Neuroblastomas. Cancer Res. 1992, 52, 1364–1368.
- Dzieran, J.; Garcia, A.R.; Westermark, U.K.; Henley, A.B.; Sánchez, E.E.; Träger, C.; Johansson, H.J.; Lehtiö, J.; Arsenian-Henriksson, M. MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E1229–E1238.
- Eggert, A.; Ikegaki, N.; Liu, X.G.; Brodeur, G.M. Prognostic and biological role of neurotrophin- receptor TrkA and TrkB in neuroblastoma. Klin. Padiatr. 2000, 212, 200–205.
- Ho, R.; Minturn, J.E.; Simpson, A.M.; Iyer, R.; Light, J.E.; Evans, A.E.; Brodeur, G.M. The effect of P75 on Trk receptors in neuroblastomas. Cancer Lett. 2011, 305, 76–85.
- Cai, S.; Chen, Q.; Xu, Y.; Zhuang, Q.; Ji, S. Atorvastatin inhibits pancreatic cancer cells proliferation and invasion likely by suppressing neurotrophin receptor signaling. Transl. Cancer Res. 2020, 9, 1439–1447.
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking nerve growth factor signaling reduces the neural invasion potential of pancreatic cancer cells. PLoS ONE 2016, 11, e0165586.
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130.
- Xu, J.B.; Yang, X.C.; Guo, J.H.; Chen, J.Y.; Shang, Y.; Liu, W.J.; Wang, T.-M.; Zou, L. Serum nerve growth factor level indicates therapeutic efficacy of 125I seed implantation in advanced pancreatic adenocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3385–3390.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
575
Revisions:
2 times
(View History)
Update Date:
31 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




