Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sergio Terracina and Version 2 by Sirius Huang.
Nerve growth factor (NGF) plays a dual role both in inflammatory states and cancer, acting both as a pro-inflammatory and oncogenic factor and as an anti-inflammatory and pro-apoptotic mediator in a context-dependent way based on the signaling networks and its interaction with diverse cellular components within the microenvironment.
- apoptosis
- cancer stem cells
- metastasis
- epigenetic
- inflammation
- microenvironment
- neurotrophins
- NGF
- tumor
1. Introduction
Inflammatory processes play a multifaceted role in tumor development and progression [1][2][3][4][5][6][7][1,2,3,4,5,6,7]. While the immune system’s inflammatory response is typically a defense mechanism against infections and tissue damage, chronic or persistent inflammation can contribute to the initiation, growth, and spread of certain types of tumors. Thus, the understanding of the relationship between inflammation and cancer could better focus on anti-inflammatory therapies and drugs that target inflammatory pathways as potential strategies for cancer prevention and treatment [8]. In recent years, a growing body of evidence has emphasized the involvement of neurotrophins (NTs) in the complex landscape of both inflammation and cancer biology, showing their significant role in determining tumor cell growth and survival, particularly in certain types of cancers expressing NT receptors on their cell surfaces [9][10][9,10]. One of the most well-studied NTs is the nerve growth factor (NGF), which binds to its specific receptor, tropomyosin-related kinase A (TrkA), expressed on various types of cancer cells, including those derived from the brain, prostate, breast, and pancreas, among others [11][12][13][11,12,13]. NGF contributes to inflammation by acting as a signaling molecule that stimulates immune cells to release cytokines and enhances the sensitivity of sensory nerves, contributing to the perception of pain and hypersensitivity in inflamed tissues [14]. NTs promote tumor growth by stimulating cancer cell proliferation and suppressing apoptosis, which physiologically helps eliminate damaged or unwanted cells from the body [15]. Furthermore, NTs can stimulate the production of pro-angiogenic factors, modulate the tumor microenvironment (TME), and induce the epithelial–mesenchymal transition (EMT), leading to increased cell mobility and invasiveness [16].
Overall, the activation of NGF signaling pathways in cancer cells can contribute to aggressive tumor growth, metastasis, and therapy resistance. Originally identified for its pivotal role in neuronal development and function, NGF has emerged as a critical player in the growth and survival of various tumor types. The intriguing aspect of NGF’s contribution to cancer lies in its dual role, acting as both an oncogenic factor, fueling tumor cell growth, and as a pro-apoptotic mediator, promoting tumor cell death under certain circumstances [17][18][19][17,18,19]. The context-dependent actions of NGF in cancer underscore the complexity of the signaling networks in which it operates and its interaction with diverse cellular components within the TME. Overall, NGF’s involvement in both inflammation and tumors further highlights its complex role in regulating physiological processes and disease states. Understanding the intricate interactions of NGF with various cell types and pathways is crucial for developing targeted therapies that can modulate its effects in different disease contexts, offering a potential therapeutic strategy to inhibit tumor progression and improve patient outcomes. However, further research is needed to fully understand the complexities of NGF signaling in different cancer types and to develop effective and safe targeted therapies.
2. NGF and Inflammation and Tumor Growth
2.1. Nerve Growth Factor and Neurotrophins
NGF is a crucial neurotrophic factor responsible for the growth, survival, and maintenance of neuronal and non-neuronal cells [20][21][20,21]. Discovered in the 1950s, it was one of the first NTs identified and extensively studied for its role in the development and function of the nervous system [22][23][22,23]. NGF belongs to the family of NTs, which includes other proteins like Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), and Neurotrophin-4/5 (NT-4/5) [24][25][24,25]. NGF is synthesized as a precursor, called pro-NGF, which after processing generates the mature NGF molecule. NGF is primarily produced by various cell types, including immune cells, endothelial cells, and tissues within the nervous system [14][26][27][28][14,26,27,28]. Pro-NGF has a physiological role that goes beyond that of a simple precursor, especially in the nervous system, where it may possess pro-apoptotic activity [29]. Indeed, pro-NGF regulates apoptosis and inflammation and is associated with several neurodegenerative diseases, myocardial infarction, and diabetes [30][31][32][33][34][30,31,32,33,34]. The actions of NGF are mediated through its interaction with specific receptors. There are two primary receptors associated with NGF:- (1)
-
TrkA is the high-affinity receptor for NGF; its activation triggers a cascade of intracellular signaling events, including the MAPK/ERK pathway and the PI3K/Akt pathway [35][36][35,36]. These pathways play essential roles in cell growth, differentiation, and survival. TrkA is expressed on the surface of neurons and other cell types, enabling NGF to exert its neurotrophic effects;
- (2)
-
p75 neurotrophin receptor (p75NTR) has a lower affinity for NGF but plays a modulatory role in NGF signaling [20][37][20,37]. It can interact with TrkA and enhance its binding affinity for NGF, influencing the cellular responses elicited by NGF. p75NTR is mostly involved in processes like cell death, survival decisions, and axonal growth.
2.2. Inflammation
Inflammation is a natural response by the body’s immune system to protect against harmful stimuli like pathogens, irritants, or damaged cells. It is a complex biological process that aims to eliminate the initial cause of cell injury, clear out damaged cells, and initiate tissue repair [56]. Actually, in medicine, inflammation, a term coined by the Romans, lacks a precise, universally accepted definition, varying in interpretation based on context and individual perspectives [57]. It often carries a negative connotation as an uncontrolled reaction likened to a destructive wildfire, requiring immediate containment. However, overshadowed in this view is the fundamental role that inflammation plays in both maintaining health and ensuring survival. Inflammation involves a complex interplay of cells, chemicals, and molecular signals including blood vessel dilatation (causing redness and heat) and increased permeability, allowing immune cells and fluids to move from the bloodstream into the tissues (leading to swelling); the cellular release of chemicals such as histamine, cytokines, and prostaglandins, which help to trigger the immune response and promote healing; and finally the activation of immune cells which migrate to the affected area to destroy pathogens or damaged cells [58]. Cytokines play a pivotal role in orchestrating or modulating inflammation, confirming not only the presence and magnitude of inflammation but also guiding treatment decisions. Inflammation can be distinguished as acute and chronic. Acute inflammation is the body’s immediate and short-term response to an injury or infection [59]. It is characterized by symptoms like redness, swelling, heat, pain, and sometimes loss of function [60][61][60,61]. Chronic inflammation, on the other hand, is long-term and can last for weeks, months, or even years [62]. It occurs when the immune system’s response persists, often due to underlying health conditions, such as autoimmune disorders, ongoing infections, obesity, or prolonged exposure to irritants like smoke. Lifestyle factors like stress, poor diet, lack of exercise, and environmental toxins can contribute to chronic inflammation [63]. While inflammation is a vital part of the body’s defense mechanism, chronic inflammation can be a problematic event leading to tissue damage and various diseases including diabetes, cancer, cardiovascular diseases, eye disorders, arthritis, obesity, autoimmune diseases, and inflammatory bowel disease [64]. Interestingly, inflammation is at the base of various conditions and a plethora of etiopathogenetic events; for example, hepatitis (liver inflammation) has been associated with viral infections, excessive alcohol consumption, certain medications, or autoimmune responses [62][65][66][67][68][62,65,66,67,68]. Long-term inflammation of the liver can lead to cirrhosis and liver cancer [69][70][69,70]. Inflammatory states and diseases cover a wide range of conditions affecting various parts of the body, varying in severity and requiring different treatments, including medications, lifestyle changes, and sometimes, in more severe cases, surgical interventions or specialized therapies. The widespread use of anti-inflammatory medications assumed to counteract all inflammatory responses potentially may hinder the body’s ability to fully recover [71][72][71,72]. Indeed, not all situations warrant an inflammatory response (such as blunt trauma and exposure to toxins), but since inflammation affects both unhealthy and healthy tissues without discrimination, it should be treated when it has the potential to persist or spread uncontrollably, causing prolonged damage. Effective management often entangles a multidisciplinary approach that requires the support of specialized healthcare professionals in this specific condition. Actually, it has been suggested that an effective way to guide therapy for inflammation is to assess a combination of markers associated with inflammation and fibrosis, such as C-reactive protein, ferritin, serum amyloid A (SAA), pro-calcitonin, and transforming growth factor-β (TGF-β, a significant contributor to fibrosis), alongside cytokine profiling [73].2.3. Role of NGF in Carcinogenesis
NGF plays a significant role in various aspects of human health, including its involvement in tumors. Overall, evidence indicates that NGF is unable to generate cell carcinogenesis alone, both in normal neuronal and non-neuronal cells/tissues; however, it could be a major determinant in the case of co-expression with pro-carcinogenic molecules [74]. Quite intriguingly, NGF was initially discovered by R. Levi-Montalcini nearly 60 years ago in the context of a transplantation experiment involving a malignant mouse sarcoma [75][76][75,76].NGF as a Tumor Growth Facilitator or Suppressor
Depending on the tumor’s origin, pro-survival signaling can be facilitated through TrkA and/or p75NTR receptors [77]. In breast cancer, NGF plays a crucial role in stimulating proliferative signaling via TrkA and pro-survival signaling through p75NTR [35]. Furthermore, the activation of p75NTR in breast cancer promotes increased resistance to cell death induced by chemotherapeutic treatments. On the other hand, the role of p75NTR in prostate cells is distinct since p75NTR mediates cell death and acts as a tumor suppressor in the case of normal prostate cells [78]. In prostate cancer, the expression of p75NTR is lost, contributing to tumor progression, death evasion, uncontrolled proliferation, and metastasis to distant sites [79]. Interestingly, other mechanisms were found in recent studies. For example, NGF plays a significant role in liver cancer progression and metastasis, exerting wide influences on liver cancer cell polarity and motility by regulating signaling pathways involved in cell movement, cytoskeletal organization, and cellular polarity [80]. Heightened NGF disrupts cell polarity, boosts cell movement, triggers changes related to cell transition, rearranges the cell’s structural framework, and protects cells from apoptosis and detachment-induced cell death [80]. Table 1 reports the main role of NGF and its receptors in various cancers.While the primary role of NGF is related to the development and function of nerve cells, it also plays a main role in inflammation. Inflammation, as stated before, is a complex biological response triggered by the body’s immune system to protect against harmful stimuli, such as tissue damage and pathogens. In this scenario, NGF can regulate the innervation and neuronal activity of peripheral neurons, inducing the release of immune-active cytokines, neuropeptides, and neurotransmitters [14][187][188][189][14,187,188,189]. Furthermore, NGF can also directly influence innate and adaptive immune responses through its interaction with various cells involved in the immune response, including mast cells, lymphocytes, and macrophages [28][190][28,190]. Actually, NGF has a variety of effects that can be either pro-inflammatory or anti-inflammatory depending on the expression of its receptors, which are dynamically regulated in immune cells depending on their state of differentiation and functional activity [191][192][191,192]. The seeming ambiguity is mainly due to the role of NGF as an endogenous molecule capable of triggering immune responses while also initiating pathways that control inflammation and prevent excessive tissue damage so that altered expression of its receptors could hinder NGF’s ability to engage the regulatory feedback processes for finally sustaining the perpetuation of inflammation in conditions such as chronic inflammatory diseases or autoimmune disorders [14]. Additionally, as NGF can contribute to the sensitivity and pain associated with the neurogenic inflammation of tissues, a potential role of NTs has been suggested as novel treatment strategies in chronic inflammatory diseases [193][194][193,194]. More specifically, NGF has intricate connections with neuroinflammation, which involves complex interactions between immune cells, glial cells, and various signaling molecules. Microglia act as the resident immune cells of the central nervous system (CNS) and can become activated in response to injury or inflammation [195]. NGF can modulate the activation and function of glial cells, particularly microglia and astrocytes, influencing their release of inflammatory mediators [196]. It can both promote and dampen the release of various cytokines depending on the context, contributing to the fine-tuning of the inflammatory response in the CNS [157][197][198][157,197,198] (see Figure 1).Table 1. Detailed overview of the role of NGF and its receptors in various types of tumors. While NGF’s primary function is related to neural development and function, its relationship with tumors is complex and multifaceted. The involvement of NGF in tumors is not as straightforward as in normal nerve growth, and its effects on different types of tumors can vary. Acetylcholine, Ach; A disintegrin and metalloprotease 17, ADAM17; protein kinase B, Akt; extracellular signal-regulated kinase, ERK; F-box-only protein 22, FBOXO22; hypoxia-inducible factor 1 subunit alpha, HIF1α; nerve growth factor, NGF; non-small-cell lung cancer, NSCLC; neurotrophic tyrosine receptor kinase, NTRK; nuclear factor kB, NF-kB; p75 pan-neurotrophin receptor, p75NTR; programmed death-ligand 1, PD-L1; rearranged during transfection, RET; small nuclear ribonucleoprotein polypeptide A, SNRPA; tissue inhibitor of metalloproteinases, TIMP; tropomyosin receptor kinase, Trk; vascular endothelial growth factor, VEGF. (*) MYCN is amplified in 20% of neuroblastomas and correlates with aggressive phenotype and poor prognosis. (**) Perineural invasion driven by the TME has been identified as a key pattern of several malignancies including breast, pancreatic, and prostate cancers.Kind of Tumor Role of NGF References Brain tumors -
Pro-NGF, NGF, and TrkA are expressed in numerous brain tumor cells (especially glioblastoma) and can promote cell survival and growth.
-
p75NTR proteolysis is required for brain tumor proliferation.
-
NGF concentrated in centrosome can phosphorylate TrkA, which may in return phosphorylate tubulin and promote mitotic spindle assembly, causing mitogenic effects in glioma cells.
-
In ependymoblastoma tumors, NGF exerts a marked action on differentiation rather than proliferation in vitro.
-
The potential of pro-NGF, NGF, and its receptors as clinical biomarkers and therapeutic targets has been highlighted.
[81][82][83][84][85][,8286,83],84,85[,8687][88][89][81,87,88,89] Breast Cancer -
NGF is both synthesized and released by breast cancer cells.
-
NGF exerts mitogenic, antiapoptotic, and angiogenic influences on breast cancer cells by engaging distinct signaling pathways that encompass the participation of TrkA and NGFR/p75NTR receptors.
-
Pro-NGF signaling has been linked to breast cancer invasion and metastasis.
-
NGF and its receptors have been identified as diagnostic and prognostic tools.
-
NGF and its receptors are promising therapeutic targets for breast cancer.
-
Norepinephrine/β2-Adrenergic Receptor pathway promotes cell proliferation and NGF production in triple-negative breast cancer.
-
Neurotoxin inhibition of sympathetic neural signaling in mammary tumors using 6-hydroxydopamine or genetic deletion of NGF or β2-adrenoceptor in triple-negative breast cancer tumor cells enhances the therapeutic effect of anthracycline chemotherapy by reducing metastasis in xenograft mouse models.
-
Electroacupuncture promotes apoptosis and inhibits axonogenesis by activating the p75NTR receptor for triple-negative breast xenograft in mice.
-
In rat models, it has been found that NGF from breast cancer may mediate spinal bone pain from metastasis via axonal growth and up-regulation of pain-associated neuropeptides.
[26][90][91][92][93][94][95][96][97][98][99][26,90,91,92,93,94,95,96,97,98,99] Colorectal Cancer -
NGF can stimulate the growth and survival of colon cancer cells and influence their invasive behavior.
-
NGF has been identified as a potential therapeutic target for the treatment of colon cancer.
-
NGF receptors may play a key role in androgen’s effect on hormone-sensitive tumor cells.
-
NGF has been included in new immunogenomic prognostic risk scores for colorectal cancer.
[17][47][100]][17,47[,100101],101[102],102[103,103] Gastric Cancer -
In gastric cancer, NGF and Trk receptor mRNA expression are down-regulated.
-
NGF Trk receptors may elicit cell apoptosis by a Ras or Raf signal transduction pathway.
-
SNRPA (small nuclear ribonucleoprotein polypeptide A) enhances tumor cell growth in gastric cancer through modulating NGF expression.
-
ACh-NGF positive feedback loop may be the basis for the abnormal innervation observed in the TME and acts through the Trk receptors.
-
p75NTR inhibits the invasive and metastatic abilities of gastric cancer cells by down-regulating uPA and matrix metalloproteinase 9 proteins and up-regulating TIMP1 protein via the NF-kB signal transduction pathway.
-
p75NTR may be used as a new potential therapeutic target in metastatic gastric cancer.
-
Individual and co-expression patterns of NGF and heme oxygenase-1 may predict shorter survival of gastric carcinoma patients.
[104][105][106][107][108][104,105,106,107,108] Head and Neck Cancer -
NGF can stimulate morphological differentiation, adhesion, proliferation, and migration in head and neck cancer.
-
NGF controls cancer cell migration through Akt phosphorylation, suggesting a possible therapeutic role of Akt inhibitors.
-
NGF and TrkA are highly expressed in cases of head and neck carcinomas with and without perineural invasion and are associated with improved tumor cell survival. (**)
-
p75NTR and TrkC receptors demonstrate a different immunoreactivity profile in comparison to TrkA and TrkB receptors in the normal human pituitary gland and adenomas.
-
p75NTR and pattern of invasion predict poor prognosis in oral squamous cell carcinoma.
-
Pro-NGF may be a potential diagnostic biomarker for thyroid cancer.
-
In thyroid cancer, increased expression of the TrkA receptor has been correlated with tumor progression and lymph node invasion.
[10][44][109][110][111][112][113][114][115][116][117][10,44,109,110,111,112,113,114,115,116,117] Leukemias -
NGF induces the ERK signaling pathway through TrkA receptors stimulating the production and survival of immune cells, but the actual impact on hematological diseases is still to be determined.
[118][119][120][121][118,119,120,121] Liver Cancer -
NGF and TrkA are highly expressed in hepatocarcinoma tissue (especially in males).
-
p75NTR may provide a mechanism for selective apoptosis of hepatic stellate cells.
-
NGF and its receptors may play a role in cellular interactions involving hepatocarcinoma cells, hepatic stellate cells, arterial cells, and nerve cells in cancer tissues.
-
NGF regulates liver cancer cell polarity and motility associated with the invasion and metastasis process.
-
Various therapies for liver cancer have been found to act on NGF pathways or influence the pro-NGF/NGF balance.
-
NGF level evaluation may be useful to predict hepatic dysfunction after irradiation and has been studied as a possible biomarker for liver cancer.
[80][122][123][,123124][],124125],125[126][127][128[,126129],127[130],128[131],129[,130132,131][133][80,122,132,133] Lung Cancer -
NGF has been linked to lung cancer progression, promoting the growth and survival of lung cancer cells and contributing to the development of chemoresistance.
-
TrkA is increased in squamous cell carcinoma, NGF and pro-NGF are increased in both squamous cell carcinoma and in adenocarcinoma, and p75NTR is increased across all lung cancer histological subtypes compared to normal lung.
-
NGF might play a role in the interaction between lung cancer cells and nerve fibers, leading to increased tumor growth.
-
NGF and chemokines secreted by apoptotic astrocytes cause the formation of an inflammatory and immunosuppressive microenvironment, enabling the formation of a pre-metastatic niche in lung cancer brain metastases.
-
Evidence of Trk fusion in NSCLC suggests the potential of NGF receptors as targets for further therapeutical applications.
[83][134][135][136][137][138][139][83,134,135,136,137,138,139] Ovarian Cancer -
Ovarian cancer is marked by elevated levels of NGF and TrkA.
-
NGF is involved in perineural invasion. (**)
-
Through its interaction with the TrkA receptor, NGF decreases transcription of miR-145 levels, causing an increase in oncogenic proteins involved in promoting angiogenesis and cell proliferation and migration, as well as inhibiting apoptosis and influencing various molecules such as cyclooxygenase-2, ADAM17, and calreticulin, all essential for ovarian cancer progression.
-
MicroRNAs may be associated with NGF/TrkA activation and alter key protein levels.
-
The onset condition of ovarian cancer can be diagnosed through the detection of high or low expression of NGF and its receptors.
-
TrkA may be considered a new potential tumor marker.
-
NGF is also associated with increased resistance to chemotherapy in ovarian cancer cells.
-
Blocking neurotrophin action could be a therapeutic target in treating ovarian cancer.
-
Metformin treatment decreases the expression of c-MYC, β-catenin, and VEGF induced by NGF/TrkA while increasing oncosuppressor miRs, such as miR-145 and miR-23b.
[140][141][142][143][144][145][146[149][140,141][,142147][,143148,144],145,146,147,148,149] Neuroblastoma -
NGF promotes the survival and growth of neuroblastoma cells by binding to TrkA on the surface of cancer cells.
-
TrkA and TrkC are overexpressed in biologically favorable neuroblastomas: their expression was associated with an absence of N-myc amplification, lower disease stage, lower patient age, differentiated tumors, and a greater likelihood of spontaneous regression or responding well to therapy.
-
TrkB is mainly expressed in unfavorable, aggressive neuroblastomas.
-
p75 plays an important role in enhancing both the sensitivity of Trk receptors to low levels of ligand, as well as enhancing ligand-induced differentiation in TrkA/p75 but not TrkB/p75 cells.
-
Targeting Trk receptors is a potential therapeutic strategy for neuroblastoma treatment.
-
MYCN (*) targets estrogen receptor alpha (Erα) and thereby NGF signaling to maintain an undifferentiated and aggressive phenotype.
-
RET and TrkA physically interact and can induce reciprocal activation in response to ligand activation.
[24][150][151][152][153][154][155][156][24,150,151,152,153,154,155,156] Pancreatic Cancer -
NGF exerts both stimulatory and inhibitory effects on pancreatic cancers with the effect based on the expression levels and the ratio of TrkA and p75NTR.
-
NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/glycogen synthase kinase (GSK) signal pathway.
-
TrkA expression in pancreatic cancer is a marker of tumor aggressiveness.
-
Elevated p75NGFR expression is associated with a favorable prognosis.
-
NGF overexpression combined with TrkA may contribute to perineural invasion by activating the Warburg effect, promoting tumor-derived exosomal miRNA-21 expression, prompting the hyperplasia of nerves, and inhibiting tumor cell apoptosis. (**)
-
In pancreatic cancer, the high-glucose microenvironment promotes the invasion ability and raises the expression of NGF by upregulating HIF1α.
-
NGF has been successfully used for diagnostic, therapeutic, and prognostic purposes in pancreatic cancer.
-
Atorvastatin may exert an anti-tumor effect in pancreatic cells via the inhibition of NGF and other neurotrophin signaling pathways.
-
In a mouse model, anti-NGF treatment beginning at 4 weeks may increase inflammation and negatively impact disease, while treatment starting at 8 weeks (after disease onset) reduces neural inflammation, neural invasion, and metastasis.
[13][15][16][157][158][159][160][161][162][163][164][165][166][167][13,15,16,157,158,159,160,161,162,163,164,165,166,167] Pediatric tumors -
NGF Trk receptors play a key role in pediatric tumors, especially in brain cancers.
-
Trk receptors play a key role in transducing the mitogenic effects of NGF and Trk inhibitors have been proven to be an important therapeutic tool for the treatment of NTRK fusion cancers.
-
NGF administration may be an effective and safe adjunct therapy in children with optic atrophy due to optic gliomas.
[28][36]36[82][168],82[169],168[170][28,,169,170] Prostate Cancer -
NGF and its receptors are found in prostate cancer cells.
-
NGF is released by both epithelial pancreatic cells and cancer-associated fibroblasts.
-
NGF may be able to suppress tumor growth via an indirect effect, probably innervation or maturation of the tumor neo-vasculature.
-
Aberrations and/or derangement of NGF signaling contribute to tumor growth and progression, enhancing the invasive potential of prostate cancer cells and promoting the development of cancer cells’ neuroendocrine features.
-
Expression of p75NTR reduces NGF-induced cell growth by activation of programmed cell death.
-
Cross-talk between androgen receptors and NGF receptors in prostate cancer cells may have implications for a new therapeutic approach.
-
Pro-NGF correlates with the Gleason score and is a potential driver of nerve infiltration in prostate cancer.
-
Cancer-associated fibroblasts can activate Yes-associated protein (YAP1)/TEA domain (TEAD1) signaling and increase the secretion of NGF, therefore promoting perineural invasion. (**)
-
FBXO22 mediates the NGF/TrkA signaling pathway and stimulates macrophage M2 polarization in prostate cancer bone metastases, promoting cell activity and osteogenic lesions.
[77][171][172][173][174][175][176][77,171,172,173,174,175,176] Skin tumors -
NGF and its receptor seem to play a role in most skin tumors including malignant melanoma.
-
In basal cell carcinoma and cutaneous squamous cell carcinoma, increased levels of NGF and TrkA, B, and C may reflect unique survival pathways.
-
P75NTR may play a mechanistic role in invasive melanomas demonstrating perineural invasion. (**)
-
Higher levels of Trk receptors in cutaneous squamous cell carcinoma cells may predict perineural invasion.
-
Increased p75NTR expression in cutaneous squamous cell carcinoma perineurally may allow p75NTR immunohistochemical staining to be used for detecting sites of perineural invasion.
-
NGF and TrkA are overexpressed in cervical squamous cell carcinoma.
-
PD-L1 and NGF are co-expressed on spindle cells in the microenvironment of Merkel carcinoma, and TrkA receptors seem to play a major role.
-
P75NTR has been identified as a useful marker to distinguish spindle cell melanoma from other spindle cell neoplasms of sun-damaged skin.
[54][177][178][179]185][186][54,177,178,179[180][181],180[182][183],181[184],182,183,184[,185,186] On the other hand, NGF exhibits neuroprotective effects by mitigating the harmful consequences of neuroinflammation and supporting neuronal survival and function, potentially counteracting the detrimental effects of excessive inflammation on neurons [199][200][199,200]. Furthermore, dysregulation of NGF signaling has been associated with various neurological disorders characterized by neuroinflammation, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and neuropathic pain conditions [201][202][201,202]. In these disorders, altered NGF levels or signaling pathways contribute to the progression of neuroinflammation and neuronal damage, so it has been suggested that modulating NGF levels or its interactions within the CNS may offer potential avenues for managing neuroinflammatory conditions and related neurological disorders [203][204][203,204]. Unfortunately, inflammation plays a multifaceted role in tumor development and progression. While the immune system’s inflammatory response is typically a defense mechanism against infections and tissue damage, chronic or persistent inflammation can contribute to the initiation, growth, and spread of certain types of tumors in several ways [69]. Cycles of tissue damage and subsequent repair processes can create an environment conducive to genetic mutations and abnormalities, increasing the likelihood of cancerous changes in cells [205]. Inflammation can indeed sustain tissue damage and attract immune cells to the site of tissue damage. Hallmarks of cancer-associated inflammation include the presence of infiltrating leukocytes, cytokines, chemokines, growth factors, lipid messengers, and matrix-degrading enzymes [206]. Some of these immune cells, like certain types of macrophages and lymphocytes, can produce factors that support tumor growth and suppress the immune system’s ability to eliminate cancer cells [207]. Inflammatory signals can stimulate angiogenesis for oxygen provision and nutrients to the tumor cells, aiding their proliferation and survival [10][208][10,208]. Managing chronic inflammation, either through lifestyle changes or medication, may play a role in reducing the risk of certain cancers or improving treatment outcomes [209].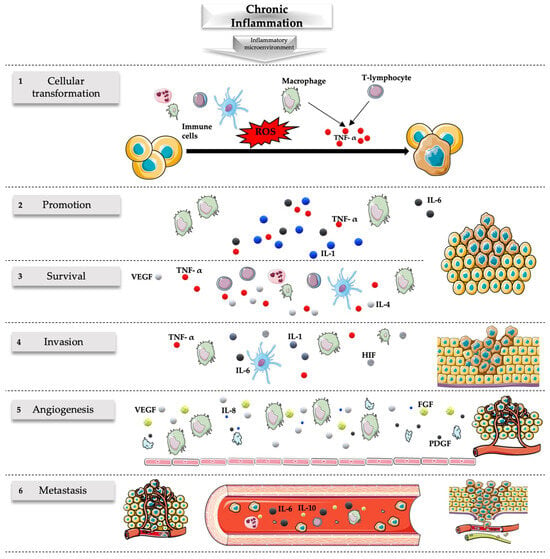 Figure 1. Role of chronic inflammation in cancer. Chronic inflammation originating from persistent stimuli has been associated with many steps of the carcinogenesis process including transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis. (1) Cellular transformation is favored by the mutagenic action of ROS released by the immune cells and the action of TNF-α released by macrophages and T lymphocytes. (2) Carcinogenesis is promoted by various cytokines released during the chronic inflammatory process, including IL-1, IL-6, and TNF-α released by macrophages. (3) The survival of the tumor is associated with an ineffective response of the immune system associated with the defective action of inflammatory cells (like the release of IL-4 and IL-5 by T cells associated with T-helper 2 but not T-helper 1 responses) related to the release of various molecules like TNF-α, VEGF, Fas ligand, and transforming growth factor-β. (4) The invasion is favored by numerous molecules during inflammatory states; some of the most important are those associated with hypoxia including HIF, TNF-α, IL-1, and IL-6. (5) Inflammatory cells, especially macrophages, but also endothelial cells and platelets stimulate vascular growth through the release of many angiogenic factors (e.g., VEGF, IL-8, FGF, PDGF), sustaining the nutritional needs of the tumor and favoring its survival and migration. (6) Numerous cytokines and factors released during inflammation can lead to metastatic events of tumors including IL-6 and IL-10. The inflammatory microenvironment has a major role in this setting with implications for the prevention and treatment of cancer. FGF, fibroblast growth factor; HIF, hypoxia-inducible factor; IL, interleukin; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/4.0/, accessed on 21 January 2024).
Figure 1. Role of chronic inflammation in cancer. Chronic inflammation originating from persistent stimuli has been associated with many steps of the carcinogenesis process including transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis. (1) Cellular transformation is favored by the mutagenic action of ROS released by the immune cells and the action of TNF-α released by macrophages and T lymphocytes. (2) Carcinogenesis is promoted by various cytokines released during the chronic inflammatory process, including IL-1, IL-6, and TNF-α released by macrophages. (3) The survival of the tumor is associated with an ineffective response of the immune system associated with the defective action of inflammatory cells (like the release of IL-4 and IL-5 by T cells associated with T-helper 2 but not T-helper 1 responses) related to the release of various molecules like TNF-α, VEGF, Fas ligand, and transforming growth factor-β. (4) The invasion is favored by numerous molecules during inflammatory states; some of the most important are those associated with hypoxia including HIF, TNF-α, IL-1, and IL-6. (5) Inflammatory cells, especially macrophages, but also endothelial cells and platelets stimulate vascular growth through the release of many angiogenic factors (e.g., VEGF, IL-8, FGF, PDGF), sustaining the nutritional needs of the tumor and favoring its survival and migration. (6) Numerous cytokines and factors released during inflammation can lead to metastatic events of tumors including IL-6 and IL-10. The inflammatory microenvironment has a major role in this setting with implications for the prevention and treatment of cancer. FGF, fibroblast growth factor; HIF, hypoxia-inducible factor; IL, interleukin; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/4.0/, accessed on 21 January 2024).
