Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Éverton do Nascimento Alencar | -- | 2973 | 2024-01-30 13:43:01 | | | |
| 2 | Lindsay Dong | -23 word(s) | 2950 | 2024-01-31 03:20:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dourado, D.; Miranda, J.A.; De Oliveira, M.C.; Freire, D.T.; Xavier-Júnior, F.H.; Paredes-Gamero, E.J.; Alencar, �.D.N. Curcumin Inorganic Nanoparticles and its Anti-cancer Potential. Encyclopedia. Available online: https://encyclopedia.pub/entry/54530 (accessed on 07 February 2026).
Dourado D, Miranda JA, De Oliveira MC, Freire DT, Xavier-Júnior FH, Paredes-Gamero EJ, et al. Curcumin Inorganic Nanoparticles and its Anti-cancer Potential. Encyclopedia. Available at: https://encyclopedia.pub/entry/54530. Accessed February 07, 2026.
Dourado, Douglas, Júlio Abreu Miranda, Matheus Cardoso De Oliveira, Danielle Teixeira Freire, Francisco Humberto Xavier-Júnior, Edgar Julian Paredes-Gamero, Éverton Do Nascimento Alencar. "Curcumin Inorganic Nanoparticles and its Anti-cancer Potential" Encyclopedia, https://encyclopedia.pub/entry/54530 (accessed February 07, 2026).
Dourado, D., Miranda, J.A., De Oliveira, M.C., Freire, D.T., Xavier-Júnior, F.H., Paredes-Gamero, E.J., & Alencar, �.D.N. (2024, January 30). Curcumin Inorganic Nanoparticles and its Anti-cancer Potential. In Encyclopedia. https://encyclopedia.pub/entry/54530
Dourado, Douglas, et al. "Curcumin Inorganic Nanoparticles and its Anti-cancer Potential." Encyclopedia. Web. 30 January, 2024.
Copy Citation
Curcumin is a natural compound that has been widely investigated thanks to its various biological properties, including antiproliferative. This molecule acts on different cancers such as lung, breast, pancreatic, colorectal, etc. However, the bioactive actions of curcumin have limitations when its physicochemical properties compromise its pharmacological potential. As a therapeutic strategy against cancer, curcumin has been associated with inorganic nanoparticles. These nanocarriers are capable of delivering curcumin and offering physicochemical properties that synergistically enhance anticancer properties.
curcuminoids
nanotechnology
oncology
1. Introduction
Cancer is categorized as a leading cause of mortality worldwide [1]. It is a pathological state that results from a build-up of genetic abnormalities and is characterized by the unchecked growth, proliferation, and differentiation of cells [2]. Current treatments are based on curative, reconstructive, and/or palliative care [3][4]. Notwithstanding the progress made in the last few decades, the treatment of cancer might be resistant to traditional chemotherapeutic agents. Hence, new targeted drugs remain a significant endeavor in cancer therapy [5]. Recently, there has been a greater focus on employing natural chemicals in chemotherapy to raise the therapeutic index of certain anticancer molecules and to act as a complementary treatment [6]. In this context, curcumin emerges as a potential bioactive with extensive anticancer potential against different types of cancer [7]. Curcumin is a major natural compound obtained from the rhizome of Curcuma longa L. [8]. Despite its high biological potential, it has biopharmaceutical limitations that compromise its clinical use in cancer therapy [9].
Nanoparticles are a nanotechnology tool that has been successfully applied in delivering anticancer molecules with biopharmaceutical limitations [10][11][12]. These can be used to treat cancer due to their specific advantages such as biocompatibility, reduced toxicity, enhanced permeability and retention (EPR) effect, and precise targeting [13][14]. In this perspective, different nanoparticles of organic nature (i.e., polymeric and lipid) for curcumin delivery were developed as anticancer therapeutic alternatives [15][16][17].
Other nanoparticles for curcumin delivery that deserve greater attention are inorganic nanoparticles. These are nanocarriers made of metallic structures, metallic oxides, among others [18]. These nanoparticles are more advantageous than those of an organic nature when it comes to their optimized physicochemical properties, which make a difference in cancer therapy [19]. They can successfully carry curcumin and show synergy with this natural compound in cancer therapy [20][21][22].
2. Curcumin and Its Anticancer Effects
Different natural compounds obtained from plants have shown anticancer activity. Among these, polyphenols are a class of natural plant chemicals that have demonstrated anticancer properties [23]. These bioactives derive from phenylalanine and contain an aromatic ring with one or more hydroxyl groups. They include a large class of antioxidants such as flavonoids, phenolic acids and their derivatives, lignans, and stilbenes [24]. Numerous mechanisms relate to polyphenols´ anticancer effects, such as the following: (i) removing cancer cells by altering signaling pathways; (ii) inhibiting cell cycle events; (iii) inducing apoptosis; and (iv) regulating the activities of enzymes involved in tumor cell proliferation [25].
In this scenario, scholars find curcumin, a polyphenol isolated from the rhizome of Curcuma longa L. [8]. Its chemical structure consists of two aromatic rings containing hydroxyl and methoxy groups, which are linked through a chain containing seven carbons of an α,β-unsaturated β-diketone moiety [26][27]. This molecule shows high tolerability and safety in physiological environments (i.e., human models) [28][29][30][31], as well as significant biological potential for therapeutic application. Among its potentialities, curcumin acts both in chemoprevention and directly in cancer cells [9][32]. In vitro and in vivo studies have shown the anticancer activity of curcumin against (i) breast, (ii) lung, (iii) prostate, (iv) pancreas, and (v) colorectal cancer, among other types [7][33][34][35].
Curcumin induces apoptosis while inhibiting the proliferation and invasion of tumors by suppressing various cellular signaling pathways. More specifically, curcumin can (i) restrict proliferation, reducing the cell cycle through inhibition of the Wnt/β-catenin pathway, increasing the levels of p53, p21, and p27, and inhibiting the levels of CDK4 and Cyclin D1 [35]. It can (ii) increase E-cadherin levels and decrease N-cadherin, vimentin, fibronectin, slug, and snail levels through suppression of the TGF-β/Smad2/3 pathway, inhibiting migration and invasion [36]. Further, it can (iii) stimulate the production of ROS through the activation of the p38 MAPK, JNK, and ERK pathways. Additionally, curcumin can (iv) set ferroptosis, increasing the levels of TFRC, FTL, and FTH1, and (v) promote apoptosis by enhancing the expression or cleavage of apoptotic proteins (Bax, Cleaved-caspase-3, Cleaved-caspase-9, and Cleaved-PARP) and by inhibiting the expression of anti-apoptotic proteins (Bcl-2) [37]. Curcumin (vi) can enhance the expressions of Beclin1, Atg5, Atg3, and LC3B-II/I and promote autophagy by the PI3K/Akt/mTOR pathway. Furthermore, curcumin can (vii) reduce the levels of Oct4, Sox2, and Nanog suppressing stemness through the inhibition of JAK/STAT3 pathways; (viii) suppress the TLR4/NF-κB signaling pathway attenuating inflammation (TNF-α, IL-6, and IL-1β); and (ix) mitigate angiogenesis through the inhibition of the expressions of VEGF, CD31, αSMC, iNOS, and COX-2 [7][38].
Although curcumin reveals promising properties, it presents critical limitations, such as (i) quick systemic elimination; (ii) significant first-pass intestinal (phase I) and hepatic (phase II) metabolism; (iii) instability in intestinal pH; (iv) poor intestinal permeability; and (v) low water solubility [39][40][41][42]. These aspects have been key in limiting curcumin’s clinical acceptance as a therapeutic agent [43].
One of the strategies to improve its biological efficacy (i.e., anticancer) has been its complexation with metals [44]. The α,β-unsaturated β-diketo moiety of curcumin is reported to be a strong chelating agent; it interacts with various metal ions [45]. Based on these advantageous interactions, developing inorganic-based nanoparticles, whose nanostructures provide important characteristics for increasing the effectiveness of cancer therapy, is key to curcumin delivery [21].
3. Curcumin-Based Inorganic Nanoparticles—Physicochemical Characteristics
3.1. Metal Nanoparticles
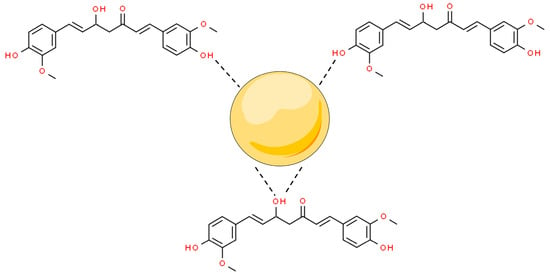
Metal nanoparticles (MNPs) are nanomaterials that are made of one chemical element. They can be synthesized from their metal precursors and modified with several functional groups. This allows them to be conjugated with antibodies, ligands, and drugs of interest presenting a wide range of applications [46][47]. Among their applications, MNPs can modulate (i) apoptosis and (ii) cell cycle arrest; and inhibit (iii) tumor angiogenesis, (iv) metastasis, and (v) inflammation to stop cancer proliferation. Moreover, metal nanoparticles have shown synergistic potential with marketed anticancer drugs, improving their bioactivity and bioavailability [20]. In this scenario, curcumin has been conjugated with MNPs, by capping the surface of the nanostructures mediated by interactions with the hydroxyl moieties of the two phenolic groups and one enolic group from this molecule (Figure 1).

Figure 1. Overall scheme of curcumin hydroxyl from enol and phenol groups’ interaction to metal nanoparticles’ (MNPs’) surfaces. Based on Amaldoss et al., 2022; Wanninger et al., 2015; and Prasad et al., 2021 [21][44][45].
MNPs can be built from different elements. In the biomedical field, gold is extensively used thanks to its advantageous properties. One could mention its high electrical conductivity and reflectivity, low cytotoxicity, biocompatibility, optical properties, malleability, and resistance to corrosion and oxidation. Further, it presents a high degree of size and shape control, besides a relatively bio-inert surface, which can be easily modified [48].
Based on these properties, Mahalunkar and colleagues [49] developed folate–curcumin-loaded gold–polyvinylpyrrolidone nanoparticles (FA–CurAu-PVP NPs). Polyvinylpyrrolidone (PVP) is a non-toxic, non-ionic polymer used in NP synthesis [50]. PVP acts as a surface stabilizer, growth modifier, nanoparticle dispersant, and reducing agent depending on the specific synthetic conditions and material system [51].
These authors [49] obtained FA-CurAu-PVP NPs by LBL (layer-by-layer) assembly. AuNPs, AuPVP NPs, CurAu-PVP NPs, and FA–CurAu-PVP NPs presented hydrodynamic diameters of 36.85 nm, 62.50 nm, 72.56 nm, and 358.7 nm, respectively. The increase in the particle size of AuNPs and AuPVP NPs is related to partial cross-linking between the particles during the conjugation process. The increase in particle size after functionalization with folate (FA) was expected, as reported in the literature [52]. The coating of the nanoparticles with PVP and functionalization with FA were confirmed using UV-Vis by monitoring the wavelength scan at each stage of synthesis. TEM analysis of FA-CurAu-PVP NCs showed structures with discrete spherical outlines and monodisperse size distribution (~250 nm). The droplet size obtained by TEM (solid diameter) was smaller than the hydrodynamic diameter from DLS, indicating a large electrical double layer in suspension. The solid diameter obtained by TEM was ideal for the passive targeting of tumors [49].
Alibolandi and colleagues [53] also developed gold nanoparticles; however, they were stabilized by dendrimers (PAMAM) complexed with PEG and functionalized with MUC-1 aptamer. PAMAM dendrimers are well-defined, highly branched, nanoscaled macromolecules with numerous active amine groups on the surface [54]. These compounds are soluble in the aqueous medium, whereas the three-dimensional interior hollow environment could entrap hydrophobic components, including Au-NP and hydrophobic chemotherapeutic agents [55].
3.2. Metal Oxide Nanoparticles
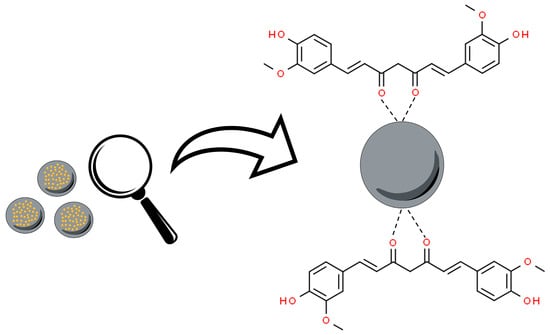
Metal oxide nanoparticles (MOx) are a fascinating class and diverse form of nanomaterials. These nanostructures are formed from metallic elements and oxygen under different conditions, generating different structural forms. Metal oxide nanoparticles have been associated with curcumin, from the interaction of the nanoparticle surface with the keto–enol functionality of this molecule (Figure 2) [56].

Figure 2. Overall scheme of curcumin’s oxygen atoms from the keto–enol groups’ interaction to metal oxide nanoparticles (MOx). Based on Bhandari et al., 2016 [56].
In this sense, Yallapu and partners developed magnetic metal oxide curcumin nanoparticles (MagNP-CUR) through the method of co-precipitation of Fe2+ and Fe3+ salts [57]. Magnetic nanoparticles (MagNPs) have been used because they are easy to prepare, chemically functional, have small sizes, excellent biocompatibility, stability, efficient drug conjugation, and remarkable magnetic capacity [58]. The production of iron-based MagNPs has been preferred compared to other metals, mainly due to biocompatibility and low toxic effects, which are essential due to the accumulation of the toxic effects of MNPs [59].
3.3. Mesoporous Silica Nanoparticles (MSNPs)
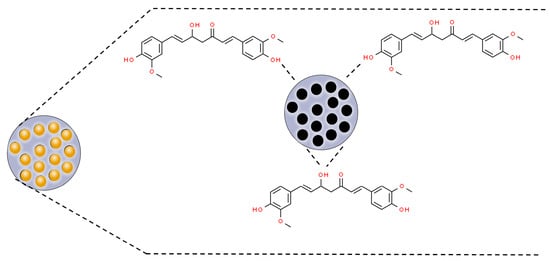
Mesoporous silica nanoparticles (MSNPs) are inorganic nanoparticles or mesoporous forms of silica with particle sizes between 30 and 300 nm that can promote endocytosis by target cells with minimal toxicity [60]. MSNPs are of special interest because of their excellent biocompatibility, high drug-loading capacity, rigid framework, well-defined pore structure, easily controllable morphology, and tunable surface chemistry [61][62]. The porous structure of mesoporous silica materials provides cavities that can host and release a great variety of biomolecules and therapeutic agents, such as anticancer drugs [63][64][65]. In this perspective, curcumin has been associated with MSNPs through hydrogen bonds with silica matrix groups (SI-OH) (Figure 3) [66][67][68].

Figure 3. Scheme of curcumin incorporation to mesoporous nanoparticles’ (MSNPs’) pores by hydrogen bonds with SI-OH groups. Based on Jambhrunkar et al., 2014; Lungare et al., 2016; and Ribeiro et al., 2022 [66][67][68].
3.4. Metal–Organic Frameworks (MOFs)
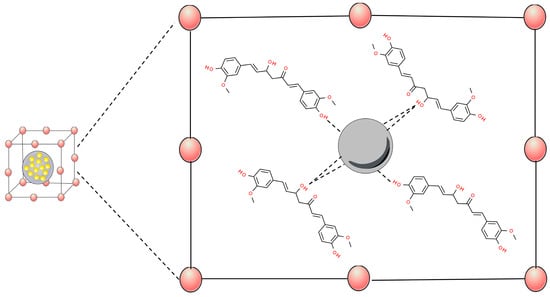
Metal–organic frameworks (MOFs) are a new type of crystalline porous material that is self-assembled by the coordination of metal cations/clusters with organic linkers [69][70]. MOFs have exhibited promising catalytic potentials towards many types of reactions, owing to their designable metal–oxo clusters bridging organic linkers, modifiable structure, and intrinsic porosities [71]. This nanomaterial has been widely developed as a carrier for the effective delivery of drugs to tumor tissues [72]. These systems have been associated with curcumin through interactions between the phenol and enol sites of this molecule, showing strong evidence of chelation-type bonds with the MOFs (Figure 4) [73].

Figure 4. Scheme of metal–organic frameworks (MOFs)’ interactions of metals to curcumin hydroxyl groups. Based on Munasinghe et al., 2023 [73].
4. Curcumin In Vivo Cancer Studies
4.1. Pancreatic Cancer
Pancreatic cancer (PC) remains one of the greatest challenges in oncology, with just over 11% of patients alive 5 years following diagnosis. Chemotherapy and surgery are the two main treatments for PC. Nevertheless, at diagnosis, only 15–20% of individuals are candidates for surgery [74]. Most PC patients have distant metastases when they are first diagnosed. As a result, excising the main lesion by surgical treatment is unlikely to improve the prognosis.
Curcumin-loaded magnetic nanoparticles were developed, evaluated in vitro (cellular uptake, cell proliferation, and clonogenic assay), and in vivo in a PC model [57]. For the in vivo model, HPAF-II human pancreatic cancer cells were inoculated into male athymic nude (nu/nu) mice for 13 days. The animals were treated with 20 µg of curcumin dispersed in 100 µL of 0.1% Tween® 20, while 20 µg of curcumin loaded in magnetic nanoparticles were dispersed in 100 µL PBS. Magnetic nanoparticles without curcumin and surfactant were evaluated to eliminate possible biases. Tumor size was monitored between 7 and 40 days. Immunohistochemistry and immunofluorescence of the tumor tissues were assessed. At the end of the treatment, curcumin-loaded metallic nanoparticles showed 71.2% of tumor inhibition, while free curcumin only inhibited 35.9%. The tumor increased over days in the control groups. Such an inhibitory profile of nanoparticles is related to the potentiation of the drug and the release modulation of curcumin by the system, increasing bioavailability for a larger period. Such hypotheses were corroborated by the immunohistochemically and immunofluorescence assays performed by the authors.
4.2. Lung Cancer
Lung cancer is a type of cancer that starts when abnormal cells grow out of control in the lungs. It is a health problem that can cause serious harm and it has one of the highest incidence rates of mortality [75][76]. Given this scenario, studies on molecules with anticancer potential have been conducted. Among these, curcumin appears to be an important candidate, as studies suggest that it inhibits the growth of lung cancer cells through multiple pathways, inducing apoptosis, and inhibiting cell proliferation and epigenetic changes [7][77].
4.3. Breast Cancer
Breast cancer is the most common malignant tumor in women in the world. It accounts for as much as 36% of oncological patients. Furthermore, its incidence is constantly increasing in all regions of the world [78]. The molecular characteristics of breast cancer are possible targets for therapies. These include the activation of human epidermal growth factor receptor 2 (HER2) and hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) [79].
Given curcumin’s anti-breast cancer potential and the damage caused to tumor cells by inorganic nanoparticles, curcumin-based metal oxide nanoparticles were developed and their performance against breast cancer was evaluated [80]. Phenylboronic acid-functionalized ZnO nanoparticles (PBA-CURC-ZnO-NC) were synthesized. Initially, researchers evaluated the impact of these nanoparticles on cellular viability, intracellular reactive oxygen species, potential mitochondrial membrane, and cell death examination in an in vitro perspective to better assess the mechanism of action of this system against cancer cells. Subsequently, the effect of this system in an in vivo solid tumor model was assessed. To this end, Ehrlich ascites carcinoma (EAC) was injected into mice for tumor development. PBA-CURC-ZnO-NCs showed pronounced anticancer activity compared to empty nanoparticles and free curcumin. However, in terms of tumor volume alone free curcumin and PBA-CURC-ZnO-NCs showed no significant changes.
Taking a different formulation route, Mahalunkar et al. studied functionalized folate–curcumin-loaded gold–polyvinylpyrrolidone (FA-Curc-AU-PVP) nanoparticles for targeted delivery in breast cancer models [49]. In vitro tests such as viability and cell cycle investigation were performed. The in vivo approach of this study involved tumors stimulated from 4T1 mammary carcinoma injection in female BALB/C mice. After tumor growth, mice were treated by intratumoral administration (10 mg/kg) of free and nano-encapsulated curcumin (FA-Curc-AU-PVP nanoparticles) for 2 weeks. Tumor volume was used as an antitumor efficacy parameter. No significant decrease in tumor volume was observed after free curcumin treatment; however, FA-Curc-AU-PVP nanoparticles significantly inhibited tumor growth. The above data suggest that FA-Curc-AU-PVP nanoparticles increased the efficacy of curcumin at a dose as low as 10 mg/kg body weight. Such responses were attributed to the functionalization of nanoparticles, which allows recognition in folic acid receptors, promoting tumor targeting and consequently drug potentiation [49].
In the same pathway, Sahne and colleagues [22] developed graphene oxide nanoparticles with a single layer of CMC conjugated to PVP and functionalized with folic acid (Cur-FA-CMC/PVP GP NPs). The researchers assessed the in vivo effectiveness of these particles in treating breast cancer. To conduct the study, they induced a 4T1 tumor model in BALB/C mice and administered 200 μL of Cur-FA-CMC/PVP GO NPs (equivalent to a curcumin dose of 4 mg/kg). They examined drug uptake in tumor cells using immunohistochemistry analysis and assessed the antitumor efficacy by monitoring daily changes in tumor size and body weight. Upon completion of the study, they examined the tumor to assess apoptosis, tumor blood vessels, and inflammatory response. The results revealed a notable concentration of particles at the tumor site, a favorable in vivo pharmacokinetic profile, and an effective tumor targeting and accumulation. The accumulation of particles induced apoptosis, leading to the inhibition of tumor growth.
4.4. Colorectal Cancer
Colorectal cancer (CRC) is a disease that is highly prevalent and recurrent. This cancer accounts for approximately 10% of all cancer cases and is the second leading cause of cancer-related deaths worldwide [81]. Furthermore, it is estimated that colorectal cancer will increase by 60% in 2030, leading to a mortality rate of over 1.1 million [82].
Given this scenario, molecules with anticancer potential such as curcumin have been investigated. Curcumin can interrupt the cell cycle, in addition to accelerating cell death, inhibiting the spread of colorectal cancer [83].
Accordingly, Alibolandi et al. developed a curcumin theragnostic nanomedicine [53]. Theragnostic merges the ability to target and deliver therapeutic agents with imaging for diagnostic purposes. Nanomaterials such as gold and iron oxide nanoparticles are often studied for these purposes [84]. In this study, in vitro cytotoxicity, in vivo cytotoxicity, and CT scanning assays were performed to evaluate Apt-PEG-AuPAMAM-CUR efficiency as a theragnostic agent [53]. First, to assess toxicity and the impact of MUC-1 aptamer, two cell lines MUC-1 positive (HT29 and C26, human and murine colon cancer) and one cell line MUC-1 negative (CHO, Chinese hamster ovary) were used. Further, a C26 tumor-bearing mice model was used for anticancer activity. After 14 days of subcutaneous inoculation, mice were treated intravenously with free curcumin, PEG-AuPAMAM-CUR, Apt-PEG-AuPAMAM-CUR (curcumin equivalent to 2 mg/kg), or saline 0.9% as negative control twice per week for three weeks.
5. Summary
Natural products have always made contributions to medicine, and it is no different in cancer therapy. Natural compounds such as polyphenols have been linked to anticancer potential. Curcumin is a polyphenol molecule of natural origin with high anticancer therapeutic potential. However, its biopharmaceutical limitations may compromise its therapeutic efficacy. This molecule has been associated with nanoparticles, a promising strategy for increasing the apparent solubility, stability, targeting, permeability enhancement, and selectivity for cancer cells. Among the universe of nanoparticles, inorganic nanoparticles stand out, which have optimized physicochemical properties when compared to the organic ones commonly used in recent years.
References
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271.
- Diori Karidio, I.; Sanlier, S.H. Reviewing cancer’s biology: An eclectic approach. J. Egypt. Natl. Canc Inst. 2021, 33, 32.
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702.
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 102375.
- Xuan, W.; Haiyun, Z.; Xiaozhuo, C. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160.
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect 2021, 11, 5–13.
- Yang, Z.-J.; Huang, S.-Y.; Zhou, D.-D.; Xiong, R.-G.; Zhao, C.-N.; Fang, A.-P.; Zhang, Y.-J.; Li, H.-B.; Zhu, H.-L. Effects and Mechanisms of Curcumin for the Prevention and Management of Cancers: An Updated Review. Antioxidants 2022, 11, 1481.
- Celani, L.A.-O.; Egito, E.A.-O.; Azevedo, Í.M.A.-O.; Oliveira, C.A.-O.X.; Dourado, D.A.-O.; Medeiros, A.A.-O.X. Treatment of colitis by oral negatively charged nanostructured curcumin in rats. Acta Cir. Bras. 2022, 37, 1678–2674.
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic potential and limitations of curcumin as antimetastatic agent. Biomed. Pharmacother. 2023, 163, 114758.
- Dourado, D.; Batista, F.P.; Philadelpho, B.O.; de Souza, M.L.; de Cerqueira e Silva, M.B.; de Grandis, R.A.; Miranda, P.A.; Colauto, N.B.; Pereira, D.T.; Formiga, F.R.; et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 2112.
- Gong, G.; Fu, B.; Ying, C.; Zhu, Z.; He, X.; Li, Y.; Shen, Z.; Xuan, Q.; Huang, Y.; Lin, Y.; et al. Targeted delivery of paclitaxel by functionalized selenium nanoparticles for anticancer therapy through ROS-mediated signaling pathways. RSC Adv. 2018, 8, 39957–39966.
- Hsiao, C.-H.; Huang, H.-L.; Chen, Y.-H.; Chen, M.-L.; Lin, Y.-H. Enhanced antitumor effect of doxorubicin through active-targeted nanoparticles in doxorubicin-resistant triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2022, 77, 103845.
- Raheem, M.A.; Rahim, M.A.; Gul, I.; Zhong, X.; Xiao, C.; Zhang, H.; Wei, J.; He, Q.; Hassan, M.; Zhang, C.Y.; et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano 2023, 12, 100152.
- Gavas, S.; Quazi, S.; Karpiński, T.A.-O. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173.
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884.
- Hu, H.; Liao, Z.; Xu, M.; Wan, S.; Wu, Y.; Zou, W.; Wu, J.; Fan, Q. Fabrication, Optimization, and Evaluation of Paclitaxel and Curcumin Coloaded PLGA Nanoparticles for Improved Antitumor Activity. ACS Omega 2023, 8, 976–986.
- Valencia, M.S.; da Silva Júnior, M.F.; Xavier-Júnior, F.H.; de Oliveira Veras, B.; de Albuquerque, P.B.S.; de Oliveira Borba, E.F.; da Silva, T.G.; Xavier, V.L.; de Souza, M.P.; das Graças Carneiro-da-Cunha, M. Characterization of curcumin-loaded lecithin-chitosan bioactive nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100119.
- López-Lorente, Á.I.; Valcárcel, M. Analytical Nanoscience and Nanotechnology. In Comprehensive Analytical Chemistry; Valcárcel, M., López-Lorente, Á.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 3–35.
- Pugazhendhi, A.; Edison, T.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111.
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D.; et al. Metal nanoparticles in cancer: From synthesis and metabolism to cellular interactions. J. Nanosctructure Chem. 2023, 13, 321–348.
- Amaldoss, M.J.N.; Yang, J.-L.; Koshy, P.; Unnikrishnan, A.; Sorrell, C.C. Inorganic nanoparticle-based advanced cancer therapies: Promising combination strategies. Drug Discov. Today 2022, 27, 103386.
- Sahne, F.; Mohammadi, M.; Najafpour, G.D. Single-Layer Assembly of Multifunctional Carboxymethylcellulose on Graphene Oxide Nanoparticles for Improving in Vivo Curcumin Delivery into Tumor Cells. ACS Biomater. Sci. Eng. 2019, 5, 2595–2609.
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170.
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9.
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631.
- Abd Wahab, N.A.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679.
- Hafez Ghoran, S.A.-O.; Calcaterra, A.A.-O.; Abbasi, M.; Taktaz, F.A.-O.; Nieselt, K.; Babaei, E.A.-O. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236.
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598.
- Cheng, A.-L.; HSU, C.-H.; Lin, J.-K.; Hsu, M.-M.; Ho, Y.-F. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, e2900.
- Dcodhar, S.; Sethi, R.; Srimal, R. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian. J. Med. Res. 1980, 138, 632.
- Sahebkar, A.; Mohammadi, A.; Atabati, A.; Rahiman, S.; Tavallaie, S.; Iranshahi, M.; Akhlaghi, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013, 27, 1883–1888.
- Adeluola, A.; Zulfiker, A.H.M.; Brazeau, D.; Amin, A. Perspectives for synthetic curcumins in chemoprevention and treatment of cancer: An update with promising analogues. Eur. J. Pharmacol. 2021, 906, 174266.
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.O. Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol. Head. Neck Surg. 2011, 145, 58–63.
- Sun, S.; Fang, H. Curcumin inhibits ovarian cancer progression by regulating circ-PLEKHM3/miR-320a/SMG1 axis. J. Ovarian Res. 2021, 14, 158.
- Tian, S.; Liao, L.; Zhou, Q.; Huang, X.; Zheng, P.; Guo, Y.; Deng, T.; Tian, X. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol. Lett. 2021, 21, 286.
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354.
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 2018, 233, 4634–4642.
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740.
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702.
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282.
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119.
- Dourado, D.; Oliveira, M.C.d.; Araujo, G.R.S.d.; Amaral-Machado, L.; Porto, D.L.; Aragão, C.F.S.; Alencar, E.d.N.; Egito, E.S.T.d. Low-surfactant microemulsion, a smart strategy intended for curcumin oral delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129720.
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). J. Evid. Based Complement. Altern. Med. 2015, 2015, 678218.
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin--synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002.
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094.
- Tiwari, A.P.; Rohiwal, S.S. Chapter 2—Synthesis and Bioconjugation of Hybrid Nanostructures for Biomedical Applications. In Hybrid Nanostructures for Cancer Theranostics; Ashok Bohara, R., Thorat, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41.
- Saleh, T.A. Chapter 8—Properties of nanoadsorbents and adsorption mechanisms. In Interface Science and Technology; Saleh, T.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 233–263.
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56.
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302.
- Jadhav, S.V.; Nikam, D.S.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Ningthoujam, R.S.; Salunkhe, A.B.; Pawar, S.H. Studies on colloidal stability of PVP-coated LSMO nanoparticles for magnetic fluid hyperthermia. New J. Chem. 2013, 37, 3121–3130.
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905.
- Panda, J.J.; Kaul, A.; Kumar, S.; Alam, S.; Mishra, A.K.; Kundu, G.C.; Chauhan, V.S. Modified dipeptide-based nanoparticles: Vehicles for targeted tumor drug delivery. Nanomedicine 2013, 8, 1927–1942.
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75.
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29.
- Kesharwani, P.; Choudhury, H.; Meher, J.G.; Pandey, M.; Gorain, B. Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Prog. Mater. Sci. 2019, 103, 484–508.
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 59–64.
- Yallapu, M.M.; Ebeling, M.C.; Khan, S.; Sundram, V.; Chauhan, N.; Gupta, B.K.; Puumala, S.E.; Jaggi, M.; Chauhan, S.C. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol. Cancer Ther. 2013, 12, 1471–1480.
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry 2019, 5, 42.
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334.
- Paul, S.; Hmar, E.B.L.; Pathak, H.; Sharma, H.K. An overview on nanocarriers. In Nanocarriers for Drug-Targeting Brain Tumors; Kumar, L., Pathak, Y.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–204.
- Lind, A.; Spliethoff, B.; Lindén, M. Unusual, vesicle-like patterned, mesoscopically ordered silica. Chem. Mater. 2003, 15, 813–818.
- Xu, C.; Lei, C.; Yu, C. Mesoporous silica nanoparticles for protein protection and delivery. Front. Chem. 2019, 7, 290.
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579.
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2019, 30, 1902634.
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49.
- Jambhrunkar, S.; Karmakar, S.; Popat, A.; Yu, M.; Yu, C. Mesoporous silica nanoparticles enhance the cytotoxicity of curcumin. Rsc Adv. 2014, 4, 709–712.
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293.
- Ribeiro, T.d.C.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.d.A.; Planeta, C.d.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976.
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714.
- Zhang, F.; Zhang, J.; Zhang, B.; Zheng, L.; Cheng, X.; Wan, Q.; Han, B.; Zhang, J. CO(2) controls the oriented growth of metal-organic framework with highly accessible active sites. Nat. Commun. 2020, 11, 1431.
- Phang, W.J.; Jo, H.; Lee, W.R.; Song, J.H.; Yoo, K.; Kim, B.; Hong, C.S. Superprotonic conductivity of a UiO-66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation. Angew. Chem. Int. Ed. Engl. 2015, 54, 5142–5146.
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232.
- Munasinghe, V.K.; Manawadu, D.; de Silva, R.M.; de Silva, K.N. Impact of active sites on encapsulation of curcumin in Metal Organic Frameworks. Mater. Res. Express 2023, 10, 035102.
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34.
- WHO. Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 3 December 2023).
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328.
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569.
- Hong, R.; Xu, B. Breast cancer: An up-to-date review and future perspectives. Cancer Commun. 2022, 42, 913–936.
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172.
- WHO. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer#:~:text=Key%20facts,people%20aged%2050%20and%20above (accessed on 3 December 2023).
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454.
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641.
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
719
Revisions:
2 times
(View History)
Update Date:
31 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




