Nitrogen and phosphorus were the major drivers of phytoplankton growth, competition, and succession, and directly affect primary productivity in lakes
[12]. Excess nutrients could contribute to lake algal blooms
[13]. Lakes’ spring algal bloom has been expanding to the middle and high latitudes, and the scale, frequency, and intensity of its occurrence are all increasing under the dual pressures of climate change and human activities
[14]. The algal blooms in lakes at mid to high latitudes arounds the world are also showing an increasing trend. Most of the studies focused on the mechanism of algal blooms in low-latitude lakes, with a lack of studies on the driving mechanism of algal blooms in mid–high-latitude lakes, especially those with seasonal ice and freeze-thaw phenomena. Therefore, it was important to analyze the mechanism of nutrient transport and transformation during freeze-thaw processes on spring algal bloom in lakes
[15]. The freeze-thaw processes of lakes include freeze-thaw, freeze-up, and thawing
[16]. During this period, the physical (water temperature, solar radiation, gas release, etc.), chemical (dissolved oxygen, CO
2 concentration, etc.), and hydrology factors (hydrodynamic conditions, water velocities, water circulation, etc.) of the lakes would change significantly, which will directly or indirectly drive the migration and transformation of nutrients
[17]. The freeze-thaw effect on the migration and transformation of nutrients in lakes will affect the stability and development of the entire lake ecosystem
[18]. During the freezing period of the Ulansuhai Lake in Inner Mongolia, due to the thickness of the snow cover and the shallow depth of the lake, the organisms at the bottom of the water body are able to carry out photosynthesis to promote the migration and transformation of nutrients; as represented by Woods Lake, the nutrient concentration replaces the temperature of the water body as an important controlling factor affecting the stability of the lake ecosystem (
Table 1)
[19][20]. The growth and melting of ice sheets altered the growth of phytoplankton by affecting physical and biogeochemical processes in the water beneath the ice
[21]. The study of Norfolk Lake in the UK and Rappbode Reservoir in Germany found that the increase of nutrient concentration caused by ice sheet freezing led to the decrease of plant abundance and biomass in the water
[22][23]. Therefore, the effects of the freeze-thaw processes on the nutrient-transport mechanism, transparency, and dissolved oxygen, and the physiological and ecological characteristics of algae in the water column should be considered
[24].
Table 1. Eutrophication in selected high-latitude lakes.
| No. |
Country |
Lake |
Algae Bloom State |
References |
| 1 |
USA |
Great-salt-sea |
Escalation |
[25][26] |
| 2 |
China |
Hulun |
Sharply escalate |
[27][28] |
| 3 |
Canada |
Winnipeg |
Escalation |
[29][30] |
| 4 |
UK |
Lough Neagh |
In grave difficulty |
[31][32] |
2.1. Effects of Freeze-Thaw Processes on Nutrient Transport in Lakes
Nutrients were mainly distributed on the surface and bottom sediment layers of the water during the non-freezing period of lakes, which was an obvious vertical stratification phenomenon
[33]. The dissolved oxygen concentration at the bottom of the water column was relatively low, while the content of organic matter and particulate matter was higher than that at the top of the water column
[34]. The formation of ice sheets promoted the transportation of nutrients in ice concentration into the water, resulting in higher nutrient concentrations in the water than during the non-freezing period
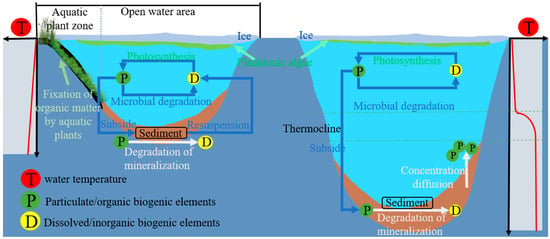
[35]. Of particular note is the formation of thermocline in deep-water lakes located in cold or temperate regions during freezing and thawing. It is difficult to exchange material between the upper mixed layer (epilimnion) and the lower stagnant layer (hypolimnion) within the lake. Large quantities of particulate organic matter and nutrients are difficult to resuspend into the upper layers of the water column through re-suspension after settling to the bottom of the lake. On the other hand, in shallow lakes, wind, waves, and turbulence can reach the bottom of the lake directly before the ice cap forms. There is an impact on organic matter and nutrients deposited on the lake bottom. These substances can enter the overlying water column through re-suspension, creating a nutrient cycle on the sediment–water inner surface (
Figure 1)
[36].
Figure 1. Patterns of nutrient cycling in shallow (left) and deep-water (right) lakes during the freezing period.
When the lake was in the frozen period, the presence of ice sheets and snow promoted significant differences in the physical and chemical environment compared to other periods
[37]. The water flow rate was slow, while the nutrient concentration varied greatly in multiple media. Nutrient concentration showed a “C-shaped” distribution in sub glacial water bodies
[38]. The concentration of ammonia nitrogen and nitrate nitrogen in water was higher than those in sediment, while the tendency of available phosphorus was opposite
[37]. The distribution characteristics of nitrogen and phosphorus at the sediment water interface were relatively different
[39]. The concentration of ammonia nitrogen and nitrate nitrogen decreased with the increase of sedimentation depth. However, the concentration of effective phosphorus showed a tendency to increase and then decrease with the increase of sedimentation depth
[40]. The presence of lake ice promotes the slowed rheological behavior of ice water, changing disturbance between sediment, and promoting a different distribution of nutrients between ice, water, and sediment compared to other periods
[41]. There was a critical value for external factors such as flow velocity and disturbance in water bodies under ice caps, and both above and below this threshold will have different effects on nitrogen and phosphorus releasing
[42].
During the thawing period, nutrients in snow quickly entered the water body, which resulted in a sudden increasing of nutrient concentration in the water body
[12]. The increasing of water temperature accelerated the metabolism of algae
[16]. During the thawing period, the water flow rate increased, and the nutrient cycling rate and biogeochemical reaction rate both accelerated
[43]. In addition, studies had shown that the comprehensive eutrophication index of water during the freezing and thawing periods was higher than that during non-freezing period
[35]. These variations will lead to the spring algal blooms
[44].
2.2. Effects of Freeze-Thaw Processes on Nutrient Transformation
Nitrogen and phosphorus, as important components of biogeochemical cycles in lakes, are the material basis for the growth and reproduction of phytoplankton and microorganisms
[45]. At present, studies showed that a variety of factors such as temperature, dissolved oxygen content, acidity, alkalinity, and solar radiation were subject to change. Regrettably, there were fewer studies on the polymorphic transformation of nitrogen and phosphorus in lakes with the freezing and thawing process. The response of each substance was difficult to quantify, which greatly restricted an in-depth understanding of the mechanism of spring algal bloom in mid–high latitude lakes
[46].
The main chemical reaction mechanisms of nitrogenous nutrients in lakes included anaerobic denitrification, anaerobic ammonium oxidation, aerobic denitrification, and anaerobic methane oxidation
[47]. The transformation of nitrogen forms in water mainly includes processes of ammonification, nitrification, and denitrification
[48]. The existence of freeze-thaw processes in lakes led to lower water temperature and dissolved oxygen concentration in the lake
[5]. Anaerobic and low-temperature environments led to a decrease in microbial activity, promoting a decrease in the rates of nitrification, denitrification, and ammonification reactions
[49]. Anaerobic environment also promoted further reduction of nitrate into nitrogen and nitrous oxide
[50]. However, the contribution of freezing and thawing processes to ammonification and nitrification can not be specifically quantified.
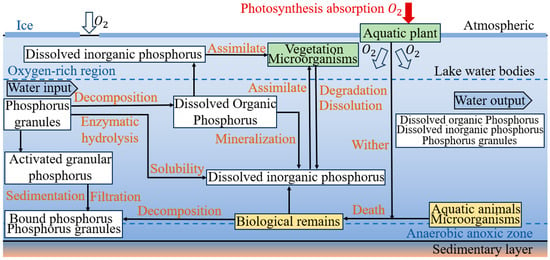
Phosphorus is an essential macronutrient for phytoplankton growth, which plays a more important role in phytoplankton succession than nitrogen in lakes. The occurrence forms of phosphorus included orthophosphates (
𝐻2𝑃𝑂4−,
𝐻𝑃𝑂42−,
𝑃𝑂4+), polymerized phosphates (
𝑃2𝑂74−,
𝑃3𝑂105−), and organophosphates (phosphatidylinositol)
[51][52]. The rate phosphorus migration and its transformation in lakes was higher than that of nitrogen and silicon
[53]. The phosphorus content decreased below the critical value required for algae growth (2 μg/L) due to the long-term low-temperature and hypoxic environment during the frozen period
[52]. The process of converting organic phosphorus into inorganic phosphorus and orthophosphate into adenosine triphosphate (ATP) was greatly inhibited due to the decrease in microbial activity during the frozen period
[53]. Phosphate can form insoluble precipitates with metal cations
𝐹𝑒+3,
𝐴𝑙+3,
𝐶𝑎+2,
𝑀𝑔+2, and the reaction speed was also affected by the temperature and oxygen content (
Figure 2). Phosphate exhibited vertical stratification due to the decomposition of dead vegetation residues by microorganisms during the frozen period
[54]. The anaerobic environment promoted the transformation of insoluble Fe(ON)
3 into soluble Fe(ON)
2, providing a material-source basis for the improvement of primary productivity in spring
[55]. Some anaerobic microorganisms in the frozen sediments accelerated the conversion of organic to inorganic phosphorus in the sediments. This phenomenon also increases phosphorus levels in the overlying water column
[56]. Hence, the complex phosphorus transformation process during the frozen period can better explain the spring-algal-bloom phenomenon compared to nitrogen.
Figure 2. Mechanisms of phosphorus-containing nutrient transformation in lakes during freezing and thawing processes.
3. Effect of Freeze-Thaw Processes on Transparency and Dissolved Oxygen
The presence of snow and ice layers weakened the intensity of solar radiation entering sub-glacial water bodies, promoting a decrease in sub-glacial light intensity to inhibit algal photosynthesis
[57][58]. The lake ice also temporarily buffered atmospheric sedimentation and reduced wind disturbance, suppressing the resuspension of sediment
[59].
Ice caps are influenced by a number of factors during their formation. The freezing temperature of the ice, the rate of ice growth, and the salinity of the water together determine the density, crystal structure, and internal microstructure of the ice. They lead to a decrease in the transparency of the ice, making less light receivable under the ice
[60]. The weakening of photosynthesis led to a further decrease in dissolved-oxygen content
[61]. In addition, the nutrient concentration in the ice and the freezing separation coefficient also increased with the decreasing of freezing temperature, promoting the release of nutrients from the ice into the water body
[62]. Ice caps redistributed nutrients between water bodies and ice layers through freezing, salt discharge, and melting dilution
[63]. The research found that the formation of ice sheets redistributed nutrients among ice, water, and sediment
[64]. The ice sheet weakened the disturbance of wind, blocks the exchange of substances between the atmosphere and water, and reduced the re-suspension of particles caused by wind
[63][64]. The pollutants in the overlying water became more uniform during the frozen period, which promoted the increasing of transparency
[64][65][66]. Hence, transparency of different lakes reacts differently during freeze-thaw processes, while it all led to a decreasing of dissolved oxygen due to the ice sheets.
4. Effect of Freeze-Thaw Processes on Algae Physiology
Phytoplankton, as the basis of material circulation and energy flow in lake ecosystems, plays an important role in maintaining the balance of the entire ecosystem
[67]. Freezing and thawing had direct or indirect effects on the physiological and ecological characteristics of planktonic algae from physical (temperature, light intensity, dissolved sample content), chemical (nutrient salt concentration, metabolic rate) and hydrological (hydrodynamic conditions, water circulation)
[66].
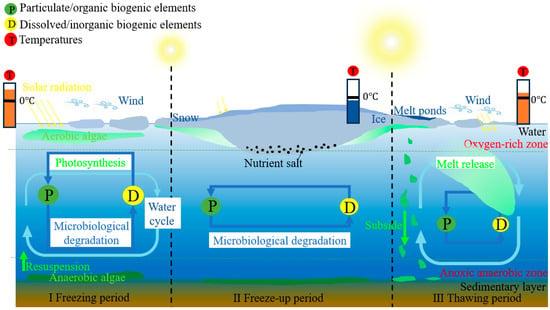
According to the previous research, the freeze-thaw process greatly limited the activity of underwater organisms
[68]. However, scholars found that the diversity of benthic phytoplankton species during the frozen period was still relatively high in recent years
[69][70][71]. Currently, the research has found that the freezing period promoted a decreasing of phytoplankton diversity over time
[72]. However, the driving mechanism of phytoplankton population succession during the freezing period was not clear (
Figure 3). Cyanobacteria had the greatest dominance during the freeze-up period; with the further reduction in temperature and the extension of the freeze-up time, the cyanobacteria entered into a dormant period
[73]. Diatoms had a clear dominance during the freeze-up period, which had a direct relationship to their physiological characteristics of regulating the water, sugar, and fat in the cells to increase the ability of drought resistance
[74]. During the thawing period, cyanobacteria and green algae dominate, due to maximal photosynthesis
[75].
Figure 3. Mechanisms of lake-algal-bloom occurrence during freezing and thawing processes.