
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | PhD Snezhana Mourouzidou | -- | 3704 | 2024-01-29 08:02:50 | | | |
| 2 | Catherine Yang | Meta information modification | 3704 | 2024-01-29 09:18:53 | | |
Video Upload Options
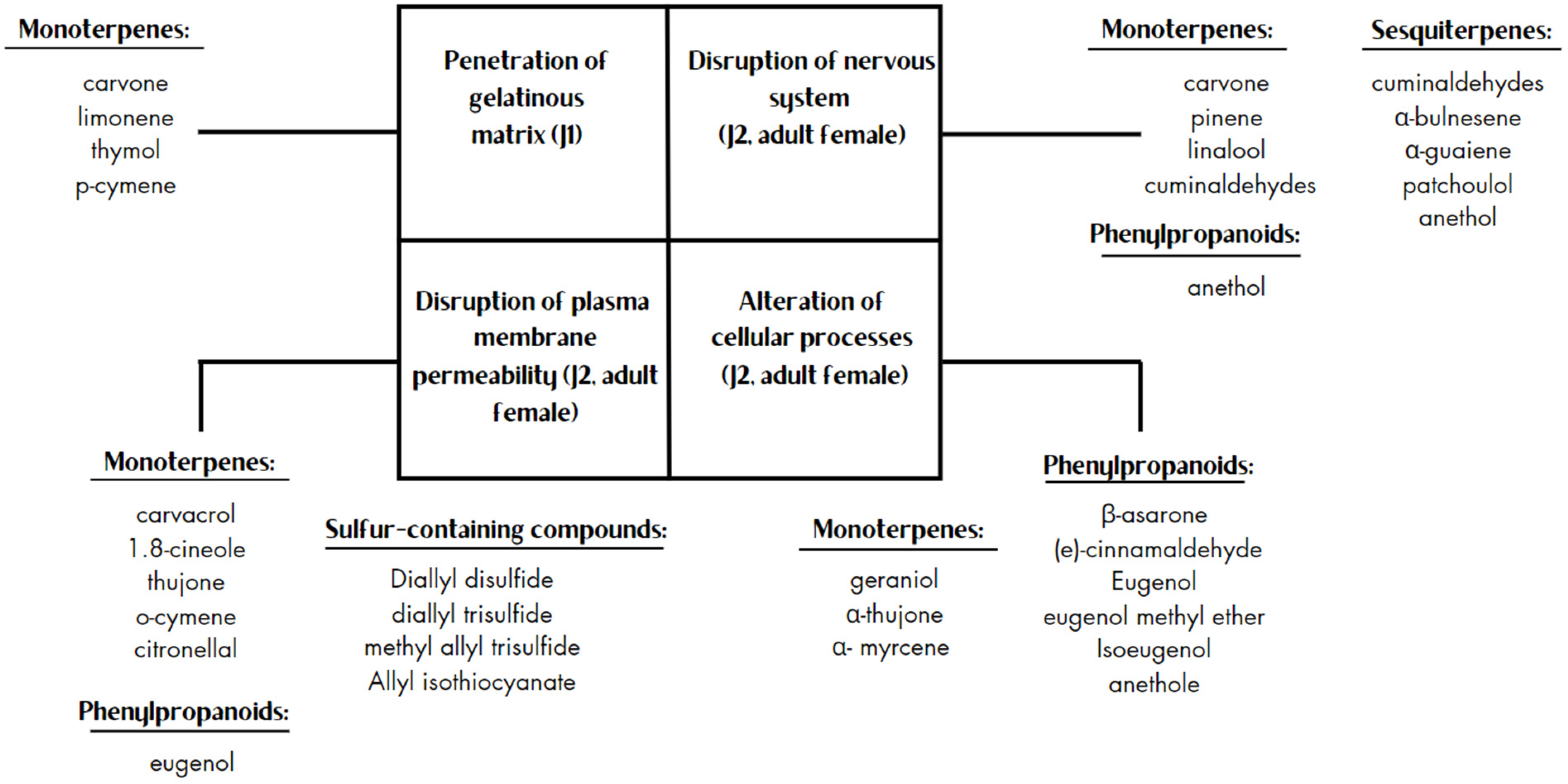
The Meloidogyne genus is widely recognized for its significant economic and scientific importance within the group of plant-parasitic nematodes. The chemical management of nematodes presents its challenges and heavily depends on employing soil fumigants containing toxic and costly nematicides. However, plant-derived essential oils offer promising alternatives, demonstrating a wide range of biological activities that affect nematodes through a range of mechanisms, including disrupting their nervous systems, inducing detrimental effects on plasma membrane permeability, penetrating the gelatinous matrix of nematode eggs, and disturbing intracellular redox status. Most of the extracted essential oils were predominantly sourced from the Lamiaceae family (32%), followed by Asteraceae (11%), Apiaceae (9%), and Poaceae (8%), and with genera Thymus, Mentha, Ocimum, Artemisia, Cymbopogon being the most common. The nematicidal activity of EOs primarily arises from their chemical groups, such as terpenes, phenylpropanoids, and organosulfur compounds.
1. Introduction
2. Essential Oils, Chemical Groups of Their Components, and Their Mode of Action
| Plant Source of the Essential Oil | Active Components | Target Life Stage | Mode of Action | Crop | Experiment | Meloidogyne spp. | Experiment Details | Author |
|---|---|---|---|---|---|---|---|---|
| Lamiaceae | ||||||||
| Lavandula Intermedia (3 species: Abrialis, Cerioni and Sumiens) |
linalool | J2, eggs | J2 mortality, egg-hatching inhibition, reduction of galls, eggs | tomato | in vitro, pot, greenhouse, soil | M. incognita | EO: 24.9 μg/mL−1, 1.2 μg/mL−1, 17.4 μg/mL−1. | [27] |
| Lavandula officinalis, Mentha arvensis, Thymus serpyllum, Ocimum basilicum | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
| Monarda didyma, Monarda fistulosa | γ-terpinene, o-cymene, carvacrol | Eggs, J2 | J2 mortality, egg-hatching inhibition, reduction of galls and eggs in soil | tomato | in vitro, soil | M. incognita | EO: 1.0 μL mL−1, 12.5 μL mL−1 for 24 h (J2 mortality) 500 and 1000 μg mL−1 for 24, 48 h (egg hatching) |
[29] |
| Mentha longifolia | piperitone oxide | J2, eggs | J2 mortality, Egg hatch inhibition | M. graminicola | EO: 15.62 to 1000 ppm for 96 h | [30] | ||
| Mentha longifolia | i-menthone | Egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Mentha spicata L. | carvone, limonene | J2, eggs | J2 mortality, reduction of galls and eggs | Coleus | in vitro, greenhouse | M. javanica | EO: 1000, 2000, 3000, 4000, and 5000 ppm (v/v) for 24 h, 48 h, 72 h |
[32] |
| Mentha spicata L. | J2 | J2 mortality, reduction of galls | pepper | in vitro, greenhouse, plastic house | M. incognita | EO: 5% (v/v) for 72 h | [33] | |
| Mentha spicata | carvone | Egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Menta piperita | carvone | J2 | J2 mortality | in vitro | M. hapla | [31] | ||
| Nepeta cateria | J2 | J2 mortality | banana | orchard | M. incognita | EO: 1.2 mL/L | [34] | |
| Basilicum L. | sabinene, myrcene, trans-caryophyllene | J2 | J2 mortality, reduction of galls | pepper | in vitro, greenhouse, plastic house | M. incognita | EO: 5% (v/v) for 72 h | [33] |
| Ocimum sanctum L. | eugenol methyl ether | J2 | J2 mortality | in vitro | M. incognita | EO: 1230 mg/L for 24 h | [35] | |
| Ocimum basilicum | i-linalool | J2 | J2 mortality | in vitro | M. hapla | [31] | ||
| Origanum onites | carvacrol | egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Origanum onites | carvacrol | egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Pogostemon cablin Benth | α-guaiene, patchoulol, α-bulnesene | J2 | J2 mortality, J2 immobility | in vitro | M. incognita | EO: 250 μg/mL−1, 31.25 μg/mL−1 for 24 h |
[36] | |
| Pogostemon cablin | α-guaiene | J2 | J2 mortality, paralysis | pepper | in vitro, greenhouse, plastic house | M. incognita | EO: 387.77 μg mL−1 for 48 h | [37] |
| Salvia officinalis | thujone | egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Thymus citriodorus | geraniol | J2, eggs | Biological cycle arrest, J2 paralysis, J2 mortality | tomato | in vitro, pot | M. incognita | EO: 50 µL kg−1 soil | [38] |
| Teucrium polium | limonene, α-pinene, β-pinene | J2 | J2 mortality | in vitro | M. incognita | EO: 4000 and 8000 ppm (v/v) for 24 h | [39] | |
| Thymus linearis Benth | thymol, carvacrol | J2, eggs | J2 mortality, Egg hatch inhibition | in vitro | M. incognita | Rainy season: 5 μL/mL for 72 h (J2 mortality), 2 μL/mL for 72 h (egg hatching) Winter season: 8 μL/m for 72 h (J2 mortality),2 μL/mL for 72 h (egg hatching) |
[40] | |
| Thymus vulgaris L. | thymol, ρ-cymene | J2, eggs | J2 mortality, reduction of galls and eggs | Coleus | in vitro, greenhouse | M. javanica | EO: 1000, 2000, 3000, 4000, and 5000 ppm (v/v) for 24 h, 48 h, 72 h |
[32] |
| Zataria multiflora | J2 | J2 mortality | banana | orchard | M. incognita | EO: 1.2 mL/L | [34] | |
| Plant Source of the Essential Oil | Active Components | Target Life Stage | Mode of Action | Crop | Experiment | Meloidogyne spp. | Experiment Details | Author |
|---|---|---|---|---|---|---|---|---|
| Acoraceae | ||||||||
| Acorus calamus | β-asarone | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 524.45 μg mL−1 for 24 h | [37] | |
| Amaranthaceae | ||||||||
| Dysphania ambrosioides | (z)-ascaridole, e-ascaridole, p-cymene | Eggs, J2 | J2 mortality, egg-hatching inhibition, reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 500 μg mL−1 for 48 h | [41] |
| Amaryllidaceae | ||||||||
| Allium sativum | diallyl disulfide (DADS), diallyl trisulfide (DATS), methyl allyl trisulfide | Eggs, J2 | J2 mortality, egg-hatching inhibition | Tomato | in vitro, pots | M. javanica | EO: 0.025 μg mL−1 for 72 h Hydrolat: 0.125 μg mL−1 for 72 h |
[42] |
| Allium sativum | diallyl disulfide (DADS), diallyl trisulfide (DATS) | Eggs, J2 | J2 mortality, egg-hatching inhibition, reduction of galls and eggs | Tomato | in vitro, greenhouse, pots | M. incognita | EO: 500 μg mL−1 Components: 62 μg mL−1 |
[42] |
| Anacardiaceae | ||||||||
| Schinus terebinthifolius | terpinen-4-ol, γ-terpinene, α-terpineol | Eggs, J2 | J2 mortality, egg-hatching inhibition | lettuce | in vitro, field | M. javanica | [43] | |
| Apiaceae | ||||||||
| Coriandrum sativum | linalool | J2 | J2 mortality | in vitro, lab | M. hapla | [31] | ||
| Cuminum cyminum | γ-terpinen-7-al, α-terpinen-7-al, cumin aldehydes | J2, Eggs | J2 mortality, egg-hatching inhibition, J2 paralysis, egg differentiation, reduction of nematode population in soil | Tomato | in vitro, pots | M. javanica | EO: 62.5 μL/L, 2000 μL/L for 48 and 96 h of immersion |
[44] |
| Daucus carota | carotol, daucol, daucene | J2, Eggs | J2 mortality, egg-hatching inhibition | in vitro | M. incognita | EO: 2500 ppm for 96 h | [45] | |
| Ferula oopoda | J2 | J2 mortality | Banana | Orchard | M. incognita | EO: 1.2 mL/L | [34] | |
| Foeniculum vulgare | anethole | J2 | J2 mortality | in vitro, lab | M. hapla | [31] | ||
| Ridolfia segetum | (z)-β-ocimene, β-pinene | J2, Eggs | J2 mortality, J2 mobility, and egg-hatching inhibition | in vitro, lab | M. javanica | EO: 16 μL/mL for 72 h | [46] | |
| Asteraceae | ||||||||
| Artemisia absinthium | borneol acetate, β-terpineol | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 937.52 μg mL−1 for 48 h | [37] | |
| Artemisia absinthium | - | Galls, eggs | Reduction of galls and eggs | Tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] |
| Artemisia nilagirica | α-thujone, α-myrcene, linalyl isovalerate, camphor, caryophyllene oxide, eucalyptol | J2, Eggs | J2 mortality, egg-hatching inhibition, reduction of galls, eggs, and nematodes in soil | Tomato | in vitro, greenhouse | M. incognita | EO: 20 μg/mL for 48 h | [20] |
| Achillea santolina | J2 | J2 mortality | Banana | Orchard | M. incognita | EO: 1.2 mL/L | [34] | |
| Achillea wilhelmsii | 1,8-cineole, limonene, α-pinene, β-pinene | J2 | J2 mortality | in vitro | M. incognita | EO: 4000 and 8000 ppm (v/v) for 24 h | [39] | |
| Tanacetum falconeri Hook. f. | cis-dehydromatricaria ester-1 | J2 | J2 mortality | in vitro | M. incognita | EO: 1% (w/v) for 24 h | [47] | |
| Tanacetum polium | (e)-caryophyllene, limonene, α-pinene, β-pinene | J2 | J2 mortality | in vitro | M. incognita | EO: 4000 and 8000 ppm (v/v) for 24 h | [39] | |
| Brassicaceae | ||||||||
| Brassica nigra | allyl isothiocyanate (AITC) | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 47.7 μg mL−1 for 72 h | [21] | |
| Burseraceae | ||||||||
| Commiphora myrrha | furanoeudesm-1,3-diene, curcerene | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 1000 μg mL−1 for 24 h | [37] | |
| Fabaceae | ||||||||
| Piptadenia viridiflora | benzaldehyde | J2 | J2 mortality | in vitro | M. incognita | EO: 1000 μg mL−1, Component: 100 and 200 μg mL−1 for 48 h |
[48] | |
| Tephrosia toxicaria | β-caryophyllene, germacrene D, a-humulene, bicyclogermacrene | Eggs, J2 | J2 mortality, egg-hatching inhibition | in vitro | M. javanica/M. enterolobii | EO: 50, 100, 200, 400, 600, 800 μg mL−1 for 48 h | [49] | |
| Trifolium incarnatum | (z)-3-hexenyl acetates, (Z)-3-hexane-1-ol, (E) -ocimene, furanoeudesm-1,3-diene | J2 | J2 mortality, reduction of galls | chili pepper (Capsicum annuum L.) | in vitro, greenhouse, plastic house | M. incognita | EO: 3% and 5% (v/v) for 48 h | [33] |
| Hypericaceae | ||||||||
| Hypericum perforatum | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
| Lauraceae | ||||||||
| Cinnamomum cassia | (e)-cinnamaldehyde | Eggs, J2 | J2 mortality, J2 paralysis, reduction of galls and eggs | Soybean | in vitro, in greenhouse pots | M. incognita | EO: 62 μg/mL−1 Component: 208 μg/mL−1 for 48 h |
[23] |
| Cinnamomum zeylanicum Blume | eugenol | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 391 mg/L for 24 h | [35] | |
| Cinnamomum zeylanicum | (e)-cinnamaldehyde, eugenol | Eggs, J2 | J2 mortality, egg-hatching inhibition, reduction of galls and eggs | in vitro, pots | M. incognita | EO: 49 μg/mL−1, Components: 529 and 768 μg/mL−1 for 48 h |
[48] | |
| Laurus nobilis L. | linalool, 1, 8-cineole, α-pinene, β-pinene, α-terpinyl acetate | Eggs, J2 | J2 mortality, egg-hatching inhibition | in vitro | M. incognita | EO: 0.80 μg/mL−1 for 96 h | [45] | |
| Myrtaceae | ||||||||
| Eucalyptus citriodora | citronellal | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 746.48 μg mL−1 for 24 h | [37] | |
| Eucalyptus citriodora | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
| Melaleuca alternifolia | β-terpineol, γ-terpinene | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 404.13 μg mL−1 for 24 h | [37] | |
| Myrtus communis | α-pinene, 1,8-cineol | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 932.65 μg mL−1 for 48 h | [37] | |
| Syzygium aromaticum | eugenol | J2 | J2 mortality | in vitro | M. graminicola | Component: 500 ppm for 48 h | [50] | |
| Pinaceae | ||||||||
| Pinus nigra | a-pinene, c-verbenol | J2 | J2 mortality | in vitro | M. javanica | EO: 1 μg mL−1 Compounds: 0.5 μg mL−1 |
[51] | |
| Piperaceae | ||||||||
| Piper nigrum | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
| Poaceae | ||||||||
| Cymbopogon flexuosus | citral | J2 | J2 mortality | in vitro | M. graminicola | Component: 500 ppm for 48 h | [50] | |
| Cymbopogon martinii | geraniol | J2 | J2 mortality | in vitro | M. graminicola | Component: 500 ppm for 48 h | [50] | |
| Cymbopogon nardus | citronellal, geraniol | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 325.41 μg mL−1 for 24 h | [37] | |
| Cymbopogon schoenanthus (L.) Spreng | piperitone | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 524 mg/L for 24 h | [35] | |
| Vetiveria zizanioides (L.) (extract) |
sesquiterpene acid 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid, 6-isopropenyl-4,8a-dimeth yl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol | J2 mortality | in vitro | M. graminicola | EO: 0.95 mg/mL for 72 h | [52] | ||
| Rutaceae | ||||||||
| Citrus bergamia | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
| Citrus reticulata | limonene | Eggs, J2 | J2 mortality, egg-hatching inhibition | in vitro | M. incognita | Component: 1500 μg/mL−1 for 96 h | [53] | |
| Citrus sinensis | l-limonene | J2 | J2 mortality, J2 paralysis | in vitro | M. incognita | EO: 353.20 μg mL−1 for 24 h | [37] | |
| Verbenaceae | ||||||||
| Lippia citriodora | citral | Egg | Egg hatch inhibition | in vitro | M. hapla | [31] | ||
| Zingibiraceae | ||||||||
| Hedychium coccineum | e-neradiol, davanone B, spathulenol, eucalyptol | Eggs, J2 | J2 mortality, egg-hatching inhibition | in vitro | M. incognita | EO: 0.25 μg/mL for 24 h 1 μg/mL for 96 h |
[54] | |
| Zingiber officinale | Galls, eggs | Reduction of galls and eggs | tomato | in vitro, pots | M. incognita | EO: 3% and 5% (v/v) | [28] | |
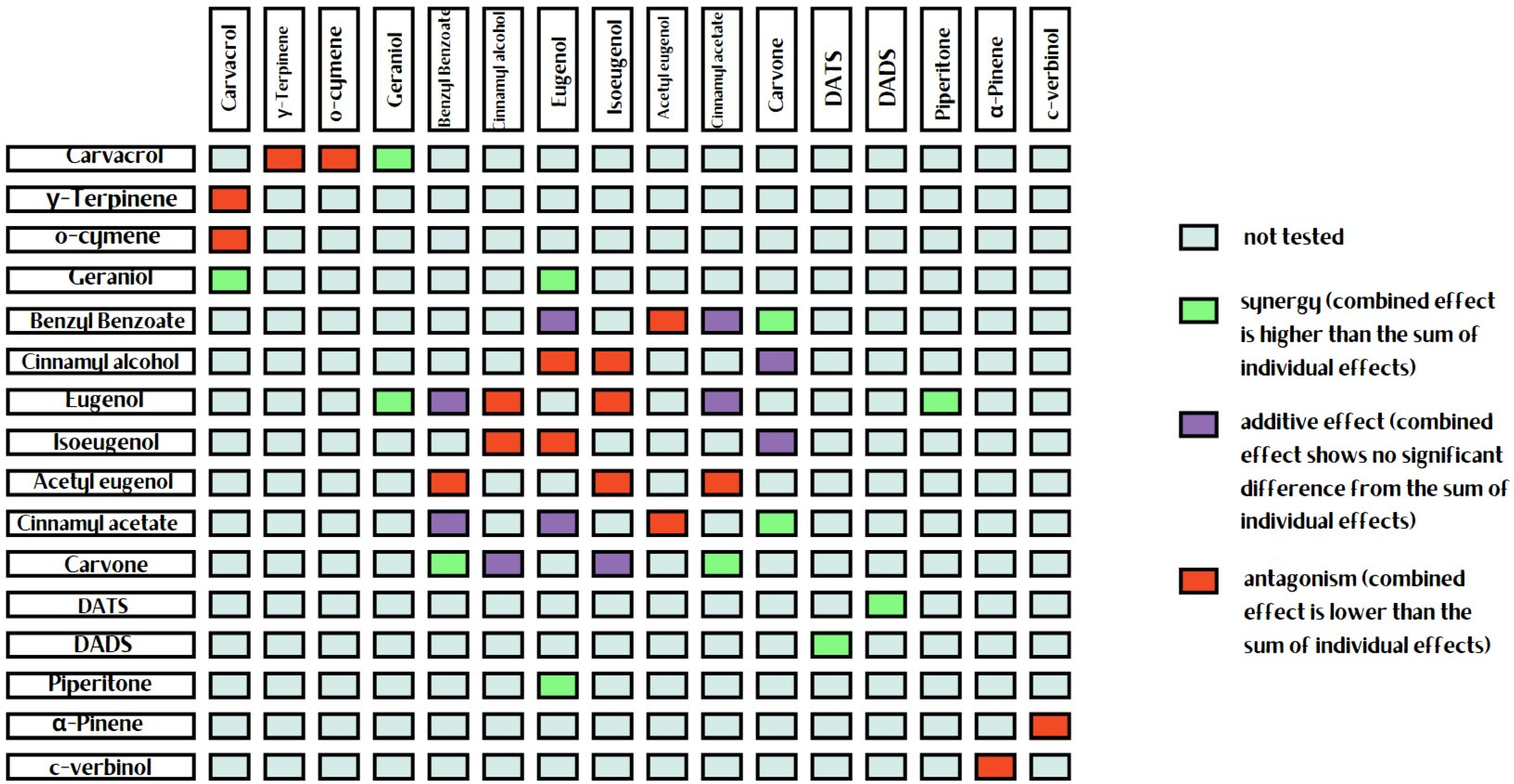
3. Synergistic and Antagonistic Interactions among Essential Oil Components
References
- Bernard, G.C.; Egnin, M.; Bonsi, C. the Impact of Plant-Parasitic Nematodes on Agriculture and Methods of Control. In Nematology-Concepts, Diagnosis and Control; IntechOpen: London, UK, 2017.
- Sikandar, A.; Jia, L.; Wu, H.; Yang, S. Meloidogyne enterolobii Risk to Agriculture, Its Present Status and Future Prospective for Management. Front. Plant Sci. 2023, 13, 1093657.
- Rusinque, L.; Camacho, M.J.; Serra, C.; Nóbrega, F.; Inácio, M.L. Root-Knot Nematode Assessment: Species Identification, Distribution, and New Host Records in Portugal. Front. Plant Sci. 2023, 14, 1230968.
- Przybylska, A.; Obrępalska-Stęplowska, A. Plant Defense Responses in Monocotyledonous and Dicotyledonous Host Plants During Root-Knot Nematode Infection. Plant Soil 2020, 451, 239–260.
- Bridge, J.; Starr, J.L. Plant Nematodes of Agricultural Importance: A Color Handbook; CRC Press: London, UK; Elsevier/Academic Press: Boston, IL, USA, 2010.
- Lamelas, A.; Desgarennes, D.; López-Lima, D.; Villain, L.; Alonso-Sánchez, A.; Artacho, A.; Latorre, A.; Moya, A.; Carrión, G. The Bacterial Microbiome of Meloidogyne-Based Disease Complex in Coffee and Tomato. Front. Plant Sci. 2020, 11, 136.
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal Activity of Essential Oils: A Review. Phytochem. Rev. 2012, 11, 371–390.
- Pandey, R.; Kalra, A.; Tandon, S.; Mehrotra, N.; Singh, H.N.; Kumar, S. Essential Oils as Potent Source of Nematicidal Compounds. J. Phytopathol. 2000, 148, 501–502.
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309.
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant Secondary Compounds in Soil and their Role in Belowground Species Interactions. Trends Ecol. Evol. 2020, 35, 716–730.
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol Activity of Aromatic and Medicinal Plants and their Bioactive Components against Soil-Borne Pathogens. Plants 2023, 12, 706.
- Ayub, M.A.; Goksen, G.; Fatima, A.; Zubair, M.; Abid, M.A.; Starowicz, M. Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations 2023, 10, 27.
- Noriega, P. Terpenes in Essential Oils: Bioactivity and Applications. In Terpenes Terpenoids—Recent Advances; IntechOpen: London, UK, 2020.
- Murti, Y.; Jain, D.; Semwal, B.; Singh, S.; Janmeda, P.; Bhaskar, P. Innovative Methods for Extraction of Essential Oils from Medicinal Plants. Int. J. Second. Metab. 2023, 10, 190–230.
- Ntalli, N.G.; Caboni, P. Botanical Nematicides: A Review. J. Agric. Food Chem. 2012, 60, 9929–9940.
- Echeverrigaray, S.; Zacaria, J.; Beltrão, R. Nematicidal Activity of Monoterpenoids against the Root-Knot Nematode Meloidogyne incognita. Phytopathology 2010, 100, 199–203.
- Padilla-Montaño, N.; de León Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, A Nortriterpen Quinone Isolated from Natural Sources. Foods 2021, 10, 591.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475.
- Rasoul, M. Evaluation of Nematicidal Effects of Monoterpenes against Root-Knot Nematode, Meloidogyne incognita. J. Plant Prot. Pathol. 2013, 4, 445–456.
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Altitude-Related Changes in the Phytochemical Profile of Essential Oils Extracted from Artemisia nilagirica and their Nematicidal Activity against Meloidogyne incognita. Ind. Crop. Prod. 2019, 139, 111472.
- Dutta, A.; Mandal, A.; Kundu, A.; Malik, M.; Chaudhary, A.; Khan, M.R.; Shanmugam, V.; Rao, U.; Saha, S.; Patanjali, N.; et al. Deciphering the Behavioral Response of Meloidogyne incognita and Fusarium oxysporum toward Mustard Essential Oil. Front. Plant Sci. 2021, 12, 714730.
- Ohri, P.; Kaur, S. Effect of Phenolic Compounds on Nematodes. A Review. J. Appl. Nat. Sci. 2010, 2, 344–350.
- Jardim, I.N.; Oliveira, D.F.; Silva, G.H.; Campos, V.P.; De Souza, P.E. (E)-Cinnamaldehyde from the Essential Oil of Cinnamomum cassia Controls Meloidogyne incognita in Soybean Plants. J. Pest Sci. 2018, 91, 479–487.
- Heywood, V.H.; Brummitt, R.K.; Culham, A.; Seberg, O. Flowering Plants Families of the World; Royal Botanic Gardens Kew: Richmond, UK, 2007.
- Fragkouli, R.; Antonopoulou, M.; Asimakis, E.; Spyrou, A.; Kosma, C.; Zotos, A.; Tsiamis, G.; Patakas, A.; Triantafyllidis, V. Mediterranean Plants as Potential Source of Biopesticides: An Overview of Current Research and Future Trends. Metabolites 2023, 13, 967.
- Bekut, M.; Brki’c, S.; Kladar, N.; Dragovi´c, G.; Gavari´c, N.; Božin, B. Potential of Selected Lamiaceae Plants in Anti (Retro) Viral therapy. Pharmacol. Res. 2018, 133, 301–314.
- D’Addabbo, T.; Laquale, S.; Argentieri, M.P.; Bellardi, M.G.; Avato, P. Nematicidal Activity of Essential Oil from Lavandin (Lavandula × intermedia Emeric Ex Loisel.) as Related to Chemical Profile. Molecules 2021, 26, 6448.
- Ozdemir, E.; Gozel, U. Nematicidal Activities of Essential Oils against Meloidogyne incognita on Tomato Plant. Fresenius Environ. Bull. 2018, 27, 4511–4517.
- Laquale, S.; Avato, P.; Argentieri, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic Activity of Essential Oils from Monarda Species. J. Pest Sci. 2018, 91, 1115–1125.
- Abhishek Gowda, A.P.; Pankaj; Shakil, N.A.; Rana, V.S.; Singh, A.K.; Bhatt, K.C.; Devaraja, K.P. Chemical Composition and Nematicidal Activity of Essential Oil and Piperitone Oxide of Mentha longifolia L. against Meloidogyne incognita. Allelopath. J. 2023, 58, 165–181.
- Felek, A.F.; Ozcan, M.M.; Akyazi, F. Effects of Essential Oils Distilled from Some Medicinal and Aromatic Plants against Root Knot Nematode (Meloidogyne hapla). J. Appl. Sci. Environ. Manag. 2019, 23, 1425.
- Hammad, E.A.; Hasanin, M.M.H. Antagonistic Effect of Nanoemulsions of Some Essential Oils against Fusarium oxysporum and Root-Knot Nematode Meloidogyne javanica on Coleus Plants. Pak. J. Nematol. 2022, 40.
- Hammad, E.A.; El-Sagheer, A.M. Comparative Efficacy of Essential Oil Nanoemulsions and Bioproducts as Alternative Strategies against Root-Knot Nematode, and Its Impact on the Growth and Yield of Capsicum annuum L. J. Saudi Soc. Agric. Sci. 2023, 22, 47–53.
- Tamoor, K.; Hafsa, H.; Maryam, H. Evaluation of some medicinal plants for the management of root-knot diseases of banana. Pak. J. Nematol. 2021, 39, 52–58.
- Eloh, K.; Kpegba, K.; Sasanelli, N.; Koumaglo, H.K.; Caboni, P. Nematicidal Activity of Some Essential Plant Oils from Tropical West Africa. Int. J. Pest Manag. 2020, 66, 131–141.
- Keerthiraj, M.; Mandal, A.; Dutta, T.K.; Saha, S.; Dutta, A.; Singh, A.; Kundu, A. Nematicidal and Molecular Docking Investigation of Essential Oils from Pogostemon cablin Ecotypes against Meloidogyne incognita. Chem. Biodivers. 2021, 18, e2100320.
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive In Vitro and In Silico Analysis of Nematicidal Action of Essential Oils. Front. Plant Sci. 2021, 11, 614143.
- Ntalli, N.; Bratidou Parlapani, A.; Tzani, K.; Samara, M.; Boutsis, G.; Dimou, M.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Thymus citriodorus (Schreb) Botanical Products as Ecofriendly Nematicides with Bio-Fertilizing Properties. Plants 2020, 9, 202.
- Ardakani, A.S.; Hosseininejad, S.A. Identification of Chemical Components from Essential Oils and Aqueous Extracts of Some Medicinal Plants and their Nematicidal Effects on Meloidogyne incognita. J. Basic Appl. Zool. 2022, 83, 14.
- Kabdal, T.; Himani; Kumar, R.; Prakash, O.; Nagarkoti, K.; Rawat, D.S.; Srivastava, R.M.; Kumar, S.; Dubey, S.K. Seasonal Variation in the Essential Oil Composition and Biological Activities of Thymus linearis Benth. Collected from the Kumaun region of Uttarakhand, India. Biochem. Syst. Ecol. 2022, 103, 104449.
- Barros, A.; Paulo Campos, V.; Lopes De Paula, L.; Alaís Pedroso, L.; Jesus Silva, F.; Carlos Pereira Da Silva, J.; Ferreira De Oliveira, D.; Humberto Silva, G. The Role of Cinnamomum Zeylanicum Essential Oil, (E)-Cinnamaldehyde and (E)-Cinnamaldehyde Oxime in the Control of Meloidogyne incognita. J. Phytopathol. 2021, 169, 229–238.
- Galisteo, A.; González-Coloma, A.; Castillo, P.; Andrés, M.F. Valorization of the Hydrolate Byproduct from the Industrial Extraction of Purple Alium sativum Essential Oil as A Source of Nematicidal Products. Life 2022, 12, 905.
- Borges, D.F.; Lopes, E.A.; Côrtes, F.R.; Visôtto, L.E.; Valente, V.M.M.; Souza, M.F. Nematicidal Potential of Essential Oils of Ageratum fastigiatum, Callistemon viminalis and Schinus terebinthifolius. Biosci. J. 2018, 34, 90–96.
- Pardavella, I.; Daferera, D.; Tselios, T.; Skiada, P.; Giannakou, I. The Use of Essential Oil and Hydrosol Extracted from Cuminum cyminum Seeds for the Control of Meloidogyne incognita and Meloidogyne javanica. Plants 2020, 10, 46.
- Kaur, A.; Chahal, K.K.; Kataria, D.; Urvashi. Assessment of Carrot Seed Essential Oil and Its Chemical Constituents against Meloidogyne incognita. J. Pharmacogn. Phytochem. 2018, 7, 896–903.
- Basaid, K.; Chebli, B.; Bouharroud, R.; Elaini, R.; Alaoui, I.F.; Kaoui, S.; De Oliveira, A.L.; Furze, J.N.; Mayad, E.H. Biocontrol Potential of Essential Oil from Moroccan Ridolfia segetum (L.) Moris. J. Plant Dis. Prot. 2021, 128, 1157–1166.
- Ismail, M.; Kowsar, A.; Javed, S.; Choudhary, M.I.; Khan, S.W.; Abbas, Q.; Tang, Y.; Wang, W. The Antibacterial, Insecticidal and Nematocidal Activities and Toxicity Studies of Tanacetum Falconeri Hook. f. Turk. J. Pharm. Sci. 2021, 18, 744–751.
- Barros, A.F.; Campos, V.P.; De Oliveira, D.F.; Silva, F.D.J.; Jardim, I.N.; Costa, V.A.; Matrangolo, C.A.R.; Ribeiro, R.C.F.; Silva, G.H. Activities of Essential Oils from Three Brazilian Plants and Benzaldehyde Analogues against Meloidogyne incognita. Nematology 2019, 21, 1081–1089.
- Moreira, F.; de Abreu Araújo, B.; Lopes, F.; Sousa, A.A.L.; Sousa, A.; Andrade, L.; Uchôa, A. Assessment of the Tephrosia toxicaria Essential Oil on Hatching and Mortality of Eggs and Second-Stage Juvenile (J2) Root-Knot Nematode (Meloidogyne enterolobii and M. javanica). Aust. J. Crop Sci. 2018, 12, 1829–1836.
- Ajith, M.; Pankaj; Shakil, N.A.; Kaushik, P.; Rana, V.S. Chemical Composition and Nematicidal Activity of Essential Oils and their Major Compounds against Meloidogyne Graminicola (Rice Root-Knot Nematode). J. Essent. Oil Res. 2020, 32, 526–535.
- Mamoci, E.; Andrés, M.F.; Olmeda, S.; González-Coloma, A. Chemical Composition and Activity of Essential Oils of Albanian Coniferous Plants on Plant Pests. Chem. Proc. 2022, 10, 15.
- Jindapunnapat, K.; Reetz, N.D.; MacDonald, M.H.; Bhagavathy, G.; Chinnasri, B.; Soonthornchareonnon, N.; Sasnarukkit, A.; Chauhan, K.R.; Chitwood, D.J.; Meyer, S.L.F. Activity of Vetiver Extracts and Essential Oil against Meloidogyne incognita. J. Nematol. 2018, 50, 147–162.
- Goyal, L.; Kaushal, S.; Dhillon, N.K.; Heena. Nematicidal Potential of Citrus reticulata Peel Essential Oil, Isolated Major Compound and Its Derivatives against Meloidogyne incognita. Arch. Phytopathol. Plant Prot. 2021, 54, 449–467.
- Arya, S.; Kumar, R.; Prakash, O.; Kumar, S.; Mahawer, S.K.; Chamoli, S.; Kumar, P.; Srivastava, R.M.; De Oliveira, M.S. Chemical Composition and Biological Activities of Hedychium coccineum Buch.-Ham. Ex Sm. Essential Oils from Kumaun Hills of Uttarakhand. Molecules 2022, 27, 4833.
- Araújo, A.M.N.; de Oliveira, J.V.; de França, S.M.; Navarro, D.M.d.A.F.; Barbosa, D.R.e.S.; Dutra, K.d.A. Toxicity and Repellency of Essential Oils in the Management of Sitophilus zeamais. Rev. Bras. Eng. Agríc. E Ambient. 2019, 23, 372–377.
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541.
- Ahmad, A.; Van Vuuren, S.; Viljoen, A. Unravelling the Complex Antimicrobial Interactions of Essential Oils—The Case of Thymus vulgaris (Thyme). Molecules 2014, 19, 2896–2910.
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and Antagonistic Interactions of Anticholinesterase Terpenoids in Salvia lavandulaefolia Essential Oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668.
- Połeć, K.; Wyżga, B.; Olechowska, K.; Hąc-Wydro, K. On the Synergy/Antagonism of Selected Terpenes in the Effect on Lipid Membranes Studied in Model Systems. J. Mol. Liq. 2022, 349, 118473.
- Jardim, I.N.; Oliveira, D.F.; Campos, V.P.; Silva, G.H.; Souza, P.E. Garlic Essential Oil Reduces the Population of Meloidogyne incognita in Tomato Plants. Eur. J. Plant Pathol. 2020, 157, 197–209.




