Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masahiro Kohzuki | -- | 2242 | 2024-01-29 06:13:22 | | | |
| 2 | Rita Xu | Meta information modification | 2242 | 2024-01-29 06:39:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kohzuki, M. Renal Rehabilitation. Encyclopedia. Available online: https://encyclopedia.pub/entry/54459 (accessed on 08 February 2026).
Kohzuki M. Renal Rehabilitation. Encyclopedia. Available at: https://encyclopedia.pub/entry/54459. Accessed February 08, 2026.
Kohzuki, Masahiro. "Renal Rehabilitation" Encyclopedia, https://encyclopedia.pub/entry/54459 (accessed February 08, 2026).
Kohzuki, M. (2024, January 29). Renal Rehabilitation. In Encyclopedia. https://encyclopedia.pub/entry/54459
Kohzuki, Masahiro. "Renal Rehabilitation." Encyclopedia. Web. 29 January, 2024.
Copy Citation
Chronic kidney disease (CKD) is a global health problem. In patients with CKD, exercise endurance is decreased, especially as renal dysfunction advances. This is due to the combined effects of protein-energy wasting, uremic acidosis, and inflammatory cachexia, which lead to sarcopenia and are aggravated by a sedentary lifestyle, resulting in a progressive downward spiral of deconditioning.
chronic kidney disease
exercise
rehabilitation
renal protection
cardio-renal syndrome
1. Introduction
Chronic kidney disease (CKD) is a global health problem. For example, the number of CKD patients in Japan is more than 11% of the total population. The number of patients undergoing hemodialysis (HD) in Japan is 349,700, corresponding to 1 in 359 of the total population in 2021 [1].
Further, CKD is associated with premature aging. Patients with CKD are characterized by frailty, osteoporosis, muscle wasting, cardiovascular hypertrophy, and vascular calcification [2]. Patients with CKD with dialysis have a very high mortality risk due to cardiovascular diseases such as chronic heart failure, and sedentary patients with CKD undergoing dialysis have an even higher mortality risk [3]. An independent, graded association has been found between a reduced glomerular filtration rate (GFR) and the risk of cardiovascular events, hospitalization, and death [4]. In addition to being a strong cardiovascular risk factor, physical inactivity is associated with an increased risk of rapid decline in renal function in patients with CKD [5].
In patients with CKD, exercise endurance is decreased, and this becomes more distinct as renal dysfunction advances. This is due to the combined effects of protein-energy wasting (PEW), uremic acidosis, and inflammatory cachexia, which lead to sarcopenia and are aggravated by a sedentary lifestyle. Collectively, these factors result in a progressive downward spiral of deconditioning [2].
Renal rehabilitation (RR) is a coordinated, multifaceted intervention designed to optimize a patient’s physical, psychological, and social functioning, as well as to stabilize, slow, or even reverse the progression of renal deterioration, improving exercise tolerance and preventing the onset and worsening of heart failure, thereby reducing morbidity and mortality. [6][7].
2. CKD and Physical Inactivity
Physical inactivity is a major health problem. Regular exercise is important for maintaining good health and preventing chronic diseases. Moreover, an association between physical inactivity and poor outcomes in patients with CKD has been well established [7][8][9]. Patients with CKD typically engage in lower levels of physical activity than the general population, which can induce a catabolic state, including reduced neuromuscular functioning, exercise tolerance, and cardiorespiratory fitness.
In addition to physical inactivity, cardiorespiratory (CR) fitness is an important consideration, as a strong predictor of mortality [10][11]. Low CR fitness has a particularly high risk of death compared to that for other common risk factors, such as dyslipidemia, hypertension, and diabetes. [12]. CR fitness is defined as the ability of the respiratory and circulatory systems to supply oxygen during physical activity, and is usually expressed as the maximal oxygen uptake (VO2 max) or peak oxygen uptake (peak VO2) during exercise testing [13]. VO2 max is the maximum rate of oxygen consumption attainable during physical exertion. A similar measure is peak VO2, which is the measurable value from a session of physical exercise. Be it incremental or otherwise, it could match or underestimate the actual VO2 max. Figure 1 shows the five major determinants of VO2 max, peak VO2, and their relationships in CKD [14]. The gears in Figure 1 represent the functional interdependence of the physiological components of the system. Pulmonary diffusion capacity, cardiac output, oxygen-carrying capacity, renal function, and other peripheral limitations such as capillary density, muscle diffusion capacity, and mitochondrial enzymes are all examples of VO2 determinants.
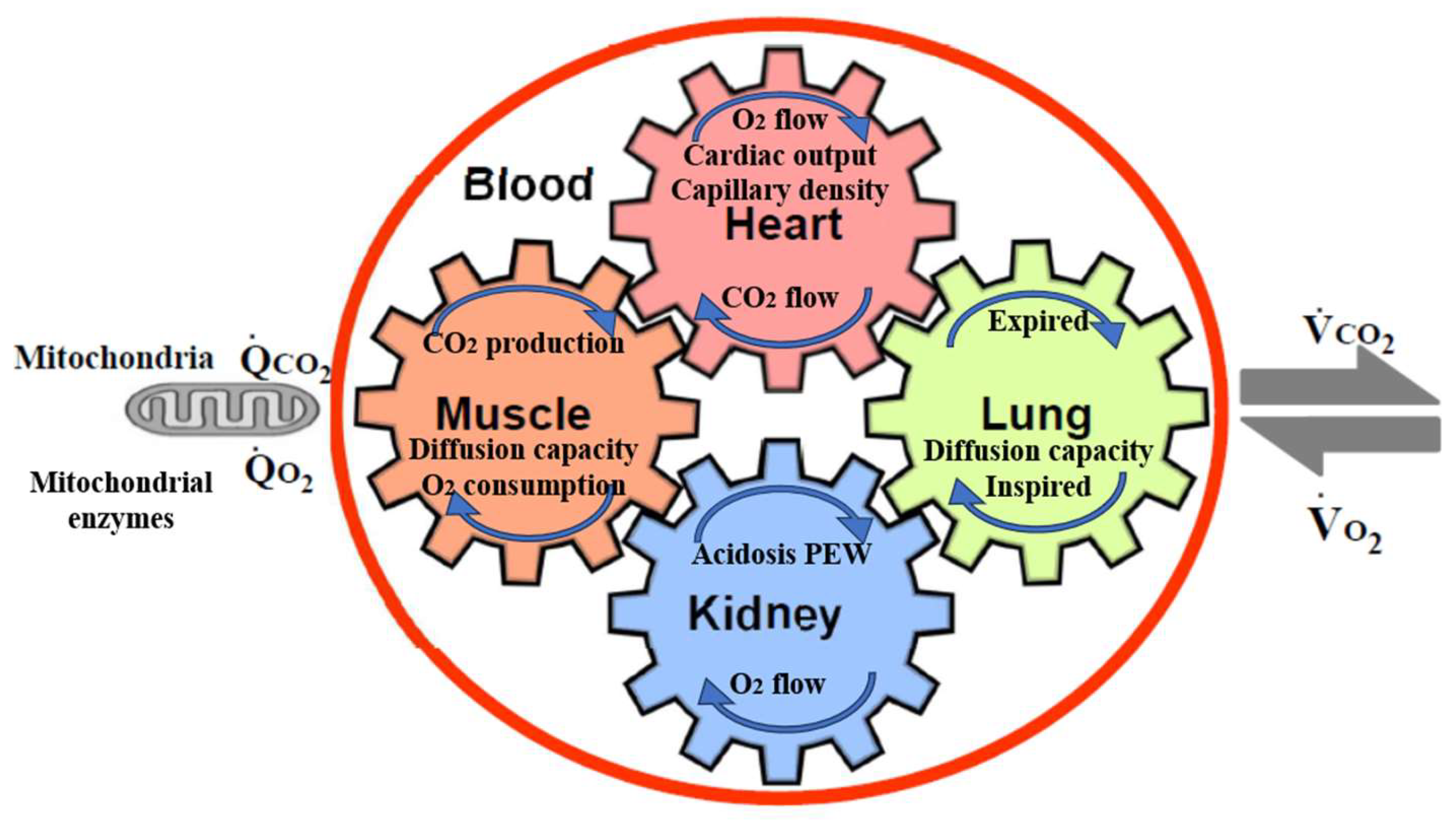
Figure 1. The five major determinants of VO2 max, peak VO2, and their relationships in CKD. The gears represent the functional interdependence of the physiological components of the system. Cardiac output, pulmonary diffusion capacity, oxygen-carrying capacity, renal function, metabolic acidosis, and other peripheral limitations, such as muscle diffusion capacity, mitochondrial enzymes, and capillary density, are all examples of VO2 max determinants. VO2, O2 uptake; VCO2, CO2 output; QCO2, CO2 production; QO2, O2 consumption by cells.
An increase in O2 utilization by the muscles (QO2) is achieved by an increase in ventilation, an increase in pulmonary blood flow by recruitment and vasodilatation of pulmonary blood vessels, an increase in cardiac output (stroke volume and heart rate), dilatation of selected peripheral vessels, and increased extraction of O2 from the blood perfusing the muscles. O2 is taken up from the alveoli (VO2) in proportion to the pulmonary blood flow and degree of O2 desaturation of hemoglobin in the pulmonary capillary blood. Metabolic acidosis in CKD patients promotes protein-energy wasting (PEW) [15], muscle protein wasting, and reducing protein synthesis [16][17]. In addition to sarcopenia by physical inactivity, PEW, metabolic acidosis, angiotensin II accumulation, and myostatin overexpression in uremia also contribute to the pathogenesis of muscle wasting, especially in CKD [18]. Erythropoietin can increase VO2 max in humans [19].
3. Chronic Effects of Exercise in CKD Animal Models
Evidence of the benefits of regular exercise in long-term conditions is accumulating. Further, the influence of chronic exercise on renal function must be considered, as acute exercise causes proteinuria, reduction in renal blood flow, and reduction in GFR. As it is shown clinically, sudden severe exercise decreases renal function [20][21]. However, such intense exercise cannot be performed for long. In other words, it is important to look at the effects of exercise over the long term. However, there is insufficient information regarding the influence of chronic exercise on renal function and the effect of exercise in pre-dialysis patients with CKD. For instance, the optimal duration and intensity of exercise for CKD patients with pre-dialysis has not yet been determined.
Since the late 1990s, my colleagues and I have published several papers in this field. Researchers assessed the renal effects of moderate treadmill chronic exercise in several CKD rat models and found that exercise does not worsen renal function and had renoprotective effects in some rat models, such as a remnant kidney model of genetic hypertensive rats [22], 5/6-nephrectomized rats [23], diabetic nephropathy rats [24], and Zucker diabetic rats [25].
4. Chronic Effect of Exercise in Patients with CKD Undergoing Dialysis
In the Dialysis Outcomes and Practice Patterns Study, patients with CKD undergoing dialysis who were regular exercisers had higher health-related quality of life (HR-QOL), sleep quality scores, and physical functioning; the study also reported fewer limitations in physical activities than those who were not regular exercisers [8]. Regular exercise was also correlated with more positive patient effects and fewer depressive symptoms [8]. Further, in models extensively adjusted for demographics and comorbidities, the mortality risk was lower with regular exercise and at facilities with more regular exercisers [8].
Meta-analyses of randomized controlled trials (RCTs) have reported that regular exercise training in patients with CKD undergoing dialysis (HD) has benefits in physical function, aerobic capacity, dialysis adequacy, depressive symptoms, and HR-QOL [26][27][28][29][30]. Additionally, a meta-analysis of combined aerobic and resistance exercises (CARE) performed during HD by Liu et al. [31] found that CARE improved the peak oxygen uptake; performance on the six-minute walking test; 60-s and 30-s sit-to-stand tests; dialysis adequacy; scores on five (out of eight) domains and the physical component summary for HR-QOL on the Medical Outcomes Study Short Form-36; blood pressure; and hemoglobin levels in patients on maintenance HD compared to those with usual care. Further, subgroup analysis showed that intradialytic CARE resulted in the amelioration of more evaluated outcomes than non-intradialytic CARE, with the exception of handgrip strength and hemoglobin levels [31]. The authors concluded that CARE is an effective way to improve physical function, aerobic capacity, HR-QOL, and dialysis adequacy in patients on maintenance HD [31].
5. Chronic Effect of Exercise in Pre-Dialysis Patients with CKD
In the first RCT on the effect of exercise in CKD patients with pre-dialysis, reported by Baria et al. [32], sedentary pre-dialysis men with CKD (creatine-based estimated GFR) were randomly assigned to home-based exercise group, a center-based exercise group, or control group. In the exercise group, aerobic exercise was done three times per week for 12 weeks. During the study period, eGFRcreat was increased by 3.6 ± 4.6 mL/min (p = 0.03) in the center-based group, but remained unchanged in the control group [32]. Further, in a single-blind randomized controlled study of the effects of moderate-intensity regular exercise on renal function and indices of cardiovascular risk in patients with stages 3–4 CKD by Greenwood et al., there was a significant difference in the rate of change in eGFRcreat between exercise and usual care groups, with the exercise group showing a slower decline in function [33].
Chen et al. reported the associations between walking, mortality, and renal replacement therapies (RRTs), such as peritoneal dialysis, HD, and kidney transplantation, in patients with stages 3–5 CKD [34]. Among 6363 patients (mean age, 70 years), 1341 (21.1%) reported walking as their most common form of exercise. The rate of mortality was lower in walking patients than in non-walking patients (2.7 vs. 5.4 per 100 person-years). Similarly, the incidence of RRT was significantly lower in walking patients than in non-walking patients (22 vs. 32.9 per 100 person-years) [34]. Further, walking was associated with lower mortality and RRT risk on multivariate regression. The adjusted sub-distribution hazard ratio (SHR) of walking was 0.67 (p < 0.001) for overall mortality and 0.79 (p < 0.001) for the risk of RRT. Further, the SHRs of overall mortality were 0.83, 0.72, 0.42, and 0.41. Thus, walking is the most popular form of exercise for patients with CKD and is associated with a lower risk of mortality and RRT. [34].
Recently, Ma et al. reported a meta-analysis of 12 RCTs, comprising 410 patients with CKD; the results indicated that regular aerobic exercise significantly improves the estimated GFR (eGFR), and the levels of serum creatinine, daily urinary protein excretion, and serum urea nitrogen in CKD patients. Further, a single exercise session of more than 30 min was associated with significantly improved eGFR (p < 0.01), including walking and running, but not cycling, as exercise modalities were associated with significantly improved serum creatinine levels (p < 0.05) in CKD patients (Figure 2 and Figure 3) [35]. Thus, regular aerobic exercise has beneficial effects on the eGFR (especially with durations longer than 30 min), serum creatinine (especially with walking or running as the modality), daily urinary protein excretion, and blood urea nitrogen levels in CKD patients [35].
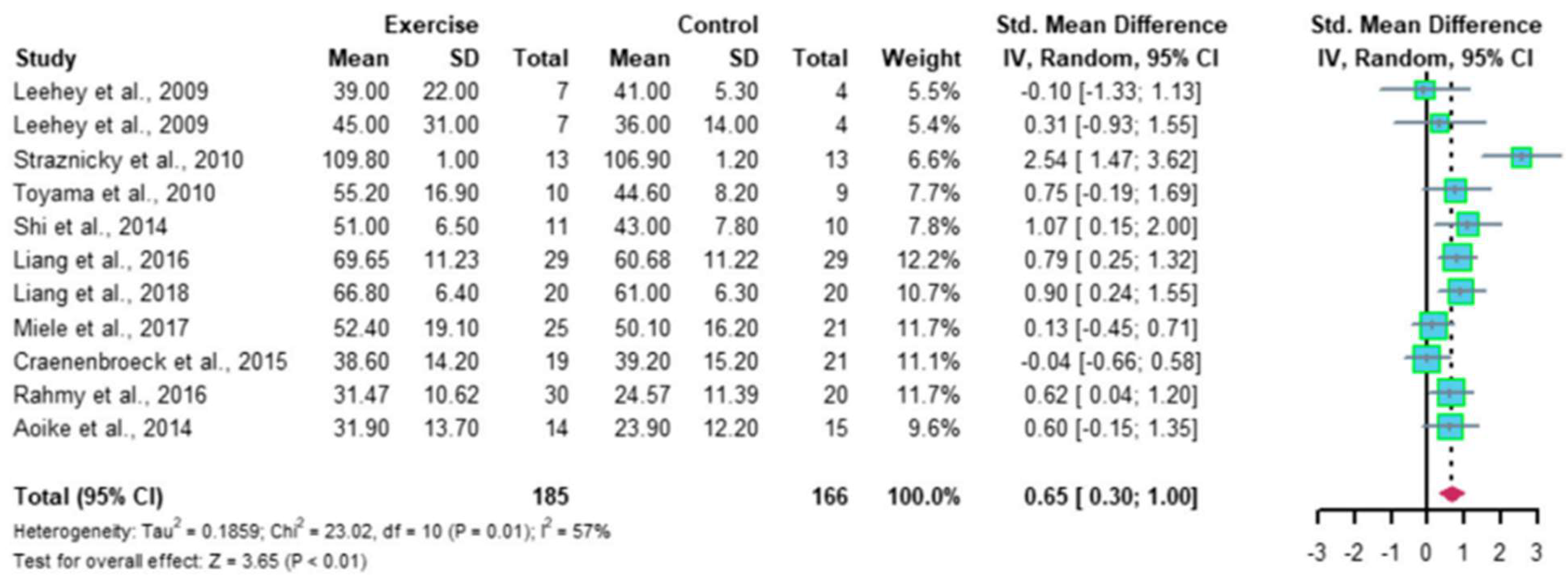
Figure 2. Meta-analysis of the effect of aerobic exercise on eGFR. The results indicate that regular aerobic exercise significantly improves the eGFR. eGFR, estimated glomerular filtration rate; CI, confidence interval; SD, standard deviation.
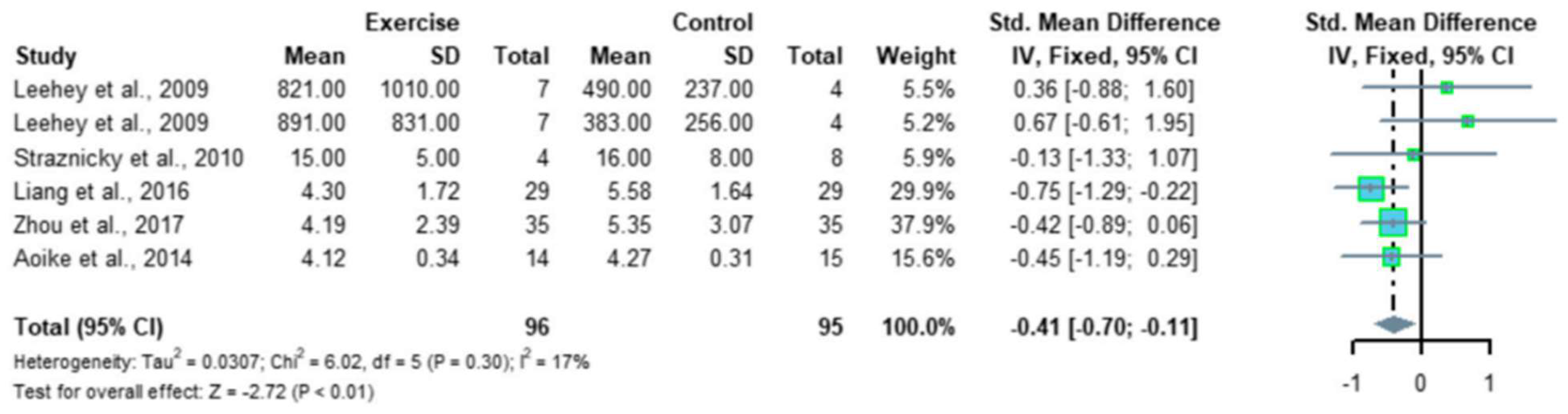
Figure 3. Meta-analysis of the effect of aerobic exercise on 24-h urinary protein excretion. The results indicate that regular aerobic exercise significantly improves the 24-h urine protein volume in patients with chronic kidney disease. CI, confidence interval; SD, standard deviation.
6. Chronic Effect of Exercise in Pre-Dialysis CKD Patients with Acute Myocardial Infarction (AMI)
CKD is common in patients with diabetes mellitus, occurring in approximately 40% of cases. Diabetes mellitus is also an important risk factor for cardiovascular diseases; however, CKD is an important mediator of this risk [36]. Kidney function is closely linked to heart function. Renal dysfunction/disease may initiate, accentuate, or precipitate cardiac dysfunction/disease, and vice versa [37].
Combined, renal dysfunction worsens the prognosis after AMI. My colleagues and I investigated the association between physical activity levels and renal function changes in AMI patients [38]. Renal function was measured using the cystatin C-based eGFR (eGFRcys), which is independent of muscle mass. Patients were stratified into a low exercise group (2335 ± 1219 steps/day) and a high exercise group (7102 ± 2365 steps/day). eGFRcys was significantly increased after 3 months of exercise in the high exercise group, whereas no significant change was observed in the low exercise group. Further, the change in eGFRcys was greater in the high exercise group (+6.7 mL/min/1.73 m2) than in the low exercise group (−2.9 mL/min/1.73 m2) [38]. The physical activity level was positively associated with renal function changes, demonstrating that high physical activity levels may suppress renal function decline in AMI patients. Figure 4 indicates the association between the number of steps and eGFRcys or eGFRcreat [38]. Pearson’s correlation analysis revealed significant correlations between the number of daily steps and both eGFR parameters. Furthermore, the coefficient was greater for the correlation between ΔeGFRcys and the number of daily steps (r = 0.55, p < 0.001) than between ΔeGFRcreat and the number of daily steps (r = 0.38, p = 0.015). As previously indicated, changes in serum creatinine levels can be caused by changes in skeletal muscles through exercise, highlighting the importance of using eGFRcys as an indicator of renal function [39][40]. A recent prospective study verified the association between physical activity levels and renal function in patients with CKD [39]. The results were similar to those in the above-mentioned study and indicated that maintaining a high level of physical activity in daily life leads to the suppression of renal function deterioration [41]. However, the study was the first to show an association between physical activity level and changes in renal function after the onset of AMI using an accelerometer and eGFRcys. The findings support the importance of interventions to maintain a high physical activity level as a strategy for renal protection in patients with AMI. Future research should verify the long-term effects of physical activity on renal function in patients with AMI.
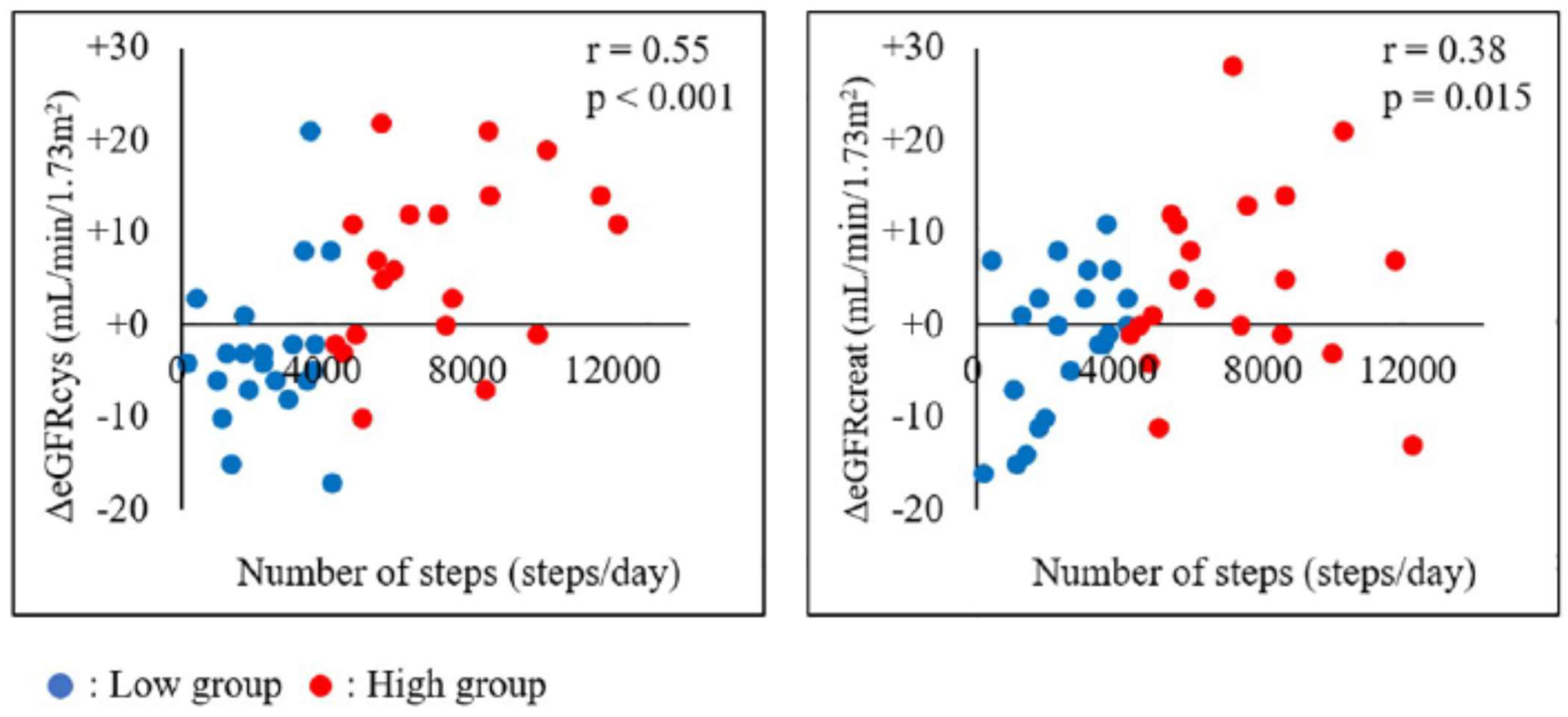
Figure 4. Association between the number of steps and ΔeGFRcys or ΔeGFRcreat. The association between the number of steps and eGFRcys or eGFRcreat in all patients is shown. Pearson’s correlation analysis revealed significant correlations between the number of steps and both eGFR parameters, with a higher correlation between ΔeGFRcys and the number of steps than between ΔeGFRcreat and the number of steps. eGFRcreat, creatine-based estimated glomerular filtration rate; eGFRcys, cystatin C-based estimated glomerular filtration rate.
There is immense potential for research in the field of cardio-nephrology, in terms of diagnosis, prognosis, complication risk evaluation, and the utilization of novel therapeutic approaches for CKD patients and associated cardiovascular complications. However, significant advancements have been made to improve patient care and outcomes in patients with CKD [42].
References
- The Japanese Society for Dialysis Therapy. Available online: https://docs.jsdt.or.jp/overview/index.html (accessed on 23 November 2023). (In Japanese).
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742.
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454.
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305.
- Johansen, K.L. Exercise in the End-Stage Renal Disease Population. J. Am. Soc. Nephrol. 2007, 18, 1845–1854.
- Kohzuki, M. Renal rehabilitation: Definition and evidence. In Renal Rehabilitation; Kohzuki, M., Ed.; Ishiyaku Publishers, Inc.: Tokyo, Japan, 2012; pp. 10–17. (In Japanese)
- Kohzuki, M. Renal rehabilitation: Present and future perspectives. In Hemodialysis; Suzuki, H., Ed.; Intech: Oakville, ON, Canada, 2013; pp. 743–751.
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transplant. 2010, 25, 3050–3062.
- Smart, N.; Steele, M. Exercise training in haemodialysis patients: A systematic review and meta-analysis. Nephrology 2011, 16, 626–632.
- Sieverdes, J.C.; Sui, X.; Lee, D.C.; Church, T.S.; McClain, A.; Hand, G.A.; Blair, S.N. Physical activity, cardiorespiratory fitness and the incidence of type 2 diabetes in a prospec-tive study of men. Br. J. Sports Med. 2010, 44, 238–244.
- Blair, S.N.; Kohl, H.W., III; Paffenbarger, R.S., Jr.; Clark, D.G.; Cooper, K.H. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401.
- Blair, S.N.; Sallis, R.E.; Hutber, A.; Archer, E. Exercise therapy—The public health message. Scand. J. Med. Sci. Sports 2012, 22, e24–e28.
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131.
- Kohzuki, M. New Ideas on Limitations to VO2max: Five Major Determinants for VO2max. Pulm. Res. Respir. Med. Open J. 2018, 5, e1–e2.
- Caso, G.; Garlick, P.J. Control of muscle protein kinetics by acid-base balance. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 73–76.
- Bailey, J.L.; Wang, X.; England, B.K.; Price, S.R.; Ding, X.; Mitch, W.E. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Investig. 1996, 97, 1447–1453.
- Mitch, W.E. Influence of metabolic acidosis on nutrition. Am. J. Kidney Dis. 1997, 29, XLVI–XLVIII.
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665.
- Kolb, E.M.; Kelly, S.A.; Middleton, K.M.; Sermsakdi, L.S.; Chappell, M.A.; Garland, T., Jr. Erythropoietin elevates VO2, max but not voluntary wheel running in mice. J. Exp. Biol. 2010, 213, 510–519.
- Bach, T.M.; Clement, D.B. Exercise induced acute renal failure in an athlete. Can. Fam. Physician 1980, 26, 591–595.
- Jackson, C.R. Exercise-induced renal failure and muscle damage. Proc. R. Soc. Med. 1970, 63, 566–570.
- Kohzuki, M.; Kamimoto, M.; Wu, X.M.; Xu, H.L.; Kawamura, T.; Mori, N.; Nagasaka, M.; Kurosawa, H.; Minami, N.; Kanazawa, M.; et al. Renal protective effects of chronic exercise and antihypertensive therapy in hyper-tensive rats with chronic renal failure. J. Hypertens. 2001, 19, 1877–1882.
- Kanazawa, M.; Kawamura, T.; Li, L.; Sasaki, Y.; Matsumoto, K.; Kataoka, H.; Ito, O.; Minami, N.; Sato, T.; Ootaka, T.; et al. Combination of Exercise and Enalapril Enhances Renoprotective and Peripheral Effects in Rats With Renal Ablation. Am. J. Hypertens. 2006, 19, 80–86.
- Tufescu, A.; Kanazawa, M.; Ishida, A.; Lu, H.; Sasaki, Y.; Ootaka, T.; Sato, T.; Kohzuki, M. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J. Hypertens. 2008, 26, 312–321.
- Ito, D.; Cao, P.; Kakihana, T.; Sato, E.; Suda, C.; Muroya, Y.; Ogawa, Y.; Hu, G.; Ishii, T.; Ito, O.; et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 2015, 10, e0138037.
- Bogataj, S.; Pajek, M.; Pajek, J.; Ponikvar, J.B.; Paravlic, A.H. Exercise-based interventions in hemodialysis patients: A systematic review with a me-ta-analysis of randomized controlled trials. J. Clin. Med. 2019, 9, 43.
- Hu, H.; Liu, X.; Chau, P.H.; Choi, E.P.H. Effects of intradialytic exercise on health-related quality of life in patients undergoing mainte-nance haemodialysis: A systematic review and meta-analysis. Qual. Life Res. 2022, 31, 1915–1932.
- Scapini, K.B.; Bohlke, M.; Moraes, O.A.; Rodrigues, C.G.; Inácio, H.F.; Sbruzzi, G.; Leguisamo, C.P.; Sanches, I.C.; Filho, H.T.; Irigoyen, M.C. Combined training is the most effective training modality to improve aerobic capaci-ty and blood pressure control in people requiring haemodialysis for endstage renal disease: Systematic review and network meta-analysis. J. Physiother. 2019, 65, 4–15.
- Sheng, K.; Zhang, P.; Chen, L.; Wu, C.; Chen, J. Intradialytic exercise in hemodialysis patients: A systematic review and meta-analysis. Am. J. Nephrol. 2014, 40, 478–490.
- Song, Y.-Y.; Hu, R.-J.; Diao, Y.-S.; Chen, L.; Jiang, X.-L. Effects of Exercise Training on Restless Legs Syndrome, Depression, Sleep Quality, and Fatigue Among Hemodialysis Patients: A Systematic Review and Meta-analysis. J. Pain Symptom Manag. 2018, 55, 1184–1195.
- Liu, Y.; Luo, X.; Deng, S.; Chen, J.; Zhang, L.; Huang, Y.; Hu, H. Combined aerobic and resistance exercise in maintenance hemodialysis patients: A meta-analysis. Semin. Dial. 2023, 36, 278–293.
- Baria, F.; Kamimura, M.A.; Aoike, D.T.; Ammirati, A.; Rocha, M.L.; de Mello, M.T.; Cuppari, L. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol. Dial. Transplant. 2014, 29, 857–864.
- Greenwood, S.A.; Koufaki, P.; Mercer, T.H.; MacLaughlin, H.; Rush, R.; Lindup, H.; O’Connor, E.M.; Jones, C.; Hendry, B.; Macdougall, I.; et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespira-tory fitness in patients with CKD: A pilot randomized controlled trial. Am. J. Kidney Dis. 2015, 65, 425–434.
- Chen, I.-R.; Wang, S.-M.; Liang, C.-C.; Kuo, H.-L.; Chang, C.-T.; Liu, J.-H.; Lin, H.-H.; Wang, I.-K.; Yang, Y.-F.; Chou, C.-Y.; et al. Association of Walking with Survival and RRT Among Patients with CKD Stages 3–5. Clin. J. Am. Soc. Nephrol. 2014, 9, 1183–1189.
- Ma, Q.; Gao, Y.; Lu, J.; Liu, X.; Wang, R.; Shi, Y.; Liu, J.; Su, H. The effect of regular aerobic exercise on renal function in patients with CKD: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 901164.
- Swamy, S.; Noor, S.M.; Mathew, R.O. Cardiovascular Disease in Diabetes and Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6984.
- Xanthopoulos, A.; Papamichail, A.; Briasoulis, A.; Loritis, K.; Bourazana, A.; Magouliotis, D.E.; Sarafidis, P.; Stefanidis, I.; Skoularigis, J.; Triposkiadis, F. Heart Failure in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6105.
- Sato, T.; Kohzuki, M.; Ono, M.; Muto, M.; Osugi, T.; Kawamura, K.; Naganuma, W.; Sato, M.; Shishito, N. Association between physical activity and change in renal function in patients after acute myocardial infarction. PLoS ONE 2019, 14, e0212100.
- Séronie-Vivien, S.; Delanaye, P.; Piéroni, L.; Mariat, C.; Froissart, M.; Cristol, J.-P. Cystatin C: Current position and future prospects. Clin. Chem. Lab. Med. 2008, 46, 1664–1686.
- Poortmans, J.R.; Gulbis, B.; De Bruyn, E.; Baudry, S.; Carpentier, A. Limitations of serum values to estimate glomerular filtration rate during exercise. Br. J. Sports Med. 2012, 47, 1166–1170.
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Weiss, N.S.; Sachs, M.C.; Ruzinski, J.; Kundzins, J.; Rock, D.; de Boer, I.H.; Ikizler, T.A.; et al. Physical Activity and Change in Estimated GFR among Persons with CKD. J. Am. Soc. Nephrol. 2014, 25, 399–406.
- Burlacu, A.; Covic, A. Special Issue: “Cardiovascular Complications in Renal Diseases”. J. Clin. Med. 2023, 12, 5307.
More
Information
Subjects:
Medicine, General & Internal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
555
Revisions:
2 times
(View History)
Update Date:
29 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




