
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rene Garcia-Contreras | -- | 4295 | 2024-01-26 23:10:10 | | | |
| 2 | Jason Zhu | Meta information modification | 4295 | 2024-01-29 02:47:06 | | |
Video Upload Options
Biotechnology and artificial intelligence have sparked a revolution in dentistry, with a focus on restoring natural tissue functions. This transformation has given rise to bioactive materials, inspired by biomimetics, aimed at replicating the processes found in nature. As synthetic biology advances, there is a heightened focus on signaling systems crucial for bio-based diagnostics and therapeutics. Dentistry now harnesses synthetic proteins for tissue regeneration and dental material enhancement. A current research priority is bacterial biofilm inhibition, vital for dental health. Given the role of Streptococcus mutans in dental caries, the development of synthetic antimicrobial peptides targeting this bacterium is underway. The balance of dental enamel between demineralization and remineralization impacts caries formation. Factors such as the presence of hydroxyapatite and salivary peptides influence enamel health. Recent studies have spotlighted salivary protein-inspired peptides for enhanced remineralization.
1. Introduction
2. Bacterial Biofilm Inhibition
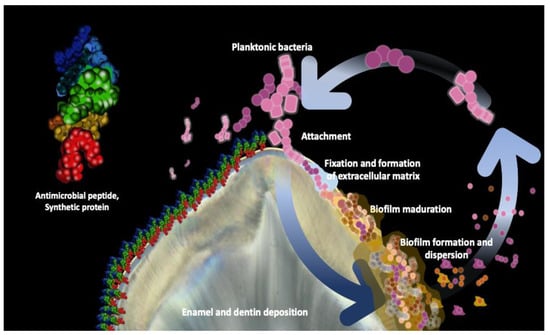
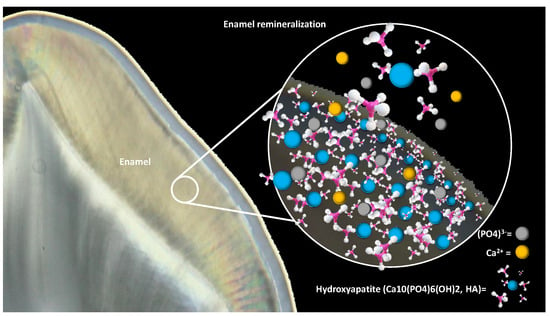
3. Enamel Remineralization
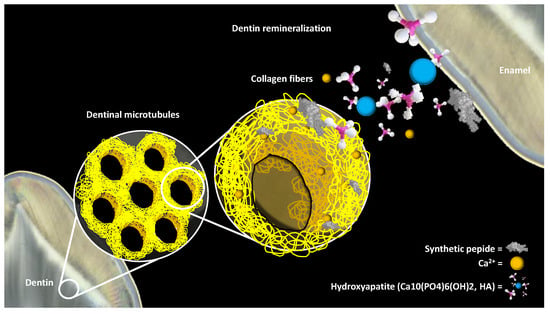
4. Stimulation of the Dentin-Pulp Complex
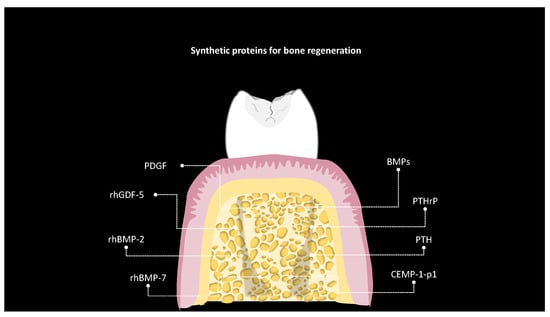
5. Bone Regeneration
6. Challenges and Limitations
7. Ethical Considerations and Regulatory Aspects
References
- Patil, S.; Albogami, S.; Hosmani, J.; Mujoo, S.; Kamil, M.A.; Mansour, M.A.; Abdul, H.N.; Bhandi, S.; Ahmed, S.S.S.J. Artificial Intelligence in the Diagnosis of Oral Diseases: Applications and Pitfalls. Diagnostics 2022, 12, 1029.
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral. Health 2023, 23, 105.
- Kumar, N.; Maher, N.; Amin, F.; Ghabbani, H.; Zafar, M.S.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Biomimetic Approaches in Clinical Endodontics. Biomimetics 2022, 7, 229.
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31.
- Hanczyc, M.M. Engineering Life: A Review of Synthetic Biology. Artif. Life 2020, 26, 260–273.
- Stein, V.; Alexandrov, K. Synthetic protein switches: Design principles and applications. Trends Biotechnol. 2015, 33, 101–110.
- Khoury, G.A.; Smadbeck, J.; Kieslich, C.A.; Floudas, C.A. Protein folding and de novo protein design for biotechnological applications. Trends Biotechnol. 2014, 32, 99–109.
- Zheng, X.; Wu, Y.; Bi, J.; Huang, Y.; Cheng, Y.; Li, Y.; Wu, Y.; Cao, G.; Tian, Z. The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy. Cell. Mol. Immunol. 2022, 19, 192–209.
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of collagen and mesenchymal stem cells in regenerative dentistry. Curr. Stem. Cell Res. Ther. 2022, 17, 606–620.
- Tavelli, L.; Chen, C.Y.; Barootchi, S.; Kim, D.M. Efficacy of biologics for the treatment of periodontal infrabony defects: An American Academy of Periodontology best evidence systematic review and network meta-analysis. J. Periodontol. 2022, 93, 1803–1826.
- Firipis, K.; Nisbet, D.R.; Franks, S.J.; Kapsa, R.M.I.; Pirogova, E.; Williams, R.J.; Quigley, A. Enhancing Peptide Biomaterials for Biofabrication. Polymers 2021, 13, 2590.
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-engineered functional materials. Adv. Healthc. Mater. 2019, 8, 1801374.
- Takahara, M.; Kamiya, N. Synthetic strategies for artificial lipidation of functional proteins. Chemistry 2020, 26, 4645–4655.
- Ahn, W.; Lee, J.H.; Kim, S.R.; Lee, J.; Lee, E.J. Designed protein-and peptide-based hydrogels for biomedical sciences. J. Mater. Chem. B 2021, 9, 1919–1940.
- Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Engineered tropoelastin and elastin-based biomaterials. Adv. Protein Chem. Struct. Biol. 2009, 78, 1–24.
- Fang, D.; Yuran, S.; Reches, M.; Catunda, R.; Levin, L.; Febbraio, M. A peptide coating preventing the attachment of Porphyromonas gingivalis on the surfaces of dental implants. J. Periodontal. Res. 2020, 55, 503–510.
- Babaji, P.; Melkundi, M.; Bhagwat, P.; Mehta, V. An in vitro Evaluation of remineralizing capacity of self-assembling peptide (SAP) P11-4 and casein phosphopeptides-amorphous calcium phosphate (CPP-ACP) on artificial enamel. Pesqui. Bras. Odontopediatria Clínica Integr. 2019, 19.
- Chen, X.; Zhou, X.C.; Liu, S.; Wu, R.F.; Aparicio, C.; Wu, J.Y. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76.
- Bagno, A.; Piovan, A.; Dettin, M.; Chiarion, A.; Brun, P.; Gambaretto, R.; Fontana, G.; Di Bello, C.; Palù, G.; Castagliuolo, I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone 2007, 40, 693–699.
- Dettin, M.; Conconi, M.T.; Gambaretto, R.; Pasquato, A.; Folin, M.; Di Bello, C.; Parnigotto, P.P. Novel osteoblast-adhesive peptides for dental/orthopedic biomaterials. J. Biomed. Mater. Res. 2002, 60, 466–471.
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753.
- Mathur, V.P.; Dhillon, J.K. Dental caries: A disease which needs attention. Indian J. Pediatr. 2018, 85, 202–206.
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmana, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722.
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759.
- Yang, Y.; Xia, L.; Haapasalo, M.; Wei, W.; Zhang, D.; Ma, J.; Shen, Y. A novel hydroxyapatite-binding antimicrobial peptide against oral biofilms. Clin. Oral Investig. 2019, 23, 2705–2712.
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56.
- Da Silva, B.R.; Conrado, A.J.S.; Pereira, A.L.; Evaristo, F.F.V.; Arruda, F.V.S.; Vasconcelos, M.A.; Lorenzón, E.N.; Cilli, E.M.; Teixeira, E.H. Antibacterial activity of a novel antimicrobial peptide KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling 2017, 33, 835–846.
- Da Silva, B.R.; De Freitas, V.A.A.; Carneiro, V.A.; Arruda, F.V.S.; Lorenzón, E.N.; De Aguiar, A.S.W.; Cilli, E.M.; Cavada, E.H.; Teixeira, E.H. Antimicrobial activity of the synthetic peptide Lys-a1 against oral streptococci. Peptides 2013, 42, 78–83.
- He, J.; Yarbrough, D.K.; Kreth, J.; Anderson, M.H.; Shi, W.; Eckert, R. Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob. Agents Chemother. 2010, 54, 2143–2151.
- Izadi, N.; Keikha, M.; Ghazvini, K.; Karbalaei, M. Oral antimicrobial peptides and new therapeutic strategies for plaque-mediated diseases. Gene Rep. 2020, 21, 100811.
- Zhou, L.; Wong, H.M.; Zhang, Y.Y.; Li, Q.L. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl. Mater. Interfaces 2019, 12, 3021–3031.
- Zhang, L.Y.; Fang, Z.H.; Li, Q.L.; Cao, C.Y. A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J. Mater. Sci. Mater. Med. 2019, 30, 1–9.
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50.
- Xu, C.; Yao, X.; Walker, M.P.; Wang, Y. Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif. Tissue Int. 2009, 84, 221–228.
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. Int. Sch. Res. Not. 2013, 2013, 684607.
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171.
- Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019, 53, 284–295.
- Kastenbom, L.; Falsen, A.; Larsson, P.; Sunnegårdh-Grönberg, K.; Davidson, T. Costs and health-related quality of life in relation to caries. BMC Oral Health 2019, 19, 187.
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative evaluation of effect of nanohydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: Double blind randomized clinical trial. Acta. Med. 2017, 60, 114–119.
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658.
- Valente, M.T.; Moffa, E.B.; Crosara, K.T.B.; Xiao, Y.; de Oliveira, T.M.; Machado, M.A.D.A.M.; Siqueira, W.L. Acquired enamel pellicle engineered peptides: Effects on hydroxyapatite crystal growth. Sci. Rep. 2018, 8, 3766.
- Yarbrough, D.K.; Hagerman, E.; Eckert, R.; He, J.; Choi, H.; Cao, N.; Le, K.; Hedger, J.; Qi, F.; Anderson, M.; et al. Specific binding and mineralization of calcified surfaces by small peptides. Calcif. Tissue Int. 2010, 86, 58–66.
- Li, Y.; Li, Y.; Bai, Q.; Wen, M.; Ma, D.; Lin, Y.; Chu, J. Recombinant amelogenin peptide TRAP promoting remineralization of early enamel caries: An in vitro study. Front. Physiol. 2023, 14, 1076265.
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Engineered salivary peptides reduce enamel demineralization provoked by cariogenic S. Mutans biofilm. Microorganisms 2022, 10, 742.
- Li, Z.C.; Xi, Q.I.N.; Qian, R.E.N.; Die, H.U.; Tian, T.I.A.N.; Ting, H.E.; Li, W.; Zhang, L.L. Rational Design of β-sheet Peptides with Self-Assembly into Nanofibres on Remineralisation of Initial Caries Lesions. Chin. J. Dent. Res. 2020, 23, 131–141.
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Modulation of Streptococcus mutans Adherence to Hydroxyapatite by Engineered Salivary Peptides. Microorganisms 2022, 10, 223.
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 2017, 96, 524–530.
- Ding, L.; Han, S.; Wang, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 7, 283–292.
- Hossein, B.G.; Sadr, A.; Espigares, J.; Hariri, I.; Nakashima, S.; Hamba, H.; Shafiei, F.; Moztarzadeh, F.; Tagami, J. Study on the influence of leucine-rich amelogenin peptide (LRAP) on the remineralization of enamel defects via micro-focus x-ray computed tomography and nanoindentation. Biomed. Mater. 2015, 10, 035007.
- Dissanayake, S.S.; Ekambaram, M.; Li, K.C.; Harris, P.W.; Brimble, M.A. Identification of key functional motifs of native amelogenin protein for dental enamel remineralisation. Molecules 2020, 25, 4214.
- Wang, D.; Deng, J.; Deng, X.; Fang, C.; Zhang, X.; Yang, P. Controlling enamel remineralization by amyloid-like amelogenin mimics. Adv. Mater. 2020, 32, e2002080.
- Chu, J.; Feng, X.; Guo, H.; Zhang, T.; Zhao, H.; Zhang, Q. Remineralization efficacy of an amelogenin-based synthetic peptide on carious lesions. Front. Physiol. 2018, 9, 842.
- Zheng, W.; Ding, L.; Wang, Y.; Han, S.; Zheng, S.; Guo, Q.; Li, W.; Zhou, X.; Zhang, L. The effects of 8DSS peptide on remineralization in a rat model of enamel caries evaluated by two nondestructive techniques. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827798.
- Han, S.; Peng, X.; Ding, L.; Lu, J.; Liu, Z.; Wang, K.; Zhang, L. TVH-19, a synthetic peptide, induces mineralization of dental pulp cells in vitro and formation of tertiary dentin in vivo. Biochem. Biophys. Res. Commun. 2021, 534, 837–842.
- Machla, F.; Angelopoulos, I.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A. Biomolecule-Mediated Therapeutics of the Dentin-Pulp Complex: A Systematic Review. Biomolecules 2022, 12, 285.
- Zou, J.; Mao, J.; Shi, X. Influencing factors of pulp-dentin complex regeneration and related biological strategies. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 350–361.
- McGuire, J.D.; Walker, M.P.; Mousa, A.; Wang, Y.; Gorski, J.P. Type VII collagen is enriched in the enamel organic matrix associated with the dentin-enamel junction of mature human teeth. Bone 2014, 63, 29–35.
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int. J. Nanomed. 2020, 15, 6631–6647.
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833.
- Li, Z.; Ren, Q.; Han, S.; Ding, L.; Qin, X.; Hu, D.; He, T.; Tian, T.; Lu, Z.; Zhang, L. Promoting effect of a calcium-responsive self-assembly β-sheet peptide on collagen intrafibrillar mineralization. Regen Biomater. 2022, 9, rbac059.
- Cloyd, A.K.; Boone, K.; Ye, Q.; Snead, M.L.; Spencer, P.; Tamerler, C. Engineered Peptides Enable Biomimetic Route for Collagen Intrafibrillar Mineralization. Int. J. Mol. Sci. 2023, 24, 6355.
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2023.
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103.
- Roberto-Rodrigues, M.; Fernandes, R.M.P.; Senos, R.; Scoralick, A.C.D.; Bastos, A.L.; Santos, T.M.P.; Viana, L.P.; Lima, I.; Guzman-Silva, M.A.; Kfoury-Júnior, J.R. Novel rat model of nonunion fracture with vascular deficit. Injury 2015, 46, 649–654.
- Díaz-Sánchez, R.M.; Yáñez-Vico, R.M.; Fernández-Olavarría, A.; Mosquera-Pérez, R.; Iglesias-Linares, A.; Torres-Lagares, D. Current approaches of bone morphogenetic proteins in dentistry. J. Oral Implantol. 2015, 41, 337–342.
- Schwarz, F.; Ferrari, D.; Sager, M.; Herten, M.; Hartig, B.; Becker, J. Guided bone regeneration using rhGDF-5-and rhBMP-2-coated natural bone mineral in rat calvarial defects. Clin. Oral Implants Res. 2009, 20, 1219–1230.
- Dupoirieux, L.; Pohl, J.; Hanke, M.; Pourquier, D. A preliminary report on the effect of dimeric rhGDF-5 and its monomeric form rhGDF-5C465A on bone healing of rat cranial defects. J. Craniomaxillofac. Surg. 2009, 37, 30–35.
- Lee, J.S.; Wikesjö, U.M.E.; Jung, U.W.; Choi, S.H.; Pippig, S.; Siedler, M.; Kim, C.K. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 in a β-tricalcium phosphate carrier into one-wall intrabony defects in dogs. J. Clin. Periodontol. 2010, 37, 382–389.
- Ho, S.K.C.; Frcd, C.; Peel, S.A.F.; Hu, Z.M. Augmentation of the maxillary sinus: Comparison of bioimplants containing bone morphogenetic protein and autogenous bone in a rabbit model. J. Can. Dent. Assoc. 2010, 76, a108.
- Ayoub, A.; Challa, S.R.R.; Abu-Serriah, M.; McMahon, J.; Moos, K.; Creanor, S.; Odell, E. Use of a composite pedicled muscle flap and rhBMP-7 for mandibular reconstruction. Int. J. Oral Maxillofac. Surg. 2007, 36, 1183–1192.
- Whitfield, J.F.; Morley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat. Endocrinol. 2002, 1, 175–190.
- Qin, L.; Raggatt, L.J.; Partridge, N.C. Parathyroid hormone: A double-edged sword for bone metabolism. Trends Endocrinol. Metab. 2004, 15, 60–65.
- Hsu, C.; He, Z.; Le Henaff, C.; Partridge, N.C. Differential effects of parathyroid hormone, parathyroid hormone-related protein, and abaloparatide on collagen 1 expression by mouse cementoblasts and mouse tooth root density. Am. J. Orthod. Dentofac. Orthop. 2023, 163, 378–388.e1.
- Safaei, M.; Mobini, G.R.; Abiri, A.; Shojaeian, A. Synthetic biology in various cellular and molecular fields: Applications, limitations, and perspective. Mol. Biol. Rep. 2020, 47, 6207–6216.
- Trump, B.D.; Cegan, J.C.; Wells, E.; Keisler, J.; Linkov, I. A critical juncture for synthetic biology: Lessons from nanotechnology could inform public discourse and further development of synthetic biology. EMBO Rep. 2018, 19, e46153.




