Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valentina Biagioli | -- | 1923 | 2024-01-26 08:18:46 | | | |
| 2 | Lindsay Dong | Meta information modification | 1923 | 2024-01-29 02:03:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Biagioli, V.; Volpedo, G.; Riva, A.; Mainardi, P.; Striano, P. Microbiota in Newborn from Birth to Weaning. Encyclopedia. Available online: https://encyclopedia.pub/entry/54392 (accessed on 07 February 2026).
Biagioli V, Volpedo G, Riva A, Mainardi P, Striano P. Microbiota in Newborn from Birth to Weaning. Encyclopedia. Available at: https://encyclopedia.pub/entry/54392. Accessed February 07, 2026.
Biagioli, Valentina, Greta Volpedo, Antonella Riva, Paolo Mainardi, Pasquale Striano. "Microbiota in Newborn from Birth to Weaning" Encyclopedia, https://encyclopedia.pub/entry/54392 (accessed February 07, 2026).
Biagioli, V., Volpedo, G., Riva, A., Mainardi, P., & Striano, P. (2024, January 26). Microbiota in Newborn from Birth to Weaning. In Encyclopedia. https://encyclopedia.pub/entry/54392
Biagioli, Valentina, et al. "Microbiota in Newborn from Birth to Weaning." Encyclopedia. Web. 26 January, 2024.
Copy Citation
Nutrients play a crucial role in enriching and diversifying the microbiota, derived not only from solid food but also from maternal dietary patterns during gestation.
microbiota
human milk oligosaccharides
complementary feeding
1. Introduction
From the first moments of life, the newborn’s body begins a mutualistic relationship with numerous microorganisms. This condition represents a true “superorganism” composed of bacteria, viruses, archaea, and unicellular eukaryotes. Bacteroidetes and Firmicutes are the major bacterial phyla, with subgroups such as Fusobacteria, Cyanobacteria, Proteobacteria, Verrucomicrobia, Actinobacteria, and a few others [1]. These microorganisms colonize our entire organism, including the integumentary system and mucosal cavities (e.g., pulmonary, oral, and vaginal); however, most are found in the gastrointestinal tract (GIT). Moreover, the colon contains more than 70% of the microorganisms colonizing the GIT [2]. The intestinal microbiota represents a “vital organ”, which, owning to the numerous connective pathways such as the neural, endocrine, immunological, and metabolic pathways, can communicate with areas that are anatomically distant from one another [3].
A balanced intestinal microbial ecosystem, called eubiosis, is effective in controlling a variety of infectious diseases. Therefore, balanced nutrition from qualitative and quantitative points of view and proper food processing to preserve their organoleptic and nutritional properties are fundamental for human health and for maintaining a healthy gut microbial composition [2]. On the contrary, the prolonged use of antibiotics causes an imbalance between the different strains that make up the microbiota, referred to as dysbiosis [4], a condition characterized by a shift in the composition of commensal bacterial strains toward a more pathogenic profile [2]. This change can lead to an increase in intestinal permeability, consequently triggering an inappropriate immune response [5]. This so-called “leaky gut” is often associated with conditions such as dysbiosis and gastrointestinal symptoms, which also appear to play a key role in the pathogenesis of autism spectrum disorder (ASD) [6][7]. Moreover, scientific evidence shows that microbiome dysbiosis correlates to the pathophysiology of allergies and autoimmune disorders [8]. Notably, the contribution of the human gut microbiota in early life is fundamental for the development and maturation of the infant mucosal and immune system (Figure 1).
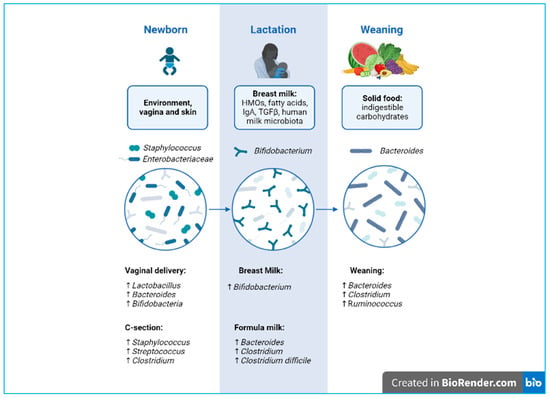
Figure 1. Developmental phases of microbiota biodiversity from delivery to weaning. Created with BioRender.com.
1.1. What Is the “Window of Opportunity”?
1.1.1. Maternal Nutrition and Infant Gut Microbiome: How Materno–fetal Exchange Influences the Development of Neonates
Pregnancy is a unique biological process that involves numerous physiological changes to support the health of both the mother and the fetus. Moreover, the placenta is a highly specialized organ, which divides the fetal and maternal environments. Historically, the placenta was considered a sterile organ, but, in recent years, new evidence has demonstrated the possible existence of a placental microbiome. In 2014, the first studies based on bacterial DNA demonstrated the existence of a placental microbiota even during full-term and normal pregnancies [9].
Metagenomics and metabolomics studies have shown that the maternal intestinal microbiota plays an important role in the transport of metabolites derived from commensal bacteria across the placental barrier and is therefore able to modulate fetal development [10]. Exogenous factors such as diet and drug intake, as well as endogenous factors such as maternal stress and alterations in the metabolic state, influence the maternal intestinal microbiota [11].
Even during a normal pregnancy, the maternal microbiota changes its composition. For example, Faecalibacterium is a producer of short-chain fatty acids (SCFAs), which tends to decrease in the last trimester of pregnancy [12]. SCFAs such as acetate, propionate, and butyrate are of fundamental importance as they provide 60–70% of the energy needs of colonocytes [13]; in particular, butyrate promotes cellular repair and regeneration processes, maintaining epithelial integrity [14][15].
The fetal microbiota composition is therefore strongly influenced by prenatal factors and strictly dependent on maternal lifestyle and habits. For example, smoking and a Western diet style rich in sugars, processed foods, and saturated fats negatively influence the fetal microbiota [16].
The intrauterine life and the first years of life represent a critical time to shape the newborn’s microbiota. In humans, the composition of the intestinal microbiota appears to stabilize around 2.5–4 years of life [17]. Before this stabilization occurs, the intestinal microbiota of the newborn appears to be much more susceptible to stimuli that modulate it [18][19].
1.1.2. Vaginal Delivery and Vertical Transmission of Microbiota
During natural birth, the fetus passes through the maternal vaginal canal, coming into contact with the microbial ecosystem present (vaginal and fecal). This has a long-term influence on the composition and development of the newborn’s microbiota [20].
1.1.3. Caesarean Section
During a cesarean (C-) section, the baby has increased contact with the mother’s integumentary system and with the microbes present in the hospital environment [21]. Contrary to vaginal births, C-section-delivered newborns show reduced microbiota diversity and richness, with significantly lower levels of bacterial genera such as Bifidobacterium, Lactobacillus, and Bacteroides, as well as higher levels of Staphylococcus. Interestingly, clinical studies highlight a relationship between C-section and immune disorders, such as allergies and asthma [22]. However, further studies are needed to clarify the long-term role of the altered microbiota.
2. Human Milk and Neonatal Brain Development
2.1. Macro- and Micronutrient Composition
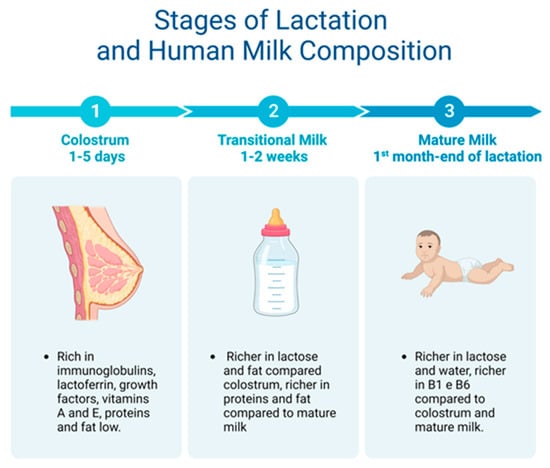
Breast milk is made up of macro- and micronutrients specialized in supporting the physiological and metabolic functions, neural development, and the intestinal microbiota of the newborn [23]. Two weeks after giving birth, human milk is considered fully mature. Mature milk contains 6.9–7.2% carbohydrates, 3–5% fats, 0.8–0.9% proteins, and 0.2% mineral components [24] (Figure 2).

Figure 2. From colostrum to mature milk and their different properties.
2.2. Human Milk Oligosaccharides
Human milk oligosaccharides (HMOs) are complex, indigestible glycans present in high quantities in breast milk [25]. HMOs act as real prebiotics as they cross the intestinal lumen and are fermented by the intestinal microbiota [26]. After lactose and lipids, HMOs are the third most abundant components of human milk, with high quantities (20–25 ng/L) in HC, gradually decreasing to 5–15 ng/L in mature milk.
HMOs prevent infection with pathogenic bacteria such as Salmonella, Campylobacter, and Listeria and promote Bifidobacterium [27], thus limiting the growth of bacteria potentially harmful to the newborn [28].
2.3. Other Bioactive Compounds
Some bioactive components of breast milk such as insulin-like growth factors (IGFs) 1 and 2 act as energy substrates for newborns, promoting the growth and development of various tissues. Among the bioactive components, there is lactoferrin, a glycoprotein that binds iron and has antimicrobial activity.
Furthermore, secretory (S)IgA and SIgG are the most abundant immunoglobulins in milk, providing support to the newborn’s immune functions [29] by preventing the adhesion of pathogens to the surface of epithelial cells. SIgA is present at concentrations up to 12 mg/L in HC.
2.4. Breast Milk Microbiome
Initially, human breast milk (HBM) was considered a sterile fluid, and the presence of bacteria was considered to be contamination or infection [24]. To date, scientific evidence has detected microbial metabolites in HC [30]. This discovery has led to growing interest in the HMB microbiome.
After the vaginal birth canal, the HBM is the second source of microbes for the newborn. Breastfed babies have been predicted to consume up to 8 × 105 bacteria each day. There are various mechanisms proposed to explain the transmission of the microbiota through breastfeeding, including contamination from the mother’s integumentary system and retrograde flow from the newborn oral cavity to the ductal tissue [24]. In support of these hypotheses, notable similarities have been found between the adult skin microbiome and the milk microbiome, in particular the presence of Corynebacterium and Staphylococcus.
3. Infant Formula
3.1. Macronutrients in Infant Formula
Even though breastfeeding is the first choice for the health of the newborn, many factors can influence the decision, such as medical, occupational, or family support problems and complications. When breastfeeding is not an option, infant formula (IF) becomes part of the newborn’s nutrition.
Most of the constituent proteins in IF derive from bovine milk, which has fewer essential amino acids than HBM. The protein content of IFs of bovine origin varies between 2 and 3 g/100 mL, higher than the protein level present in HBM (1.4–1.6 g/100 mL in HC and 0.8–1.0 g/mL in mature milk). This excess of proteins and amino acids overstimulates the β-pancreatic cells and is strongly associated with early adiposity and a greater risk of becoming overweight in adulthood [31].
3.2. Supplements in Infant Formula: Probiotics, Prebiotics, and Postbiotics
In recent years, research has increasingly focused on the effect of prebiotics and probiotics on the infant microbiota. These studies, combined with a growing interest from industries in IF, have led to the continuous improvement in IF to create a formulation that resembles HBM as much as possible. New IFs have currently been developed with enrichment in bioactive ingredients such as probiotics, prebiotics, and postbiotics.
In October 2013, the International Scientific Association for Probiotics and Prebiotics (ISAPP) re-examined the concept of probiotics, redefining them as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. This definition is inclusive of a wide range of microbes and therapeutic applications. The seven most commonly used genera in commercial products are Bifidobacterium, Saccharomyces, Streptococcus, Escherichia, Lactobacillus, Enterococcus, and Bacillus [32]. Prebiotics, on the other hand, are food substrates that, once ingested, are selectively fermented by certain microbial strains, conferring benefits to the host. The most commonly used prebiotics include galactooligosaccharides (GOS), fructooligosaccharides (FOS), and inulin.
3.3. Infant Formula, Risks, and Future Directions
The use of IF stimulates glucose metabolism, increases insulin sensitivity, and causes early advanced adiposity that persists into adulthood. However, further studies are needed to delve into the safe dosages and beneficial effects in pediatric disorders and premature infants to create more standardized protocols for this age group [33].
4. Dietary Nutrients Shape the Gut Microbiota: From Infancy to Childhood
4.1. Microbiota Maturation during Weaning
Malnourished and under- or over-nourished infants and children develop an immature intestinal microbiota, which can compromise child growth [34].
The transition to solid foods implies an increase in the intake of proteins, carbohydrates, lipids, and fibers, which leads to an increase in microbial richness and diversity. For example, a randomized control trial demonstrated that the introduction of solid foods (including meat, cereals, and fruit) led to an increase in the diversity of the gut microbiota [35].
During this transition, the count of milk-related bacteria, such as Bifidobacterium, Lactobacillaceae, and Enterobacteriaceae, decreases, while bacteria such as Bacteroides, Akkermansia, and Ruminococcaceae, which are capable of fermenting and digesting the more complex nutrients, are introduced during weaning and expand [36].
4.2. Establishment of Nutritional Habits from Solid Food Introduction
It is known that the mother’s eating habits and qualitative choice of nutrients during pregnancy are essential for the development of the newborn’s taste and olfactory preferences [37]. The olfactory system, which includes the olfactory bulbs of the brain that are already functioning at the 24th week of gestation, develops in parallel with that of taste: swallowing and breathing the amniotic fluid offers the fetus the flavors and odors of the food consumed by the mother [38].
4.3. Nutrient–Microbiota Interactions and Brain Development during Early Life
Microbial signals are crucial not only for intestinal eubiosis but also for the healthy and correct development of neuronal circuits in the brain [39]. Intestinal microbes communicate with the central nervous system (CNS), secreting signaling molecules that move through the circulatory system and crossing the intestinal epithelium and the blood–brain barrier (BBB) [40][41]. Brain development begins during fetal life and continues through adolescence through critical processes such as neurogenesis, synaptogenesis, and myelination [42].
5. Conclusions
Starting from intrauterine life, the gut microbiota composition and biodiversity are influenced by environmental factors and nutrition. Beyond the different dietary regimes, the vastness and biodiversity of the microbial world that accompanies us throughout our lives cannot be addressed with a “one-size-fits-all” approach. The period of gestation and the birth of the newborn represents a window of opportunity to modulate the health status of the newborn through noninvasive and inexpensive methods such as education on healthy nutrition for microbial biodiversity.
References
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. BioMed Res. Int. 2018, 2018, 4178607.
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12.
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022, 13, 999001.
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148.
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248.
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050.
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17.
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 2018, 10, 274.
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets. BMC Microbiol. 2023, 23, 76.
- Li, Y.; Toothaker, J.M.; Ben-Simon, S.; Ozeri, L.; Schweitzer, R.; McCourt, B.T.; McCourt, C.C.; Werner, L.; Snapper, S.B.; Shouval, D.S.; et al. In utero human intestine harbors unique metabolome, including bacterial metabolites. JCI Insight 2020, 5, e138751.
- Galley, J.D.; Mashburn-Warren, L.; Blalock, L.C.; Lauber, C.L.; Carroll, J.E.; Ross, K.M.; Hobel, C.; Coussons-Read, M.; Schetter, C.D.; Gur, T.L. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav. Immun. 2023, 107, 253–264.
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 683022.
- Roager, H.M.; Stanton, C.; Hall, L.J. Microbial metabolites as modulators of the infant gut microbiome and host-microbial interactions in early life. Gut Microbes 2023, 15, 2192151.
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41.
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9.
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812.
- Thriene, K.; Michels, K.B. Human Gut Microbiota Plasticity throughout the Life Course. Int. J. Environ. Res. Public Health 2023, 20, 1463.
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56.
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493.
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86.
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121.
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98.
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654.
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309.
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080.
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Järvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534.
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115.
- Gertosio, C.; Meazza, C.; Pagani, S.; Bozzola, M. Breastfeeding and its gamut of benefits. Minerva Pediatr. 2016, 68, 201–212.
- Arroyo, G.; Ortiz Barrientos, K.A.; Lange, K.; Nave, F.; Mas, G.M.; Aguilar, P.L.; Galindo, M.A.S.; Schlotterer, H.R.; Perrin, M.T.; Rasmussen, K.M.; et al. Effect of the Various Steps in the Processing of Human Milk in the Concentrations of IgA, IgM, and Lactoferrin. Breastfeed. Med. 2017, 12, 443–445.
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.-P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051.
- Corona, L.; Lussu, A.; Bosco, A.; Pintus, R.; Marincola, F.C.; Fanos, V.; Dessì, A. Human Milk Oligosaccharides: A Comprehensive Review towards Metabolomics. Children 2021, 8, 804.
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis. Biomolecules 2021, 11, 1000.
- Lemoine, A.; Tounian, P.; Adel-patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231.
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Gordon, J.I. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013, 339, 548–554.
- Qasem, W.; Azad, M.B.; Hossain, Z.; Azad, E.; Jorgensen, S.; Juan, S.C.S.; Cai, C.; Khafipour, E.; Beta, T.; Roberts, L.J.; et al. Assessment of complementary feeding of Canadian infants: Effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017, 17, 54.
- Schwab, C. The development of human gut microbiota fermentation capacity during the first year of life. Microb. Biotechnol. 2022, 15, 2865–2874.
- Bjerregaard, A.A.; Halldorsson, T.I.; Tetens, I.; Olsen, S.F. Mother’s dietary quality during pregnancy and offspring’s dietary quality in adolescence: Follow-up from a national birth cohort study of 19,582 mother-offspring pairs. PLoS Med. 2019, 16, e1002911.
- Podzimek, Š.; Dušková, M.; Broukal, Z.; Rácz, B.; Stárka, L.; Dušková, J. The evolution of taste and perinatal programming of taste preferences. Physiol. Res. 2018, 67 (Suppl. S3), S421–S429.
- Corkins, M.R.; Daniels, S.R.; de Ferranti, S.D.; Golden, N.H.; Kim, J.H.; Magge, S.N.; Schwarzenberg, S.J. Nutrition in Children and Adolescents. Med. Clin. N. Am. 2016, 100, 1217–1235.
- Silva, G.A.P.; Costa, K.A.O.; Giugliani, E.R.J. Infant feeding: Beyond the nutritional aspects. J. Pediatr. 2016, 92 (Suppl. S1), S2–S7.
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163.
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
612
Revisions:
2 times
(View History)
Update Date:
29 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




