
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olubukola Oluranti Babalola | -- | 3605 | 2024-01-26 07:47:09 | | | |
| 2 | Catherine Yang | Meta information modification | 3605 | 2024-01-26 07:54:26 | | |
Video Upload Options
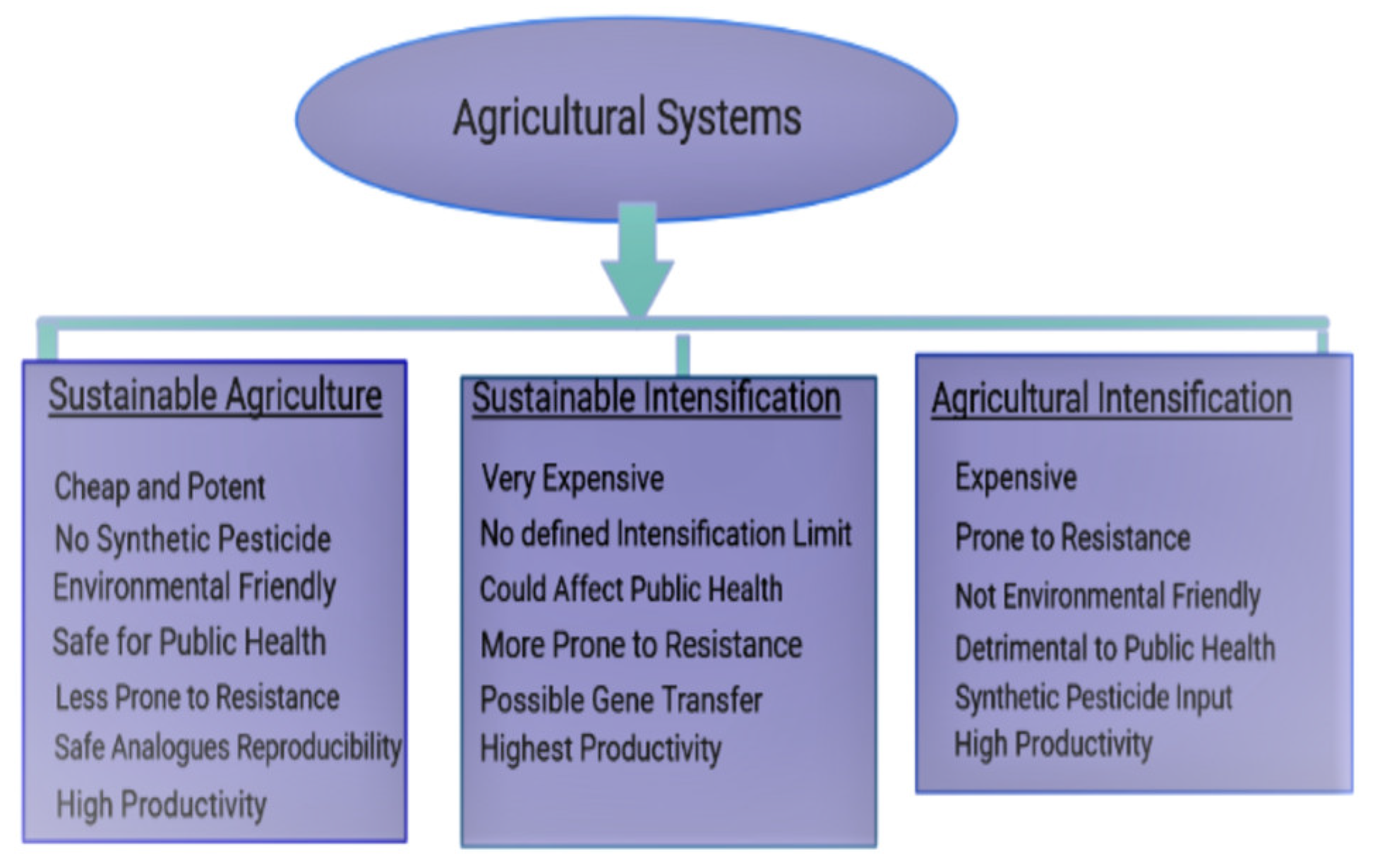
The failure of sustainable and agricultural intensifications in saving the ecosystem/public health has caused a paradigm shift to microbiome resource engineering through sustainable approaches. As agricultural intensification systems prioritize synthetic input applications over environmental health, sustainable intensification fails to define the end point of intensification, giving room for the application of “intensification” over “sustainability” to suit farmers’ needs. The complexity of a cooperative microbiome and the uncontrollable nature of its numerous influencing parameters as well as the non-specificity associated with bioinoculant application, results in the direct utilization of agroactive compounds to obtain greater preventive efficiency.
1. Introduction
2. History of Sustainable Agricultural Practices
| Rare Actinomycetes | Core Genes | Metabolites Encoded | Functionalities | Roles in Sustainable Agriculture | References |
|---|---|---|---|---|---|
| Frankia sp. | nif | Nitrogenase enzyme | Nitrogen fixation | Soil fertilization | [24] |
| Tsukamurella tyrosinosolvens | pho | Phosphatase enzyme | Phosphate solubilization | Phosphate fertilization |
[25] |
| Amycolatopsis sp. | PR1–1a & GLU | proteins | Systemic acquired resistance | Plant defence | [26] |
| Arthrobacter sp. SD3-25 | atzB, atzC & trzN | hydrolases | Atrazine & simazine pesticide biodegradation | Soil fertility and Bioaugmentation | [27] |
| mbtH, fagD | protein | Siderophores biosynthesis | Plant defence and Iron fertilization | [28] | |
| Tsukamurella tyrosinosolvens | febB, febD, yqjH, hpaC | Transport proteins | Biosynthesis of iron transporter | Plant defence and growth | [25] |
| atzF argG argH |
Hydrolase Arginine succinate synthase Arginine succinate lyase |
Urea degradation | Ammonia and amino acids biosynthesis for growth | [28] | |
| Saccharothrix sp. | SacA,B,C,E | Polyketide synthase |
Saccharochelins A–E biosynthesis | Phytoprotection | [29] |
| Amycolatopsis sp. | asrR | Type III glycopeptide | Ristomycin | Phytoprotection | [30] |
| Nocardiopsis alba BH35 | Crude filtrate | Protein | Antifungal metabolites | Phytoprotection | [31] |
| Rhodococcus ruber C1 | dmpP | Phenol hydroxylase | Phenol degradation | Biodegradation | [32] |
| Rhodococcus sp. ANT_H53B |
crtP,M,N,Nc | Diapolycopene Oxygenase, dehydrosqualene synthase & other enzymes |
C3 apocarotenoid biosynthesis | Phytoprotection and enhancement | [33] |
| Saccharothrix yanglingensis Hhs.015 | Elicitor PeSy1 | Protein | Induce plant resistance | Plant defense against Pseudomonas syringae pv. tomato DC3000 | [34] |
| Chi6769 | Protein | Chitinase biosynthesis | Phytoprotection | [35] | |
| Saccharopolyspora sp. | arsG | Arsenate reductase | Arsenic removal | Arsenic biocleansing | [36] |
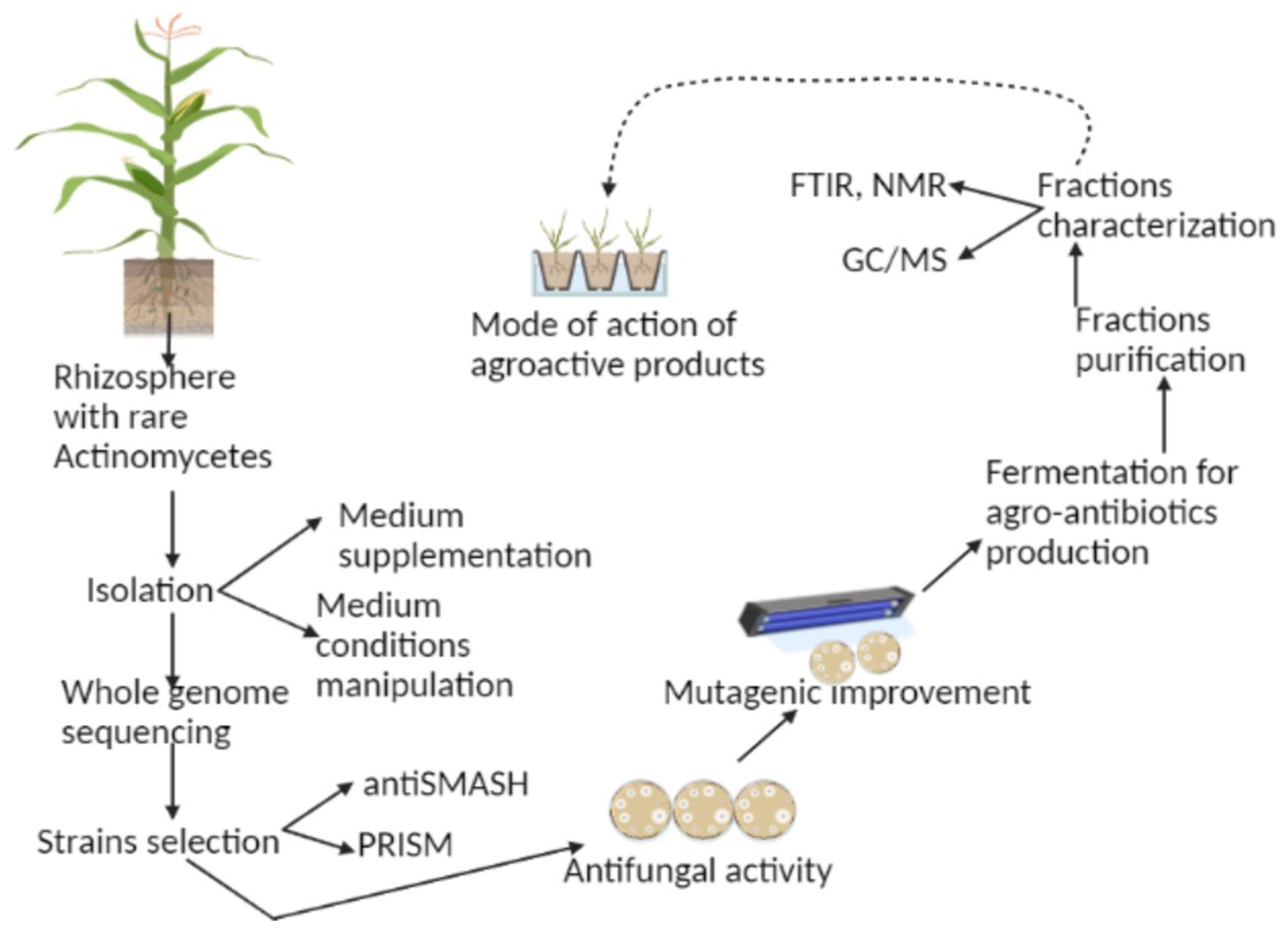
3. Rare Actinomycetes to the Rescue
4. Agro-Antibiotics Encoded by Biosynthetic Gene Clusters (BGCs) in Actinomycetes
4.1. Herbicidal Agents
4.2. Insecticidal/Acaricidal Agents
4.3. Anti-Phytopathogenic Agents
References
- Kayembe, C.; Nel, D. Challenges and opportunities for education in the Fourth Industrial Revolution. Afr. J. Public Aff. 2019, 11, 79–94.
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198.
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533.
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433–100445.
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219.
- Santoyo, G.; Gamalero, E.; Glick, B.R. Mycorrhizal-bacterial amelioration of plant abiotic and biotic stress. Front. Sustain. Food Syst. 2021, 5, 672881.
- Akinola, S.A.; Ayangbenro, A.S.; Babalola, O.O. The diverse functional genes of maize rhizosphere microbiota assessed using shotgun metagenomics. J. Sci. Food Agric. 2021, 101, 3193–3201.
- Babalola, O.O.; Kirby, B.M.; Le Roes-Hill, M.; Cook, A.E.; Cary, S.C.; Burton, S.G.; Cowan, D.A. Phylogenetic analysis of actinobacterial populations associated with Antarctic Dry Valley mineral soils. Environ. Microbiol. 2009, 11, 566–576.
- Seenivasagan, R.; Babalola, O.O. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology 2021, 10, 1111.
- Shi, L.; Wu, Z.; Zhang, Y.; Zhang, Z.; Fang, W.; Wang, Y.; Wan, Z.; Wang, K.; Ke, S. Herbicidal secondary metabolites from Actinomycetes: Structure diversity, modes of action, and their roles in the development of herbicides. J. Agric. Food Chem. 2019, 68, 17–32.
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.K. Mini review: Genome mining approaches for the identification of secondary metabolite biosynthetic gene clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556.
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707.
- Raubenheimer, D.; Rothman, J.M.; Pontzer, H.; Simpson, S.J. Macronutrient contributions of insects to the diets of hunter–gatherers: A geometric analysis. J. Hum. Evol. 2014, 71, 70–76.
- Vasey, D.E. An Ecological History of Agriculture 10000 Bc-Ad 10000; Purdue University Press: West Lafayette, IN, USA, 2002.
- Cilliers, J. The Future of Africa: Challenges and Opportunities; Springer Nature: Berlin/Heidelberg, Germany, 2021.
- Sinclair, F.; Wezel, A.; Mbow, C.; Chomba, S.; Robiglio, V.; Harrison, R. The Contribution of Agroecological Approaches to Realizing Climate-Resilient Agriculture; GCA: Rotterdam, The Netherlands, 2019.
- Sinclair, F.L.; Rosenstock, T.S.; Gitz, V.; Wollenberg, L. Agroforestry to Diversify Farms and Enhance Resilience. In 10 Best Bet Innovations for Adaptation in Agriculture: A Supplement to the UNFCCC NAP; CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS): Wageningen, The Netherlands, 2017; pp. 14–19.
- Tiwari, T.P.; Brook, R.M.; Wagstaff, P.; Sinclair, F.L. Effects of light environment on maize in hillside agroforestry systems of Nepal. Food Secur. 2012, 4, 103–114.
- Kong, Z.; Hart, M.; Liu, H. Paving the way from the lab to the field: Using synthetic microbial consortia to produce high-quality crops. Front. Plant Sci. 2018, 9, 1467–1472.
- Mahon, N.; Crute, I.; Di Bonito, M.; Simmons, E.A.; Islam, M.M. Towards a broad-based and holistic framework of Sustainable Intensification indicators. Land Use Policy 2018, 77, 576–597.
- Talaviya, T.; Shah, D.; Patel, N.; Yagnik, H.; Shah, M. Implementation of artificial intelligence in agriculture for optimisation of irrigation and application of pesticides and herbicides. Artif. Intell. Agric. 2020, 4, 58–73.
- Chukwuneme, C.F.; Babalola, O.O.; Kutu, F.R.; Ojuederie, O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020, 15, 93–105.
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Shanthi, V. Actinomycetes: Implications and Prospects in Sustainable Agriculture. In Biofertilizers: Study and Impact; Wiley: Hoboken, NJ, USA, 2021; pp. 335–370.
- Zhang, H.; Han, L.; Jiang, B.; Long, C. Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J. Microbiol. Biotechnol. 2021, 37, 109.
- Alipour Kafi, S.; Karimi, E.; Akhlaghi Motlagh, M.; Amini, Z.; Mohammadi, A.; Sadeghi, A. Isolation and identification of Amycolatopsis sp. strain 1119 with potential to improve cucumber fruit yield and induce plant defense responses in commercial greenhouse. Plant Soil 2021, 468, 125–145.
- Mawang, C.I.; Azman, A.S.; Fuad, A.S.M.; Ahamad, M. Actinobacteria: An eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol. Rep. 2021, 32, e00679.
- Jiang, B.; Long, C.; Xu, Y.; Han, L. Molecular mechanism of Tsukamurella tyrosinosolvens strain P9 in response to root exudates of peanut. Arch. Microbiol. 2023, 205, 48.
- Shen, Q.; Dai, G.; Li, A.; Liu, Y.; Zhong, G.; Li, X.; Ren, X.; Sui, H.; Fu, J.; Jiao, N.; et al. Genome-Guided Discovery of Highly Oxygenated Aromatic Polyketides, Saccharothrixins D–M, from the Rare Marine Actinomycete Saccharothrix sp. D09. J. Nat. Prod. 2021, 84, 2875–2884.
- Liu, K.; Hu, X.R.; Zhao, L.X.; Wang, Y.; Deng, Z.; Tao, M. Enhancing ristomycin a production by overexpression of ParB-like StrR family regulators controlling the biosynthesis genes. Appl. Environ. Microbiol. 2021, 87, e01066-21.
- Lu, Y.; Wang, N.; He, J.; Li, Y.; Gao, X.; Huang, L.; Yan, X. Expression and characterization of a novel chitinase with antifungal activity from a rare actinomycete, Saccharothrix yanglingensis Hhs. 015. Protein Expr. Purif. 2018, 143, 45–51.
- Zhao, T.; Gao, Y.; Yu, T.; Zhang, Y.; Zhang, Z.; Zhang, L.; Zhang, L. Biodegradation of phenol by a highly tolerant strain Rhodococcus ruber C1: Biochemical characterization and comparative genome analysis. Ecotoxicol. Environ. Saf. 2021, 208, 111709.
- Styczynski, M.; Rogowska, A.; Gieczewska, K.; Garstka, M.; Szakiel, A.; Dziewit, L. Genome-based insights into the production of carotenoids by Antarctic bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B. Molecules 2020, 25, 4357.
- Su, Y.S.; Cheng, M.J.; Wu, M.D.; Chai, C.Y.; Kwan, A.L.; Su, S.H.; Kuo, Y.H. Chemical Constituents from a Mangrove-Derived Actinobacteria Isoptericola chiayiensis BCRC 16888 and Evaluation of Their Anti-NO Activity. Chem. Biodivers. 2021, 18, e2100211.
- Nouioui, I.; Ha, S.M.; Baek, I.; Chun, J.; Goodfellow, M. Genome insights into the pharmaceutical and plant growth promoting features of the novel species Nocardia alni sp. nov. BMC Genom. 2022, 23, 70.
- Saygin, H.; Ay, H.; Guven, K.; Inan-Bektas, K.; Cetin, D.; Sahin, N. Saccharopolyspora karakumensis sp. nov., Saccharopolyspora elongata sp. nov., Saccharopolyspora aridisoli sp. nov., Saccharopolyspora terrae sp. nov. and their biotechnological potential revealed by genome analysis. Syst. Appl. Microbiol. 2021, 44, 126270.
- Subbanna, A.R.N.S.; Stanley, J.; Rajasekhara, H.; Mishra, K.K.; Pattanayak, A.; Bhowmick, R. Perspectives of Microbial Metabolites as Pesticides in Agricultural Pest Management. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland; Midtown Manhattan, NY, USA, 2020; pp. 925–952.
- Wang, K.; Ke, S.; Fang, W.; Wu, Z.; Zhang, Y. Novel Agroactive Secondary Metabolites from Actinomycetes in the Past Two Decades with Focus on Screening Strategies and Discovery. In Natural Products from Actinomycetes; Springer: Gateway East, Singapore, 2022; pp. 199–221.
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342–1359.
- Li, S.; Yang, B.; Tan, G.Y.; Ouyang, L.M.; Qiu, S.; Wang, W.; Xiang, W.; Zhang, L. Polyketide pesticides from actinomycetes. Curr. Opin. Biotechnol. 2021, 69, 299–307.
- Myronovskyi, M.; Rosenkränzer, B.; Stierhof, M.; Petzke, L.; Seiser, T.; Luzhetskyy, A. Identification and heterologous expression of the albucidin gene cluster from the marine strain Streptomyces albus subsp. chlorinus NRRL B-24108. Microorganisms 2020, 8, 237.
- Lukoseviciute, L.; Lebedeva, J.; Kuisiene, N. Diversity of polyketide synthases and nonribosomal peptide synthetases revealed through metagenomic analysis of a deep oligotrophic cave. Microb. Ecol. 2021, 81, 110–121.
- Bozhüyük, K.A.; Micklefield, J.; Wilkinson, B. Engineering enzymatic assembly lines to produce new antibiotics. Curr. Opin. Microbiol. 2019, 51, 88–96.
- Dong, X.; Lv, L.; Wang, W.; Liu, Y.; Yin, C.; Xu, Q.; Yan, H.; Fu, J.; Liu, X. Differences in distribution of potassium-solubilizing bacteria in forest and plantation soils in Myanmar. Int. J. Environ. Res. Public Health 2019, 16, 700.
- Adeniji, A.A.; Babalola, O.O. Evaluation of Pseudomonas fulva PS9. 1 and Bacillus velezensis NWUMFkBS10. 5 as Candidate Plant Growth Promoters during Maize-Fusarium Interaction. Plants 2022, 11, 324.
- El-Gawahergy, H.; Amin, D.H.; Elsayed, A.F. Mining for NRPS and PKS Genes in Actinobacteria Using Whole-Genome Sequencing and Bioinformatic Tools. In Natural Products from Actinomycetes: Diversity, Ecology and Drug Discovery; Springer: Gateway East, Singapore, 2022; pp. 393–410.
- Harir, M.; Bendif, H.; Bellahcene, M.; Fortas, Z.; Pogni, R. Streptomyces Secondary Metabolites. In Basic Biology and Applications of Actinobacteria; IntechOpen: London, UK, 2018; pp. 99–122.
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72.
- Hu, J.; Xia, Z.; Shuai, L.; Chen, J.; Zhu, Z.; Cao, L.; Xie, J.; Dai, Z.; Hu, Y.; Huang, W.; et al. Effect of pII key nitrogen regulatory gene on strain growth and butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. Appl. Microbiol. Biotechnol. 2022, 106, 3081–3091.
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439.
- Xu, X.; Han, L.; Zhao, L.; Chen, X.; Miao, C.; Hu, L.; Huang, X.; Chen, Y.; Li, Y. Echinosporin antibiotics isolated from Amycolatopsis strain and their antifungal activity against root-rot pathogens of the Panax notoginseng. Folia Microbiol. 2019, 64, 171–175.
- Fei, C.; She, R.; Li, G.; Zhang, L.; Fan, W.; Xia, S.; Xue, F. Safety and clinical efficacy of tenvermectin, a novel antiparasitic 16-membered macrocyclic lactone antibiotics. Eur. J. Pharm. Sci. 2018, 117, 154–160.
- Chen, X.; Hu, L.F.; Huang, X.S.; Zhao, L.X.; Miao, C.P.; Chen, Y.W.; Xu, L.H.; Han, L.; Li, Y.Q. Isolation and characterization of new phenazine metabolites with antifungal activity against root-rot pathogens of Panax notoginseng from Streptomyces. J. Agric. Food Chem. 2019, 67, 11403–11407.




