
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Júlio César Santos | -- | 4006 | 2024-01-25 15:49:47 | | | |
| 2 | Sirius Huang | Meta information modification | 4006 | 2024-01-26 02:13:05 | | |
Video Upload Options
The liquid biofuel bioethanol is extensively produced worldwide through the fermentation of sugars obtained from various raw materials, including lignocellulosic biomass, an abundant renewable resource. Due to its recalcitrant nature, lignocellulosic materials typically is pretreated using mechanical, chemical, physicochemical, or biological methods to enhance sugar recovery. The pretreated lignocellulosic biomass then undergoes a fermentation process, either sequentially or simultaneously with saccharification, resulting in the biofuel called second-generation ethanol. The ethanol yield is influenced by various fermentation strategies and conditions, such as inoculum concentration, medium agitation, temperature, and pH.
1. Introduction
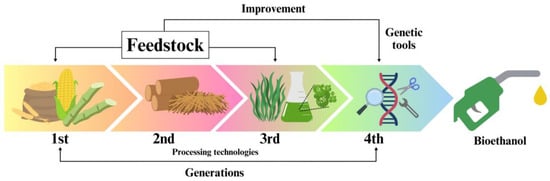
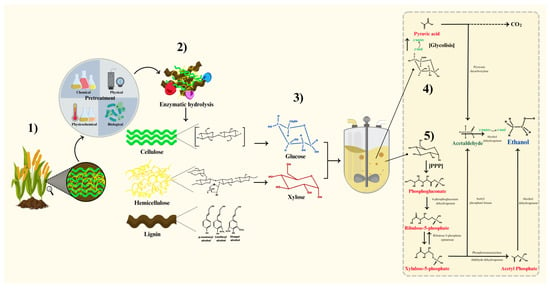
2. Breakdown of Lignocellulosic Substrates to Fermentable Sugars
2.1. Lignocellulosic Biomass Composition
| Lignocellulosic Biomass | Cellulose, % | Hemicellulose, % | Lignin, % | Reference |
|---|---|---|---|---|
| Bamboo | 47 | 23 | 28 | [40] |
| Banana waste | 28.92 | 25.23 | 10.56 | [41] |
| Barley straw | 38.43 | 28.55 | 16.26 | [42] |
| Corn cobs | 33.6 | 37.2 | 19.3 | [43] |
| Corn stover | 35–40 | 17–35 | 7–18 | [44] |
| Corn stalk | 34.5 | 27.6 | 8.7 | [45] |
| Grass | 25–40 | 35–50 | 10–30 | [46] |
| Hardwood stems | 40–55 | 24–40 | 18–25 | [47] |
| Miscanthus | 38.38 | 24.23 | 17.66 | [48] |
| Municipal solid waste | 33–49 | 9–16 | 10–14 | [21] |
| Oat straw | 31–37 | 27–38 | 14–19 | [49] |
| Pinewood | 40 | 28.5 | 27.7 | [50] |
| Rice straw | 28–36 | 23–28 | 12–15 | [44] |
| Rye straw | 30.9 | 21.5 | 22.1 | [51] |
| Softwood stems | 45–50 | 25–35 | 25–35 | [41] |
| Sugarcane bagasse | 42–48 | 19–25 | 20–42 | [41] |
| Sunflower stalk | 34.6 | 21 | 30 | [52] |
| Sweet sorghum bagasse | 34–45 | 18–27 | 14–21 | [44] |
| Wheat straw | 33–38 | 26–32 | 17–19 | [43] |
2.2. Pretreatment Methods
| Pretreatment | Feedstocks | Pretreatment Conditions | Pretreatment Results | Limitations | Bioethanol Yield | Reference |
|---|---|---|---|---|---|---|
| Mechanical | Rice straw | Dry ball milling Loading charge biomass 50 g 60 min 1700 rpm |
Sugar yields: Glucose (331 mg/g rice straw) Xylose (74 mg/g rice straw) Arabinose (14 mg/g rice straw) |
High consumption of energy. No solubilization of lignin. |
* | [70] |
| Oil palm biomass | Ball milling Loading charge biomass 20 g 50 min 250 rpm |
Size reduction (88.2%) Crystallinity reduction (46.6%) Sugar yield: Glucose 36.1% Xylose 56.4% |
Inhibitors produced as acetic acid (1.3 mg/ g of biomass). High energy consumption. |
* | [71] | |
| Physical | Wood biomass | Microwave treatment/DES Choline chloride/oxalic acid 80 °C 800 W 3 min |
Lignin removed 80% Glucose yield 22.3%. |
Inhibitors such as HMF are produced. | * | [72] |
| Sweet lime peel | 2% v/v H2SO4 Loading charge of 6% 60 min 750 W 20 kHz. |
181.5 mg sugars/g biomass | Relative low loading biomass charge High biomass content increases viscosity resulting in low chemical reaction. |
64% in acidic pH | [73] | |
| Switchgrass | Steam explosion 200 °C 10 min |
Sugar yield: Glucose 50.9% Xylose 28% Lignin removed 50% |
Inhibitors such as formic acid, acetic acid, HMF, and furfural. | * | [74] | |
| Chemical | Sugarcane bagasse | Alkaline 15% NaOH 140 °C 1 h |
Reducing sugars 5.29 g/L Lignin removed 90% |
Glucose released is negatively affected by the amount of hemicellulose and lignin present. | 0.1 g ethanol/g biomass and 0.88 g/Lh | |
| Sugarcane bagasse | Organosolv Glycerol–acid 15% 130 °C 60 min |
Glucan 65.8% | High concentration of glycerol–acid is required to improve hydrolysis. No significant influence on the lignin content. |
0.38 g ethanol/g biomass and 0.57 g/Lh | [75] | |
| Rice straw | Acid 1%H2SO4 10%(w/v) rice straw 100 °C 2 h |
Reducing sugars 14 g/L Lignin removed 11.7% Xylose 83% |
More recycling times for acid treatment hydrolysate decrease xylose yield. | Concentration of 40.6 g/L yield of 86.4% | [76] | |
| Rice straw | DES: choline chloride-ethylene glycol 150 °C 24 h Biomass loading 5% w/w |
Lignin removed 74% Glucan digestibility 87% |
Residual DES decreases the enzymatic hydrolysis of cellulose. | * | [77] | |
| Wheat straw | Alkaline-Microwave 2.75% NaOH Solid loading 10% (w/v) 23 min 100 °C |
Lignin removed 60% Total carbohydrates 82% |
High energy consumption. Rapid oxidation of carbohydrates in alkaline conditions. |
48 g ethanol/g sugar consumed | [78] | |
| Wheat straw | Ultrasound-assisted ionic liquid Triethylamine hydrogen sulfate ([TBA][H2SO4]) Sonicated at 24 kHz 130 °C 30 min |
Saccharification yield 76.1% Lignin removed 74.9% |
Sodium azide is used and represents a metabolic inhibitor. Ultrasound is not effective on a bigger scale. |
42 g/L | [79] | |
| Corn stalks | Ionic liquid 150 °C 11.5 h 2.5% arginine 420 MPa |
Lignin removed 92% Purity of cellulose reach 85% |
Arginine can inhibit cellulose degradation. Good method for fabrication of cellulose fiber but not ethanol. |
* | [80] | |
| Green coconut shells | Organosolv 80% (w/w) glycerol 1%(w/w) sulfuric acid 121 °C |
Glucose 49 g/L Lignin removed 60% |
Inhibitors such as furfural and HMF are formed. | 29.6 g/L | [81] | |
| Olive tree biomass | Combined acid–alkaline 2.4% H2SO4 130 °C 84 min Peroxide: 7% H2O2 80 °C 90 min NaOH until 11.5 pH |
Solubilization of hemicellulose 71% Lignin removed 80% Cellulose highly accessible 72% |
Overliming method is needed for removing degradation products from lignocellulosic hydrolysates. Presence of acetic acid and furfural as potential inhibitors. |
15 g ethanol/100 g biomass | [82] | |
| Oil palm trunk | Alkaline Peroxide 3% H2O2 70 °C 30 min |
Lignin removed 58% Cellulose extraction 74%. |
High phenolic compounds released. Black liquor released with high amounts of tannins and gallic acid. |
* | [83] | |
| Corn stover | Ammonia Recycle Percolation Process 170 °C 10% ammonia (v/w) 1 h |
Lignin removed 70% Xylan removed 47% |
High temperature and energy required to improve pretreatment performance. High operation cost. |
19.4 g/L | [83] | |
| Miscanthus (Miscanthus giganteus) | Acid diluted; 1% H2SO4 (v/v), 1:10 solid ratio (w/v), 121 °C for 30 min. |
Xylose 24 g/L | Presence of furfural and HMF. | 13.58 g/L; 0.148 g bioethanol/g dry biomass | [84] | |
| Biological | Paddy straw | White rot fungi Pleurotus florida 5% inoculum 25–29 °C Biomass loading 10% (w/v) 28 days |
Saccharification efficiency of 75% | High time required. No convenience in an industrial stage. Fungal residues limit enzymatic hydrolysis. |
* | [85] |
| Chlorella sp. KR-1 | Polygalacturonase, amyloglucosidase, cellulase, and β-glucanase (simultaneous) pH 5.5; and 45 °C; 0.3 N HCl. |
28.5 g of sugar released/L of hydrolysate | Inhibitors such as furfurals, HMF, and formic acid might be produced. | Ethanol yield of 0.4 g/g of fermentable sugar | [86] | |
| Scenedesmus abundans | H2SO4/amyloglucosidase, α-amylase (simultaneous) | 10.752 g of total sugars/L and 5.730 g of glucose/L of the hydrolysate | Sugar content released depends on cultivation and pretreatment performance. | Ethanol yield of 0.1 g/g of algal biomass. | [87] |
3. Sugar Recovery from Pretreated Biomass through Hydrolysis
4. Metabolic Pathways, Settings, and Factors of Fermentation Process
References
- Sadigov, R. Rapid Growth of the World Population and Its Socioeconomic Results. Sci. World J. 2022, 2022, 8110229.
- Ritchie, Hannah, and Max Roser. “Cars, Planes, Trains: Where Do CO2 Emissions from Transport Come From?” Our World in Data. Available online: https://ourworldindata.org/co2-emissions-from-transport (accessed on 28 December 2023).
- Huang, Y.; Zhang, Y.; Deng, F.; Zhao, D.; Wu, R. Impacts of Built-Environment on Carbon Dioxide Emissions from Traffic: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 16898.
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606.
- Sharma, G.; Kaur, M.; Punj, S.; Singh, K. Biomass as a sustainable resource for value-added modern materials: A review. Biofuels Bioprod. Biorefin. 2020, 14, 673–695.
- Islam, K.; Wang, H.; Rehman, S.; Dong, C.; Hsu, H.-Y.; Lin, C.S.K.; Leu, S.-Y. Sustainability metrics of pretreatment processes in a waste derived lignocellulosic biomass biorefinery. Bioresour. Technol. 2020, 298, 122558.
- Renewable Fuels Association. 2023 Ready. Set. Go! Renewable Fuels Association: Washington, DC, USA, 2023.
- Gonçalves, F.d.O.; Perna, R.F.; Lopes, E.S.; Tovar, L.P.; Filho, R.M.; Lopes, M.S. Strategies to Ensure Fuel Security in Brazil Considering a Forecast of Ethanol Production. Biomass 2023, 3, 1–17.
- De Almeida, M.A.; Colombo, R. Production Chain of First-Generation Sugarcane Bioethanol: Characterization and Value-Added Application of Wastes. BioEnergy Res. 2023, 16, 924–939.
- Carrillo-Nieves, D.; Alanís, M.J.R.; Quiroz, R.d.l.C.; Ruiz, H.A.; Iqbal, H.M.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74.
- Santos, C.I.; Silva, C.C.; Mussatto, S.I.; Osseweijer, P.; van der Wielen, L.A.; Posada, J.A. Integrated 1st and 2nd generation sugarcane bio-refinery for jet fuel production in Brazil: Techno-economic and greenhouse gas emissions assessment. Renew. Energy 2018, 129, 733–747.
- Aghaei, S.; Alavijeh, M.K.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenergy 2022, 161, 106447.
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717.
- Maity, S.; Mallick, N. Trends and advances in sustainable bioethanol production by marine microalgae: A critical review. J. Clean. Prod. 2022, 345, 131153.
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857.
- Zabed, H.; Sahu, J.; Suely, A.; Boyce, A.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501.
- Duque, A.; Álvarez, C.; Doménech, P.; Manzanares, P.; Moreno, A.D. Advanced Bioethanol Production: From Novel Raw Materials to Integrated Biorefineries. Processes 2021, 9, 206.
- Morales, M.; Arvesen, A.; Cherubini, F. Integrated process simulation for bioethanol production: Effects of varying lignocellulosic feedstocks on technical performance. Bioresour. Technol. 2021, 328, 124833.
- El Akkari, M.; Ben Fradj, N.; Gabrielle, B.; Djomo, S.N. Spatially-explicit environmental assessment of bioethanol from miscanthus and switchgrass in France. Clean. Circ. Bioecon. 2023, 6, 100059.
- Singh, A.; Chen, C.-W.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. Subcritical Water Pretreatment for the Efficient Valorization of Sorghum Distillery Residue for the Biorefinery Platform. Bioengineering 2022, 10, 38.
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128.
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815.
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282.
- Müller, C.; Scapini, T.; Rempel, A.; Abaide, E.R.; Camargo, A.F.; Nazari, M.T.; Tadioto, V.; Bonatto, C.; Tres, M.V.; Zabot, G.L.; et al. Challenges and opportunities for third-generation ethanol production: A critical review. Eng. Microbiol. 2023, 3, 100056.
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079.
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769.
- Ramachandra, T.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479.
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851.
- Dave, N.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. A critical review on production of bioethanol from macroalgal biomass. Algal Res. 2019, 42, 101606.
- Chowdhury, H.; Loganathan, B. Third-generation biofuels from microalgae: A review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44.
- Li, S.; Li, X.; Ho, S.-H. Microalgae as a solution of third world energy crisis for biofuels production from wastewater toward carbon neutrality: An updated review. Chemosphere 2022, 291, 132863.
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-generation biorefineries: A sustainable platform for food, clean energy, and nutraceuticals production. Biomass Convers. Biorefin. 2022, 12, 4215–4230.
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139.
- Sarwer, A.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Rafiq, S.; Khurram, M.S.; Ul-Haq, N.; Shah, N.S.; Din, A.A.; Ahmad, I.; et al. Suitability of Biofuels Production on Commercial Scale from Various Feedstocks: A Critical Review. ChemBioEng Rev. 2022, 9, 423–441.
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834.
- Mehmood, M.A.; Shahid, A.; Malik, S.; Wang, N.; Javed, M.R.; Haider, M.N.; Verma, P.; Ashraf, M.U.F.; Habib, N.; Syafiuddin, A.; et al. Advances in developing metabolically engineered microbial platforms to produce fourth-generation biofuels and high-value biochemicals. Bioresour. Technol. 2021, 337, 125510.
- Osman, A.I.; Qasim, U.; Jamil, F.; Al-Muhtaseb, A.H.; Abu Jrai, A.; Al-Riyami, M.; Al-Maawali, S.; Al-Haj, L.; Al-Hinai, A.; Al-Abri, M.; et al. Bioethanol and biodiesel: Bibliometric mapping, policies and future needs. Renew. Sustain. Energy Rev. 2021, 152, 111677.
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50.
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA production in integrated lignocellulose biorefineries. New Biotechnol. 2019, 49, 161–168.
- Azeez, M.A.; Orege, J.I. Bamboo, Its Chemical Modification and Products. In Bamboo—Current and Future Prospects; IntechOpen: London, UK, 2018.
- Gabhane, J.; William, S.P.; Gadhe, A.; Rath, R.; Vaidya, A.N.; Wate, S. Pretreatment of banana agricultural waste for bio-ethanol production: Individual and interactive effects of acid and alkali pretreatments with autoclaving, microwave heating and ultrasonication. Waste Manag. 2014, 34, 498–503.
- Díaz, M.J.; Moya, M.; Castro, E. Bioethanol Production from Steam-Exploded Barley Straw by Co-Fermentation with Escherichia coli SL100. Agronomy 2022, 12, 874.
- Nigam, P.S.N.; Gupta, N.; Anthwal, A. Pre-treatment of Agro-Industrial Residues. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Dordrecht, The Netherlands, 2009; pp. 13–33.
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353.
- Soni, S.K.; Sharma, A.; Soni, R. Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability 2023, 15, 3590.
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476.
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807.
- Xu, P.; Cheng, S.; Han, Y.; Zhao, D.; Li, H.; Wang, Y.; Zhang, G.; Chen, C. Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China. Front. Chem. 2020, 8, 595143.
- Rajendran, K.; Drielak, E.; Varma, V.S.; Muthusamy, S.; Kumar, G. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production—A review. Biomass Convers. Biorefin. 2018, 8, 471–483.
- Du, B.; Sharma, L.N.; Becker, C.; Chen, S.; Mowery, R.A.; van Walsum, G.P.; Chambliss, C.K. Effect of varying feedstock–pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng. 2010, 107, 430–440.
- García-Cubero, M.T.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 2009, 100, 1608–1613.
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247.
- Brigham, C. Biopolymers. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 753–770.
- Damayanti, D.; Supriyadi, D.; Amelia, D.; Saputri, D.R.; Devi, Y.L.L.; Auriyani, W.A.; Wu, H.S. Conversion of Lignocellulose for Bioethanol Production, Applied in Bio-Polyethylene Terephthalate. Polymers 2021, 13, 2886.
- Frassoldati, A.; Ranzi, E. Modeling of Thermochemical Conversion of Biomasses. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019.
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173.
- Banu, J.R.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086.
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2017, 5, 52–79.
- Bajpai, P. Wood and Fiber Fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–74.
- Malik, K.; Sharma, P.; Yang, Y.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Lignocellulosic biomass for bioethanol: Insight into the advanced pretreatment and fermentation approaches. Ind. Crops Prod. 2022, 188, 115569.
- Sharma, S.; Kumar, A. Lignin; Springer International Publishing: Cham, Switzerland, 2020.
- Akpan, E.I. Chemistry and Structure of Lignin. In Sustainable Lignin for Carbon Fibers: Principles, Techniques, and Applications; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–50.
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658.
- Hou, J.; Tang, J.; Chen, J.; Zhang, Q. Quantitative Structure-Toxicity Relationship analysis of combined toxic effects of lignocellulose-derived inhibitors on bioethanol production. Bioresour. Technol. 2019, 289, 121724.
- Kordala, N.; Lewandowska, M.; Bednarski, W. Effect of the method for the elimination of inhibitors present in Miscanthus giganteus hydrolysates on ethanol production effectiveness. Biomass Convers. Biorefin. 2023, 13, 2089–2097.
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309.
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351.
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457.
- Das, N.; Jena, P.K.; Padhi, D.; Mohanty, M.K.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefin. 2023, 13, 1503–1527.
- Hideno, A.; Inoue, H.; Tsukahara, K.; Fujimoto, S.; Minowa, T.; Inoue, S.; Endo, T.; Sawayama, S. Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of rice straw. Bioresour. Technol. 2009, 100, 2706–2711.
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball Milling Pretreatment of Oil Palm Biomass for Enhancing Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789.
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700.
- John, I.; Pola, J.; Appusamy, A. Optimization of Ultrasonic Assisted Saccharification of Sweet Lime Peel for Bioethanol Production Using Box–Behnken Method. Waste Biomass Valorization 2019, 10, 441–453.
- Bonfiglio, F.; Cagno, M.; Rey, F.; Torres, M.; Böthig, S.; Menéndez, P.; Mussatto, S.I. Pretreatment of switchgrass by steam explosion in a semi-continuous pre-pilot reactor. Biomass Bioenergy 2019, 121, 41–47.
- De Carvalho, D.M.; de Queiroz, J.H.; Colodette, J.L. Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind. Crops Prod. 2016, 94, 932–941.
- Hilares, R.T.; Swerts, M.P.; Ahmed, M.A.; Ramos, L.; da Silva, S.S.; Santos, J.C. Organosolv Pretreatment of Sugar Cane Bagasse for Bioethanol Production. Ind. Eng. Chem. Res. 2017, 56, 3833–3838.
- Hossain, A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crops Prod. 2021, 167, 113480.
- Singh, A.; Bishnoi, N.R. Enzymatic hydrolysis optimization of microwave alkali pretreated wheat straw and ethanol production by yeast. Bioresour. Technol. 2012, 108, 94–101.
- Ziaei-Rad, Z.; Pazouki, M.; Fooladi, J.; Azin, M.; Gummadi, S.N.; Allahverdi, A. Investigation of a robust pretreatment technique based on ultrasound-assisted, cost-effective ionic liquid for enhancing saccharification and bioethanol production from wheat straw. Sci. Rep. 2023, 13, 446.
- Yang, J.; Lu, X.; Zhang, Y.; Xu, J.; Yang, Y.; Zhou, Q. A facile ionic liquid approach to prepare cellulose fiber with good mechanical properties directly from corn stalks. Green Energy Environ. 2020, 5, 223–231.
- Padilha, C.E.d.A.; Nogueira, C.d.C.; Souza, D.F.d.S.; de Oliveira, J.A.; dos Santos, E.S. Valorization of green coconut fibre: Use of the black liquor of organolsolv pretreatment for ethanol production and the washing water for production of rhamnolipids by Pseudomonas aeruginosa ATCC 27583. Ind. Crops Prod. 2019, 140, 111604.
- Martínez-Patiño, J.C.; Ruiz, E.; Romero, I.; Cara, C.; López-Linares, J.C.; Castro, E. Combined acid/alkaline-peroxide pretreatment of olive tree biomass for bioethanol production. Bioresour. Technol. 2017, 239, 326–335.
- Kim, T.H.; Lee, Y.Y.; Sunwoo, C.; Kim, J.S. Pretreatment of Corn Stover by Low-Liquid Ammonia Recycle Percolation Process. Appl. Biochem. Biotechnol. 2006, 133, 41–58.
- Turner, W.; Greetham, D.; Mos, M.; Squance, M.; Kam, J.; Du, C. Exploring the Bioethanol Production Potential of Miscanthus Cultivars. Appl. Sci. 2021, 11, 9949.
- Kumar, M.N.; Ravikumar, R.; Sankar, M.K.; Thenmozhi, S. New insight into the effect of fungal mycelia present in the bio-pretreated paddy straw on their enzymatic saccharification and optimization of process parameters. Bioresour. Technol. 2018, 267, 291–302.
- Lee, O.K.; Oh, Y.-K.; Lee, E.Y. Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour. Technol. 2015, 196, 22–27.
- Guo, H.; Daroch, M.; Liu, L.; Qiu, G.; Geng, S.; Wang, G. Biochemical features and bioethanol production of microalgae from coastal waters of Pearl River Delta. Bioresour. Technol. 2013, 127, 422–428.
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633.
- Zhao, J.; Lee, J.; Wang, D. A Critical Review on Water Overconsumption in Lignocellulosic Biomass Pretreatment for Ethanol Production through Enzymic Hydrolysis and Fermentation. Energy Fuels 2023, 37, 2667–2680.
- Ranjithkumar, M.; Uthandi, S.; Kumar, P.S.; Muniraj, I.; Thanabal, V.; Rajarathinam, R. Highly crystalline cotton spinning wastes utilization: Pretreatment, optimized hydrolysis and fermentation using Pleurotus florida for bioethanol production. Fuel 2022, 308, 122052.
- Li, F.; Liu, Y.; Jia, J.; Yu, H. Combination of biological pretreatment with deep eutectic solvent pretreatment for enhanced enzymatic saccharification of Pinus massoniana. Bioresour. Technol. 2023, 380, 129110.
- Sun, E.; Zhang, Y.; Yong, C.; Qu, P.; Huang, H.; Xu, Y. Biological fermentation pretreatment accelerated the depolymerization of straw fiber and its mechanical properties as raw material for mulch film. J. Clean. Prod. 2021, 284, 124688.
- Uzuner, S.; Cekmecelioglu, D. Enzymes in the Beverage Industry. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–43.
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of Cellulose by Amorphous Carbon Bearing SO3H, COOH, and OH Groups. J. Am. Chem. Soc. 2008, 130, 12787–12793.
- Chen, H. Lignocellulose biorefinery feedstock engineering. In Lignocellulose Biorefinery Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–86.
- Wada, M.; Ike, M.; Tokuyasu, K. Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form. Polym. Degrad. Stab. 2010, 95, 543–548.
- Vasić, K.; Knez, Ž.K.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753.
- Deshmukh, M.; Pande, A.; Marathe, A. Different particle size study of castor deoiled cake for biofuel production with an environmental sustainability perspective. Heliyon 2022, 8, e09710.
- Kapoor, M.; Semwal, S.; Satlewal, A.; Christopher, J.; Gupta, R.P.; Kumar, R.; Puri, S.K.; Ramakumar, S. The impact of particle size of cellulosic residue and solid loadings on enzymatic hydrolysis with a mass balance. Fuel 2019, 245, 514–520.
- Gao, W.; Li, Z.; Liu, T.; Wang, Y. Production of high-concentration fermentable sugars from lignocellulosic biomass by using high solids fed-batch enzymatic hydrolysis. Biochem. Eng. J. 2021, 176, 108186.
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544.
- Amândio, M.S.T.; Rocha, J.M.S.; Xavier, A.M.R.B. Enzymatic Hydrolysis Strategies for Cellulosic Sugars Production to Obtain Bioethanol from Eucalyptus globulus Bark. Fermentation 2023, 9, 241.
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res. 2021, 56, 102329.
- Wang, W.; Zhang, C.; Tong, S.; Cui, Z.; Liu, P. Enhanced Enzymatic Hydrolysis and Structural Features of Corn Stover by NaOH and Ozone Combined Pretreatment. Molecules 2018, 23, 1300.
- Ostadjoo, S.; Hammerer, F.; Dietrich, K.; Dumont, M.-J.; Friščić, T.; Auclair, K. Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water. Molecules 2019, 24, 4206.
- Godbey, W.T. Fermentation, Beer, and Biofuels. In An Introduction to Biotechnology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 331–351.
- Spector, M.P. Metabolism, Central (Intermediary). In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 242–264.




