Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kong, C.; Chen, J.; Li, P.; Wu, Y.; Zhang, G.; Sang, B.; Li, R.; Shi, Y.; Cui, X.; Zhou, T. Respiratory Toxicology of Graphene-Based Nanomaterials. Encyclopedia. Available online: https://encyclopedia.pub/entry/54355 (accessed on 03 March 2026).
Kong C, Chen J, Li P, Wu Y, Zhang G, Sang B, et al. Respiratory Toxicology of Graphene-Based Nanomaterials. Encyclopedia. Available at: https://encyclopedia.pub/entry/54355. Accessed March 03, 2026.
Kong, Chunxue, Junwen Chen, Ping Li, Yukang Wu, Guowei Zhang, Bimin Sang, Rui Li, Yuqin Shi, Xiuqing Cui, Ting Zhou. "Respiratory Toxicology of Graphene-Based Nanomaterials" Encyclopedia, https://encyclopedia.pub/entry/54355 (accessed March 03, 2026).
Kong, C., Chen, J., Li, P., Wu, Y., Zhang, G., Sang, B., Li, R., Shi, Y., Cui, X., & Zhou, T. (2024, January 25). Respiratory Toxicology of Graphene-Based Nanomaterials. In Encyclopedia. https://encyclopedia.pub/entry/54355
Kong, Chunxue, et al. "Respiratory Toxicology of Graphene-Based Nanomaterials." Encyclopedia. Web. 25 January, 2024.
Copy Citation
Graphene-based nanomaterials (GBNs) consist of a single or few layers of graphene sheets or modified graphene including pristine graphene, graphene nanosheets (GNS), graphene oxide (GO), reduced graphene oxide (rGO), as well as graphene modified with various functional groups or chemicals (e.g., hydroxyl, carboxyl, and polyethylene glycol), which are frequently used in industrial and biomedical applications owing to their exceptional physicochemical properties.
graphene
nanomaterials
respiratory toxicology
1. Introduction
Graphene is a novel kind of nanomaterial composed of a single layer of carbon atoms, which was initially isolated from crystalline graphite in 2004 [1]. With a thickness of merely 0.335 nm, it is presently acknowledged as the thinnest two-dimensional material, serving as the fundamental substrate for sp2 hybridization. Surface modification, composites, and the construction of “nano-architecture” are employed to fabricate graphene-based nanomaterials (GBNs) with diverse dimensional and spatial structures. These GBNs primarily encompass pristine graphene, graphene nanosheets (GNS), graphene oxide (GO), reduced graphene oxide (rGO), as well as graphene modified with various functional groups or chemicals such as hydroxyl, carboxyl, and polyethylene glycol. Until now, GBNs have been extensively utilized in various fields, including electronics, biomedicine, pharmaceutical engineering, and tissue engineering, owing to their advantageous characteristics such as remarkable mechanical strength, exceptional electrical and thermal conductivity, high specific surface area, and valuable antimicrobial properties [2][3]. With the widespread use of GBNs, the potential for human exposure raises concerns about the safety of GBNs that showed potential behavioral, immune, pulmonary, systemic, ocular, reproductive, and developmental toxicity and genotoxicity [4][5]. Currently, reproductive, immunologic, and cytotoxicity reviews of GBNs have been conducted, but the respiratory system, as a major target of GBNs, has not yet been reported to comprehensively characterize and compare the potential impacts of different GBNs on the respiratory system. At present, toxicological studies on GBNs have focused on laboratory animal and cellular experiments, with a lack of epidemiological data on populations exposed to GBNs.
2. Exposure and Metabolism of GBNs
GBNs are known to exhibit high resistance to degradation once introduced into the natural environment, facilitating their transfer or enrichment among environmental mediators or organisms, with direct or indirect toxic effects on both aquatic and terrestrial animals and plants [6]. Several investigations have indicated that GBNs can infiltrate organisms via various routes such as skin, respiratory tract, digestive tract, and eyes, subsequently accumulating in tissues and organs such as the lung, liver, spleen, and kidney [7][8]. Although human data, case reports, or medical investigations of workers exposed to GBNs are not currently available, monitoring data in the work environment and laboratory data on GBN toxicity are important for the health risk assessment of GBNs.
At present, only a few works of literature have reported airborne GBN concentrations in occupational environments. Graphene concentrations are mostly below detection limits in workplace monitoring and in studies modeling graphene exposure [9][10]. In a large-scale graphene production workplace, occupational exposure to GBNs by workers was found to be between 909 and 6438 particles per cm3 after an average exposure of 8 h, which is equivalent to 0.38–3.86 μg·cm−3 [11]. Airborne GNS concentrations of 2.27 and 0.017 mg·m−3 were measured when collecting the products from the discharge vessel [12]. This concentration range is similar to the range that did not cause toxicity in rats during a five-day repeated exposure to nasal inhalation only of GNS (0.12–1.88 mg·m−3) [13]. Although airborne levels of GBNs in the occupational environment seem to be lower, it is noteworthy that the chronic toxicity and/or carcinogenicity of various GBNs should not be underestimated.
Animal experiments have demonstrated that the elimination of GBNs from the body is primarily contingent upon their size, with smaller GBNs being rapidly removed, while larger GBNs are more prone to retention [14]. Specifically, the larger GBNs entering via the respiratory tract tended to persist in the non-ciliated region for a long time, leading to the development of lung inflammation, edema, granuloma, and fibrosis [15]. In addition, the surface of GBNs can be modified through polyethylene glycol (PEG) functionalization, resulting in a reduction in lung inflammatory and toxic responses caused by GBNs [16]. This modification has been found to have positive biological effects, including antioxidant, anti-apoptotic, antibacterial, and anticancer effects [17]. Therefore, a comprehensive understanding of the effects of GBNs on the respiratory system is more conducive to their application and development in various fields.
3. Respiratory Toxicity of GBNs
Animal models of GBN toxicity can be constructed by diverse exposure routes, including nasal inhalation, intratracheal instillation, intravenous injection, and intraperitoneal injection, leading to the development of respiratory disorders such as lung inflammation, fibrosis, and granuloma. Furthermore, the surface modification of GBNs with various functional groups can potentially exhibit both beneficial biological effects, such as anti-inflammatory and anticancer properties, as well as exacerbate the toxic effects associated with GBNs. Consequently, to elucidate the respiratory toxicity characteristics of different types of GBNs, discern their distinctions, and establish a more comprehensive foundation for toxicity assessment in the extensive utilization of GBNs. And the respiratory toxicity induced by GBN exposure in vitro and in vivo is summarized in Figure 1.
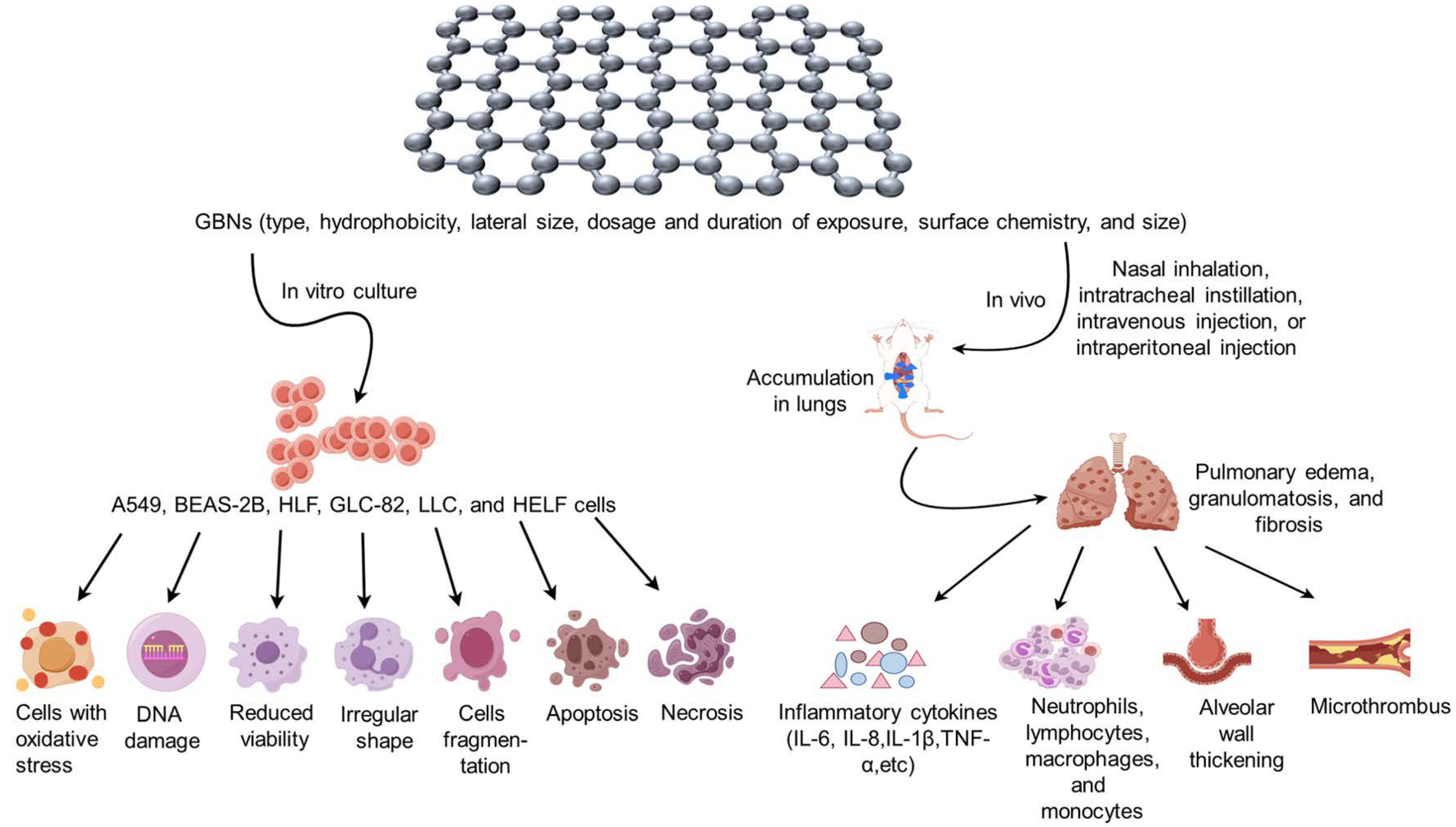
Figure 1. Respiratory toxicity induced by graphene-based nanomaterial (GBN) exposure in vitro and in vivo. When cultured in vitro with the addition of GBNs, lung cancer human alveolar basal epithelial cells (A549), human bronchial epithelial cells (BEAS-2B), human lung fibroblast cells (HLF), lung cancer cell line (GLC-82), Lewis lung carcinoma (LLC), and human embryonic lung fibroblasts (HELF) exhibited oxidative stress, DNA damage, decreased viability, irregular cell shape, cell fragmentation, apoptosis, necrosis, and other related phenomena. In the in vivo experiments, various types, sizes, dosages, and modes of exposure of GBNs were administered to the animal organism through nasal inhalation, intratracheal instillation, intravenous injection, intraperitoneal injection, etc. This resulted in the accumulation of GBNs in the lungs, causing pathological changes such as pulmonary edema, granulomatosis, and fibrosis. The study observed an increase in the expression of inflammatory cytokines, including IL-6, IL-8, IL-1β, and TNF-α, as well as an increase in the number of inflammatory cells such as neutrophils and lymphocytes. Additionally, thickening of alveolar walls and formation of microthrombus were observed.
3.1. Graphene and GNS
In vitro cellular experiments revealed that human bronchial epithelial cells (BEAS-2B) or lung cancer human alveolar basal epithelial cells (A549) exposed to graphene or GNS showed a notable reduction in cell viability, accompanied by the occurrence of apoptosis and necrosis in a dose- and time-dependent manner [18][19][20]. Animal studies indicated that, following nasal inhalation of graphene at concentrations ranging from 0.68 to 3.86 mg/m3 for 6 h per day, there was a slight increase in the number of neutrophils, lymphocytes, and monocytes in the peripheral blood on day 1, but the count of white blood cells and lymphocytes returned to a normal level by day 3. Conversely, no discernible pathological alterations such as inflammation, fibrosis, or granuloma formation were observed in the lungs of mice following a single intratracheal administration of 50 μg of graphene on days 1, 7, and 42. And well-dispersed graphene would induce more minimal toxicity in the lung than aggregated graphene [21][22]. At present, the toxicity of graphene has not been fully elucidated, so its potential toxicity is still not to be underestimated and deserves our attention.
On days 1, 28, and 90, nasal inhalation of GNS at concentrations ranging from 0.1 to 3.2 mg/m3 (lateral size < 2 μm or 2–7 μm) for 6 h per day, five days per week, did not result in any pulmonary toxicity or proinflammatory effects in rats [13][23]. However, when a single intratracheal instillation of GNS (lateral size < 2 μm) at a dose from 0.3 to 3 mg was administered on day 1, an increase in the number of neutrophils in the bronchoalveolar lavage fluid (BALF) of the rats was observed in a dose-dependent relationship, and acute multifocal alveolitis occurred, which disappeared from the lungs of the rats on day 28 [24]. But in mice, a single intratracheal administration of fewer layers (1–10 layers) of 50 μg GNS with a lateral size less than 4 μm that were phagocytosed by macrophages remained in the lungs and metastasized to the mediastinal lymph nodes, inducing high levels of inflammatory cytokines, including IL-1β, IL-6, TNF-α, MIP-2, and IL-8 in BALF during the period of 1–7 days following [25]. Additionally, the lungs of mice exhibited pathological changes such as inflammatory edema and granulomas, which were predominantly infiltrated by neutrophils and eosinophils. However, with the prolongation of time, the lung structure of the mice gradually returned to normal by the 28th day after exposure to GNS [18][25][26]. Furthermore, 4 h after a single intratracheal administration of 40 μg GNS with lateral sizes smaller than 2 μm, 5 μm, and 20 μm, the levels of neutrophil counts, macrophage colony-stimulating factor (MCSF), myeloperoxidase (MPO), and lactate dehydrogenase (LDH) expression in BALF were significantly elevated for GNS with lateral sizes of 5 μm and 20 μm compared to those with a lateral size smaller than 2 μm. And the inflammation persisted in the lungs on day 30, returning to normal on day 60 [27]. However, the research has demonstrated that even on day 90 following a single intratracheal instillation of 25–100 μg of GNS (with a lateral dimension of approximately 500 nm), GNS remains present in the lung tissue of mice and induces alterations in the lung gene profile and the expression level of Th1/Th2 cytokines, which subsequently disrupt the physiological and immune homeostasis of the organism [28]. Consequently, it can be concluded that GNS exerts a certain degree of toxicity on lung cells. However, it is important to note that the toxic effects on the lungs may vary depending on the distinct physical properties of GNS such as lay number, lateral size, exposure route, and dose, as well as animal species. When GNS with a high concentration or transverse size enters the lungs, it can induce acute inflammatory damage. However, with the prolongation of time, the lungs possess the capacity for self-repair, resulting in gradual restoration of the inflammatory lesions to their normal state. Nevertheless, due to the diminutive size of GNSs, it is difficult to eliminate them from the body, so they could accumulate in the lungs for a long time and readily traverse the gas–blood barrier during entry into the circulatory system, thereby causing an imbalance in lung immunity and the occurrence of chronic inflammation.
3.2. GO
In vitro cellular assays have demonstrated that GO exhibits dose- or time-dependent cytotoxicity and genotoxicity against a variety of cell types, including A549, BEAS-2B, human bronchial epithelial cells (16HBE), human lung fibroblast (HLF) cells, and a lung cancer cell line (GLC-82). The toxicity of GO derived from different oxidation methods exhibits variability in A549 cells, with toxicity increasing as the oxygen content rises [29]. At concentrations ranging from 1 to 100 μg/mL, GO shows a significant decrease in the viability of HLF cells, accompanied by an increase in intracellular ROS production and the occurrence of cellular morphological irregularities, fragmentation, or apoptosis [30]. But there was no significant cytotoxicity in the murine lung epithelial cell line (FE1) after exposure to GO mainly consisting of 2–3 graphene layers with a lateral size of 1–2 μm at relatively high doses (5–200 μg/mL), even if excessive ROS were generated [31].
In animal experiments, no obvious pathological alterations were observed in the lungs of rats following active nasal intranasal inhalation of 0.6–10 mg/m3 GO (0.5–5 μm lateral size) for a duration of 5 days [32]. However, exposure to 0.05–500 mg/kg GO (0.1–10 μm) or nanographene oxide (NGO) by intratracheal instillation, tail intravenous injection, or intraperitoneal injection could lead to acute and chronic pulmonary inflammatory damage with progressive dose-dependent aggravation in mice. There were obvious pathological changes in the lungs, mainly characterized by neutrophil infiltration, increased pulmonary capillary permeability, microthrombosis, alveolar wall thickening, epithelioid granulomas, pulmonary edema, and peribronchiolar collagen deposition on day 7 after exposure. And elevated levels of lactate dehydrogenase (LDH), alkaline phosphatase (ALP), TNF-α, IL-6, and IL-1β were also shown in a marked dose-dependent manner [16][33][34][35][36][37][38][39]. On day 90, it was observed that NGO had not been completely eliminated from the lungs [40]. Furthermore, the size of GO plays a significant role in its toxicity [41], as micro-sized GO particles exhibit a reduced clearance rate in the lungs and induce more severe inflammation and fibrosis compared to nano-sized GO particles [42][43][44][45].
In an animal model of ovalbumin (OVA)-induced allergic asthma, administration of GO via daily intraperitoneal injection at doses ranging from 0.04 to 4 mg/kg during the sensitization period resulted in a deterioration of airway hyperresponsiveness and collagen deposition, elevated IL-4, and decreased IFN-γ expression in the lungs on day 40 [46], indicating marked exacerbation of OVA-induced asthmatic responses, as the dose of GO was increased. Another study discovered that a single intratracheal administration of 80 μg of GO on the first day of OVA sensitization significantly reduced eosinophil accumulation and attenuated Th2 immune response in the lung on the second day following challenge (31 days after sensitization). However, this intervention also led to an increase in the level of acidic mammalian chitinase (AMCase) released by alveolar macrophages, as well as heightened airway hyperresponsiveness and airway remodeling [47].
Therefore, it can be postulated that the impact of GO on the induction of lung damage in animals is influenced by various factors, including size and oxygen content of GO, concentration of the administered dose, duration of exposure, pathways into the body, as well as characteristics of animal species, exogenous toxicant, and underlying disease.
3.3. rGO
Currently, the primary focus of in vitro cellular experiments involving rGO is the examination of the effects on FE1 and A549 cells. rGO with a lateral size of 1–2 µm did not induce significant cytotoxicity or genotoxicity in FE1 cells at relatively high concentrations (5–200 µg/mL) [31]. However, rGO with a lateral size from 50 to 700 nm enhanced the migration and invasion of A549 cells at concentrations ranging from 1 to 10 µg/mL, whereas rGO at a concentration of 20 µg/mL suppressed cell migration and invasion without affecting cell viability [48]. However, it was also discovered that concentrations equal to or greater than 5 μg/mL of rGO, with a lateral size of approximately 900 nm, significantly inhibited A549 cell viability in a dose-dependent manner. This inhibitory effect was accompanied by an increase in intracellular ROS production, activation of the NF-κB pathway, release of IL-8 expression, and induction of apoptosis [3][49][50]. The variation in cytotoxicity primarily depends on the lateral size of the rGO, whereby larger sizes have a more pronounced impact on cell viability at equivalent concentrations. Another animal experiment conducted intratracheal instillation administration of 18–162 μg of rGO (1–2 μm), which resulted in a predominantly neutrophilic inflammatory response in the lungs of mice from day 1 to day 90, with increased DNA damage in the inflammatory cells of BALF in a clear dose-effect relationship; however, no fibrosis formation was observed [9].
3.4. Functionalized GBNs
Functionalized GBNs are a subset of GBNs that are distinguished by their integration of functional groups (e.g., hydroxyl groups, epoxy groups, etc.), which are formed during the preparation process. These functional groups are attached to the graphene surface through covalent bonding, noncovalent interactions, or doping [51]. Consequently, functionalized GBNs possess a combination of properties derived from both graphene and the modified functional groups. There are two main categories into which they can be broadly classified: graphene/inorganic nanocomposites, which encompass metals and carbon nanomodified materials, and graphene/polymer composites, which involve synthetic polymers and modifications of natural polymers. Functionalized GBNs exhibit anticancer, anti-inflammatory, and anti-apoptotic properties, thereby reducing the toxicity of GBNs and enhancing their potential as drug adjuvants. Nevertheless, it should be acknowledged that the vanillin modification could exacerbate the toxic effects of GBNs [52].
3.4.1. GBN/Inorganic Nanocomposites
Respiratory toxicity studies of GBN/inorganic nanocomposites were primarily conducted on lung fibroblasts and lung cancer cells, specifically human embryonic lung fibroblasts (HELF), A549 cells, human large cell lung cancer (H460), human non-small-cell lung cancer (H1975), and Lewis lung carcinoma (LLC). According to recent studies [53][54], incubation of HELF, A549, H460, H1975, and LLC cells with graphene/nickel oxide nanocomposites (Gr/NiO NCs) and iron/platinum nanoparticle GO (FePt/GO) at concentrations ranging from 0 to 100 μg/mL for 24 h did not yield any significant alterations in the cell viability of HELF, but GBNs decrease other cancer cell viability and cell death rate in a dose-dependent manner, indicating an enhanced anticancer efficacy. To serve as a drug delivery method, composite materials consisting of synthesized graphene oxide/TiO2/doxorubicin (GO/TiO2/DOX) were incorporated into a chitosan/poly (lactic acid) (PLA) solution. The increased concentrations of GO and TiO2/DOX significantly suppressed the proliferative capacity of A549 lung cancer cells [55].
3.4.2. GBN/Polymer Composites
In the cellular experiments, it was observed that amino-modified GNSs effectively reduced inflammation and genotoxicity induced by GNSs in human-transformed type I (TT1) alveolar epithelial cells and human lung epithelial cells (16HBE14o-) [56][57][58]. On the other hand, at concentrations ranging from 1 to 100 μg/mL, PEG-modified GO showed significantly lower toxicity towards HLF cells compared to unmodified GO. In contrast, GO modified with polyethylenimine (PEI) exhibited a notable increase in its cytotoxic effect [30].
Animal experiments have demonstrated that the levels of LDH and total protein in the BALF of rats were elevated and accompanied by a predominantly neutrophilic inflammation on day 1 following the intratracheal instillation of GNS (1 mg/rat) modified with carboxy (COOH), N–H, and N=H, respectively, but this inflammation gradually diminished over a period of 28 days [24]. Additionally, after three months of administration of poly sodium 4-styrenesulfonate (PSS) within the dosage range of 0–16 mg/kg or PEG-modified GO at a dosage of 5 mg/kg via tail intravenous injection, the lungs of mice exhibited a dose-dependent chronic inflammation with macrophage infiltration, whereas PEG modification accelerated GO clearance, attenuated collagen deposition in lung tissue, and induced chronic lung fibrosis [59][60]. It can be hypothesized that the toxicity and subsequent biological effects of GBN/polymer composites primarily rely on the physicochemical characteristics of the surface-modified complexes, which determine their ability to remove GBNs and alleviate their toxicity and inflammatory responses. Consequently, these properties dominate the potential and applicability of these composites in the biomedical field.
GBN/polymer composites are mainly utilized to exploit the superior biocompatibility and hydrophilicity of GBNs, as well as other physical properties, to serve as carriers to enhance the hydrophobicity of cancer therapeutics, thereby improving the targeting of cancer therapies to cancer cells. For instance, it has been observed that a concentration of 1 μg/mL of doxorubicin (DOX)-loaded hyaluronic acid (HA)-modified Q- graphene is capable of inducing a 50% reduction in A549 cells [61]. Similarly, the inhibitory effect of anticancer drugs on the growth and proliferation of A549 cells and NSCLC cell line NCI-H460 can be enhanced by the use of oridonin-loaded chitosan GO (CS-GO), HA-functionalized GO-based gefitinib delivery system (NGO-SS-HA-Gef), and paclitaxel-loaded GO (PTX-GO). These delivery systems have concentrations ranging from 16 to 64 μg/mL for CS-GO, 1 to 400 μg/mL for NGO-SS-HA-Gef, and 10 to 150 μg/mL for PTX-GO [62][63][64].
References
- Gulzar, A.; Yang, P.; He, F.; Xu, J.; Yang, D.; Xu, L.; Jan, M.O. Bioapplications of graphene constructed functional nanomaterials. Chem.-Biol. Interact. 2017, 262, 69–89.
- Hoyle, C.; Rivers-Auty, J.; Lemarchand, E.; Vranic, S.; Wang, E.; Buggio, M.; Rothwell, N.J.; Allan, S.M.; Kostarelos, K.; Brough, D. Small, Thin Graphene Oxide Is Anti-inflammatory Activating Nuclear Factor Erythroid 2-Related Factor 2 via Metabolic Reprogramming. ACS Nano 2018, 12, 11949–11962.
- Reshma, S.C.; Syama, S.; Mohanan, P.V. Nano-biointeractions of PEGylated and bare reduced graphene oxide on lung alveolar epithelial cells: A comparative in vitro study. Colloids Surf. B Biointerfaces 2016, 140, 104–116.
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies on graphene-based nanomaterials in laboratory animals. Regul. Toxicol. Pharmacol. RTP 2017, 85, 7–24.
- Orecchioni, M.; Ménard-Moyon, C.; Delogu, L.G.; Bianco, A. Graphene and the immune system: Challenges and potentiality. Adv. Drug Deliv. Rev. 2016, 105, 163–175.
- Yan, Z.; Yang, X.; Lynch, I.; Cui, F. Comparative evaluation of the mechanisms of toxicity of graphene oxide and graphene oxide quantum dots to blue-green algae Microcystis aeruginosa in the aquatic environment. J. Hazard. Mater. 2022, 425, 127898.
- Liu, W.; Sun, C.; Liao, C.; Cui, L.; Li, H.; Qu, G.; Yu, W.; Song, N.; Cui, Y.; Wang, Z.; et al. Graphene Enhances Cellular Proliferation through Activating the Epidermal Growth Factor Receptor. J. Agric. Food Chem. 2016, 64, 5909–5918.
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275.
- Bengtson, S.; Knudsen, K.B.; Kyjovska, Z.O.; Berthing, T.; Skaug, V.; Levin, M.; Koponen, I.K.; Shivayogimath, A.; Booth, T.J.; Alonso, B.; et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS ONE 2017, 12, e0178355.
- Lee, J.H.; Han, J.H.; Kim, J.H.; Kim, B.; Bello, D.; Kim, J.K.; Lee, G.H.; Sohn, E.K.; Lee, K.; Ahn, K.; et al. Exposure monitoring of graphene nanoplatelets manufacturing workplaces. Inhal. Toxicol. 2016, 28, 281–291.
- Boccuni, F.; Ferrante, R.; Tombolini, F.; Lega, D.; Antonini, A.; Alvino, A.; Pingue, P.; Beltram, F.; Sorba, L.; Piazza, V.; et al. Workers’ Exposure to Nano-Objects with Different Dimensionalities in R&D Laboratories: Measurement Strategy and Field Studies. Int. J. Mol. Sci. 2018, 19, 349.
- Heitbrink, W.A.; Lo, L.M.; Dunn, K.H. Exposure controls for nanomaterials at three manufacturing sites. J. Occup. Environ. Hyg. 2015, 12, 16–28.
- Kim, J.K.; Shin, J.H.; Lee, J.S.; Hwang, J.H.; Lee, J.H.; Baek, J.E.; Kim, T.G.; Kim, B.W.; Kim, J.S.; Lee, G.H.; et al. 28-Day inhalation toxicity of graphene nanoplatelets in Sprague-Dawley rats. Nanotoxicology 2016, 10, 891–901.
- Loret, T.; de Luna, L.A.V.; Lucherelli, M.A.; Fordham, A.; Lozano, N.; Bianco, A.; Kostarelos, K.; Bussy, C. Lung Persistence, Biodegradation, and Elimination of Graphene-Based Materials are Predominantly Size-Dependent and Mediated by Alveolar Phagocytes. Small 2023, 19, e2301201.
- Patil, R.; Bahadur, P.; Tiwari, S. Dispersed graphene materials of biomedical interest and their toxicological consequences. Adv. Colloid Interface Sci. 2020, 275, 102051.
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281.
- Rosli, N.F.; Fojtů, M.; Fisher, A.C.; Pumera, M. Graphene Oxide Nanoplatelets Potentiate Anticancer Effect of Cisplatin in Human Lung Cancer Cells. Langmuir ACS J. Surf. Colloids 2019, 35, 3176–3182.
- Park, E.J.; Lee, G.H.; Han, B.S.; Lee, B.S.; Lee, S.; Cho, M.H.; Kim, J.H.; Kim, D.W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2015, 89, 1557–1568.
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87.
- Frontiñan-Rubio, J.; González, V.J.; Vázquez, E.; Durán-Prado, M. Rapid and efficient testing of the toxicity of graphene-related materials in primary human lung cells. Sci. Rep. 2022, 12, 7664.
- Schinwald, A.; Murphy, F.; Askounis, A.; Koutsos, V.; Sefiane, K.; Donaldson, K.; Campbell, C.J. Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology 2014, 8, 824–832.
- Shin, J.H.; Han, S.G.; Kim, J.K.; Kim, B.W.; Hwang, J.H.; Lee, J.S.; Lee, J.H.; Baek, J.E.; Kim, T.G.; Kim, K.S.; et al. 5-Day repeated inhalation and 28-day post-exposure study of graphene. Nanotoxicology 2015, 9, 1023–1031.
- Creutzenberg, O.; Oliveira, H.; Farcal, L.; Schaudien, D.; Mendes, A.; Menezes, A.C.; Tischler, T.; Burla, S.; Ziemann, C. PLATOX: Integrated In Vitro/In Vivo Approach for Screening of Adverse Lung Effects of Graphene-Related 2D Nanomaterials. Nanomaterials 2022, 12, 1254.
- Lee, J.K.; Jeong, A.Y.; Bae, J.; Seok, J.H.; Yang, J.Y.; Roh, H.S.; Jeong, J.; Han, Y.; Jeong, J.; Cho, W.S. The role of surface functionalization on the pulmonary inflammogenicity and translocation into mediastinal lymph nodes of graphene nanoplatelets in rats. Arch. Toxicol. 2017, 91, 667–676.
- Schinwald, A.; Murphy, F.A.; Jones, A.; MacNee, W.; Donaldson, K. Graphene-based nanoplatelets: A new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano 2012, 6, 736–746.
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13, 7.
- Roberts, J.R.; Mercer, R.R.; Stefaniak, A.B.; Seehra, M.S.; Geddam, U.K.; Chaudhuri, I.S.; Kyrlidis, A.; Kodali, V.K.; Sager, T.; Kenyon, A.; et al. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: A member of the graphene-based nanomaterial family. Part. Fibre Toxicol. 2016, 13, 34.
- Park, E.J.; Lee, S.J.; Lee, K.; Choi, Y.C.; Lee, B.S.; Lee, G.H.; Kim, D.W. Pulmonary persistence of graphene nanoplatelets may disturb physiological and immunological homeostasis. J. Appl. Toxicol. JAT 2017, 37, 296–309.
- Chng, E.L.; Pumera, M. The toxicity of graphene oxides: Dependence on the oxidative methods used. Chemistry 2013, 19, 8227–8235.
- Wang, A.; Pu, K.; Dong, B.; Liu, Y.; Zhang, L.; Zhang, Z.; Duan, W.; Zhu, Y. Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells. J. Appl. Toxicol. JAT 2013, 33, 1156–1164.
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagen. 2016, 57, 469–482.
- Kim, Y.H.; Jo, M.S.; Kim, J.K.; Shin, J.H.; Baek, J.E.; Park, H.S.; An, H.J.; Lee, J.S.; Kim, B.W.; Kim, H.P.; et al. Short-term inhalation study of graphene oxide nanoplates. Nanotoxicology 2018, 12, 224–238.
- Liu, J.H.; Yang, S.T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812.
- Zhang, L.; Ouyang, S.; Zhang, H.; Qiu, M.; Dai, Y.; Wang, S.; Wang, Y.; Ou, J. Graphene oxide induces dose-dependent lung injury in rats by regulating autophagy. Exp. Ther. Med. 2021, 21, 462.
- Li, Y.; Wang, Y.; Tu, L.; Chen, D.; Luo, Z.; Liu, D.; Miao, Z.; Feng, G.; Qing, L.; Wang, S. Sub-Acute Toxicity Study of Graphene Oxide in the Sprague-Dawley Rat. Int. J. Environ. Res. Public Health 2016, 13, 1149.
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8.
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769.
- El-Yamany, N.A.; Mohamed, F.F.; Salaheldin, T.A.; Tohamy, A.A.; Abd El-Mohsen, W.N.; Amin, A.S. Graphene oxide nanosheets induced genotoxicity and pulmonary injury in mice. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2017, 69, 383–392.
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Shrivastava, S.; Grácio, J.J.; Dash, D. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano 2011, 5, 4987–4996.
- Li, B.; Yang, J.; Huang, Q.; Zhang, Y.; Peng, C.; Zhang, Y.; He, Y.; Shi, J.; Li, W.; Hu, J.; et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater 2013, 5, e44.
- Taheriazam, A.; Abad, G.G.Y.; Hajimazdarany, S.; Imani, M.H.; Ziaolhagh, S.; Zandieh, M.A.; Bayanzadeh, S.D.; Mirzaei, S.; Hamblin, M.R.; Entezari, M.; et al. Graphene oxide nanoarchitectures in cancer biology: Nano-modulators of autophagy and apoptosis. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 503–522.
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-Dependent Pulmonary Impact of Thin Graphene Oxide Sheets in Mice: Toward Safe-by-Design. Adv. Sci. 2020, 7, 1903200.
- Wang, X.; Duch, M.C.; Mansukhani, N.; Ji, Z.; Liao, Y.P.; Wang, M.; Zhang, H.; Sun, B.; Chang, C.H.; Li, R.; et al. Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano 2015, 9, 3032–3043.
- Loret, T.; de Luna, L.A.V.; Fordham, A.; Arshad, A.; Barr, K.; Lozano, N.; Kostarelos, K.; Bussy, C. Innate but Not Adaptive Immunity Regulates Lung Recovery from Chronic Exposure to Graphene Oxide Nanosheets. Adv. Sci. 2022, 9, e2104559.
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515.
- Shang, S.; Li, J.; Zhao, Y.; Xi, Z.; Lu, Z.; Li, B.; Yang, X.; Li, R. Oxidized graphene-aggravated allergic asthma is antagonized by antioxidant vitamin E in Balb/c mice. Environ. Sci. Pollut. Res. Int. 2017, 24, 1784–1793.
- Shurin, M.R.; Yanamala, N.; Kisin, E.R.; Tkach, A.V.; Shurin, G.V.; Murray, A.R.; Leonard, H.D.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Graphene oxide attenuates Th2-type immune responses, but augments airway remodeling and hyperresponsiveness in a murine model of asthma. ACS Nano 2014, 8, 5585–5599.
- Liao, Y.; Wang, W.; Huang, X.; Sun, Y.; Tian, S.; Cai, P. Reduced graphene oxide triggered epithelial-mesenchymal transition in A549 cells. Sci. Rep. 2018, 8, 15188.
- Di Ianni, E.; Møller, P.; Vogel, U.B.; Jacobsen, N.R. Pro-inflammatory response and genotoxicity caused by clay and graphene nanomaterials in A549 and THP-1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 872, 503405.
- Tabish, T.A.; Pranjol, M.Z.I.; Hayat, H.; Rahat, A.A.M.; Abdullah, T.M.; Whatmore, J.L.; Zhang, S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology 2017, 28, 504001.
- Shaheen, S.; Saeed, Z.; Ahmad, A.; Pervaiz, M.; Younas, U.; Mahmood Khan, R.R.; Luque, R.; Rajendran, S. Green synthesis of graphene-based metal nanocomposite for electro and photocatalytic activity; recent advancement and future prospective. Chemosphere 2023, 311, 136982.
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Kim, J.H. Differential Immunomodulatory Effect of Graphene Oxide and Vanillin-Functionalized Graphene Oxide Nanoparticles in Human Acute Monocytic Leukemia Cell Line (THP-1). Int. J. Mol. Sci. 2019, 20, 247.
- Rajivgandhi, G.; Maruthupandy, M.; Quero, F.; Li, W.J. Graphene/nickel oxide nanocomposites against isolated ESBL producing bacteria and A549 cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 829–843.
- Ma, S.; Miao, H.; Luo, Y.; Sun, Y.; Tian, X.; Wang, F.; You, C.; Peng, S.; Tang, G.; Yang, C.; et al. FePt/GO Nanosheets Suppress Proliferation, Enhance Radiosensitization and Induce Autophagy of Human Non-Small Cell Lung Cancer Cells. Int. J. Biol. Sci. 2019, 15, 999–1009.
- Samadi, S.; Moradkhani, M.; Beheshti, H.; Irani, M.; Aliabadi, M. Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO2 composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer. Int. J. Biol. Macromol. 2018, 110, 416–424.
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Tarat, A.; Jenkins, G.J.; Doak, S.H. Few-layer graphene induces both primary and secondary genotoxicity in epithelial barrier models in vitro. J. Nanobiotechnol. 2021, 19, 24.
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Miller, M.; Lopez, S.B.; Williams, A.; Tarat, A.; Jenkins, G.J.; Doak, S.H. In Vitro Primary-Indirect Genotoxicity in Bronchial Epithelial Cells Promoted by Industrially Relevant Few-Layer Graphene. Small 2021, 17, e2002551.
- Chatterjee, N.; Yang, J.; Choi, J. Differential genotoxic and epigenotoxic effects of graphene family nanomaterials (GFNs) in human bronchial epithelial cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 1–10.
- Wen, K.P.; Chen, Y.C.; Chuang, C.H.; Chang, H.Y.; Lee, C.Y.; Tai, N.H. Accumulation and toxicity of intravenously-injected functionalized graphene oxide in mice. J. Appl. Toxicol. JAT 2015, 35, 1211–1218.
- Li, B.; Zhang, X.Y.; Yang, J.Z.; Zhang, Y.J.; Li, W.X.; Fan, C.H.; Huang, Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int. J. Nanomed. 2014, 9, 4697–4707.
- Luo, Y.; Cai, X.; Li, H.; Lin, Y.; Du, D. Hyaluronic Acid-Modified Multifunctional Q-Graphene for Targeted Killing of Drug-Resistant Lung Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 4048–4055.
- Liu, J.; Zhang, D.; Lian, S.; Zheng, J.; Li, B.; Li, T.; Jia, L. Redox-responsive hyaluronic acid-functionalized graphene oxide nanosheets for targeted delivery of water-insoluble cancer drugs. Int. J. Nanomed. 2018, 13, 7457–7472.
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276.
- Arya, N.; Arora, A.; Vasu, K.S.; Sood, A.K.; Katti, D.S. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: A reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 2013, 5, 2818–2829.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
511
Revisions:
4 times
(View History)
Update Date:
29 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





