Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura LOPEZ-GÓMEZ | -- | 2431 | 2024-01-25 13:42:01 | | | |
| 2 | Catherine Yang | Meta information modification | 2431 | 2024-01-26 01:33:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Montalbán-Rodríguez, A.; Abalo, R.; López-Gómez, L. Enteric Glial Cells and Their Involvement in PD. Encyclopedia. Available online: https://encyclopedia.pub/entry/54348 (accessed on 07 February 2026).
Montalbán-Rodríguez A, Abalo R, López-Gómez L. Enteric Glial Cells and Their Involvement in PD. Encyclopedia. Available at: https://encyclopedia.pub/entry/54348. Accessed February 07, 2026.
Montalbán-Rodríguez, Alba, Raquel Abalo, Laura López-Gómez. "Enteric Glial Cells and Their Involvement in PD" Encyclopedia, https://encyclopedia.pub/entry/54348 (accessed February 07, 2026).
Montalbán-Rodríguez, A., Abalo, R., & López-Gómez, L. (2024, January 25). Enteric Glial Cells and Their Involvement in PD. In Encyclopedia. https://encyclopedia.pub/entry/54348
Montalbán-Rodríguez, Alba, et al. "Enteric Glial Cells and Their Involvement in PD." Encyclopedia. Web. 25 January, 2024.
Copy Citation
The brain–gut axis has been identified as an important contributor to the physiopathology of Parkinson’s disease (PD). In this pathology, inflammation is thought to be driven by the damage caused by aggregation of α-synuclein in the brain. Activation and reactive gliosis are associated to the neurodegeneration produced by Parkinson’s disease in the enteric nervous system.
Parkinson’s disease
enteric glial cells
enteric nervous system
α synuclein
1. Enteric Glial Cells
Within the enteric ganglia, EGCs are in direct contact with neuronal membranes [1][2]. EGCs do not produce or present myelin sheaths, are smaller than enteric neurons [2] and outnumber them, as has been observed in humans, where the EGC population in the myenteric plexus exceeds that of neurons by six/seven times. Nevertheless, this ratio varies by species [3][4]. For example, in the ileal myenteric ganglia of guinea pigs, the EGC population is only twice that of neurons [2].
EGCs exhibit extensive heterogeneity. Furthermore, they are phenotypically plastic and adapt their functions in response to environmental signals to maintain homeostasis [5][6]. This phenotypic plasticity is reflected in pathological processes such as neuroinflammation, cancer, and/or infections, as they can acquire proinflammatory or protumorigenic phenotypes [5].
1.1. Types of Enteric Glia
Currently, subpopulations of EGCs are categorized based on their morphology and anatomical location within the intestinal wall (Table 1). The different types of enteric glia vary both between regions of the digestive tract and among species. Furthermore, the expression of glial genes is influenced by factors such as circadian rhythms and age [5]. Nevertheless, it is still unknown how the morphology relates to adult EGCs in these areas and how the mature cell structure of plexus layers influences the phenotype of enteric glia [6].
| Type | Subtype | Location | Functions |
|---|---|---|---|
| Intraganglionar | Myenteric type I | Small and extended star-shaped cells that surround the neurons in the myenteric ganglia | Modulation of enteric neuron activity |
| Oxidative stress regulation | |||
| Trophic support | |||
| Neuroinflammation regulation | |||
| Gliogenesis | |||
| Neurogenesis | |||
| Mucosal glia replenishment | |||
| Submucosal type I | Associated with neurons within submucosal ganglia | Modulation of secretory neuron activity | |
| Extraganglionar | Interganglionar type II | Located in the interganglionic fiber tracts | They propagate the signal in the glial network |
| Mucosa type III | Some follow nerve fibers, while others terminate in the mucosal epithelium | Influence the maturation of epithelial cells | |
| Potentially modulate immune responses Identification from postnatal development | |||
| Myenteric plexus/submucosal plexus type III | Located in the extraganglionic regions at the level of the myenteric and submucosal plexuses | Unknown | |
| Intramuscular type IV | Associated with nerve fibers in the circular and longitudinal muscle layers of smooth muscles | Unknown |
Enteric glia express various receptors and constantly monitor their extracellular environment. This is why these cells are almost always activated and rarely at rest. Glial activation triggers a cascade of physiological intracellular signal transduction, such as those mediated by Ca2+ or cyclic adenosine monophosphate (cAMP). The effects of these cascades are generally beneficial and work to modulate intestinal reflexes and/or maintain homeostasis [5].
Depending on its state of activation, glia can be classified as activated, reactive or dysfunctional. Activated or reactive glia have physiological and defensive functions within the ENS, and dysfunctional glia may be responsible for enteric pathologies (Table 2).
Table 2. Types of enteric glia based on their reactivity state: activated, reactive, and dysfunctional [4][5][8][9].
| Type | Characteristics | Mechanisms of Action |
|---|---|---|
| Activated | Controls the activity of surrounding cells | The enteric glial activation encoded by intracellular Ca2+ responses modulate enteric excitatory motor and secretomotor neurocircuits |
| Exerts beneficial homeostatic effects | ||
| Responds to physiological stimuli | ||
| Reactive | Responds to physiopathological disturbances of any severity | Responds to intestinal inflammation. |
| Contributes to neuronal death during acute intestinal inflammation | ||
| Changes can alter glial activities through gain or loss of functions, which can be beneficial or detrimental | Contributes to vagal anti-inflammatory effects on resident intestinal immune cells after intestinal injury | |
| Dysfunctional | Dysfunctional or maladaptive response of glial cells | Altered enteric glial networks, displaying dysfunctional responses in patients with different GI disorders, including IBD, immunological disorders of the gut or PD |
| Exerts harmful effects contributing to a disease, in addition to being permanent |
Abbreviations: GI, gastrointestinal; IBD, inflammatory bowel disease; PD, Parkinson’s disease.
1.2. Enteric Glial Markers
EGCs express various factors/proteins that can be used for their specific detection, as summarized in Table 3.
| Marker | Characteristics | Functions |
|---|---|---|
| Nuclear transcription factor (Sox-10) |
Key to the development of the neuronal crest cells and the enteric glia | Crucial role in neuronal crest cells and peripheral glia differentiation and maintenance |
| Specific marker for EGCs progenitors | Controls and modulates the expression of several key genes for early ENS development | |
| Found in glial precursors and in most of the mature and immature EGCs | Promotes the expression of various transcription factors crucial for neuronal differentiation, such as Phox2b and Ascl1 | |
| Glial fibrillary acidic protein (GFAP) |
It is found along neuronal plexuses. | There is an increased GFAP intensity when the tissue is inflamed or next to colonic cancer |
| Does not occur prenatally | ||
| All subtypes of enteric glia within the mouse ileum express GFAP, but at different levels. | ||
| Dynamic expression that varies depending on the glial state | ||
| GFAPκ is the main isoform in colonic EGCs relative to GFAPα and GFAPδ | ||
| Calcium-binding protein (S100β) |
Expressed by both progenitors and differentiated enteric glial cells | Among other functions, this protein contributes to structural support and regulation of the immune response |
| Proteolipid protein 1 (PLP1) |
In adult mice, it is expressed in both ENS plexuses, in both, the small and large intestine | Unknown |
Abbreviations: EGCs, enteric glial cells; ENS, enteric nervous system; GFAP, glial fibrillary acidic protein; PLP1, proteolipid protein 1.
Studies have shown that the nuclear factor Sox-10 is involved in gliogenesis in the intestine [4], and its absence leads to total intestinal aganglionosis [6]. However, although most mature and immature EGCs express Sox-10, there is also a small population of EGCs that express glial fibrillary acidic protein (GFAP) and are negative for Sox-10. Furthermore, the role of Sox-10 in the early stages of EGC development appears to differ from its later role in EGC development [6]. From week 6 to week 11 of gestation, there is abundant expression of Sox-10 and the calcium-binding protein B (S100β) and very little expression of GFAP [6]. Other studies indicate that during the 12th week of gestation in humans, both S100β and GFAP markers seem to be independently regulated [14][16][17]. From this point onward, the S100β signal strengthens and fully develops after 18 weeks [14].
There is activation and plasticity of EGCs when an environment of inflammation or infection is mimicked [18]. Reactive gliosis accompanied by an increased intensity of labelling of GFAP and S100β was observed in the small intestine of mice, while the number of neurons decreased [14][18]. In addition, the systemic administration of lipopolysaccharide (LPS) led to an increased intensity labelling of S100β in the proximal colon, duodenum, and cecum after 24 h [19]. GFAP intensity is also increased in all these regions after 24 h [14][19]. Incorrect regulation of GFAP has been linked to scenarios of inflammation, Crohn’s disease (CD), colon cancer, IBD or intestinal ischemia [10][14][20].
Although these are the most common markers for EGCs, studies have found other interesting ones. The typical marker of astrocytes and oligodendrocytes, proteolipid protein 1 (PLP1), a proteolipid protein form of myelin, was unexpectedly and specifically expressed by enteric glia in the adult mouse intestine. A study demonstrated that most PLP1-positive cells also coexpressed S100β, but coexpression with GFAP was less abundant [12]. Later, it was noted that PLP1-positive glial cells were also present in the stomach and cecum of postnatal and adult human. It is unclear why enteric glia present this kind of myelin gene expression since they are non-myelinating glia [14].
1.3. Functions of the Enteric Glia and Their Role in Disease
In recent years, many functions of the enteric glia have been discovered that were previously unknown, further highlighting the importance of these cells (Figure 1).
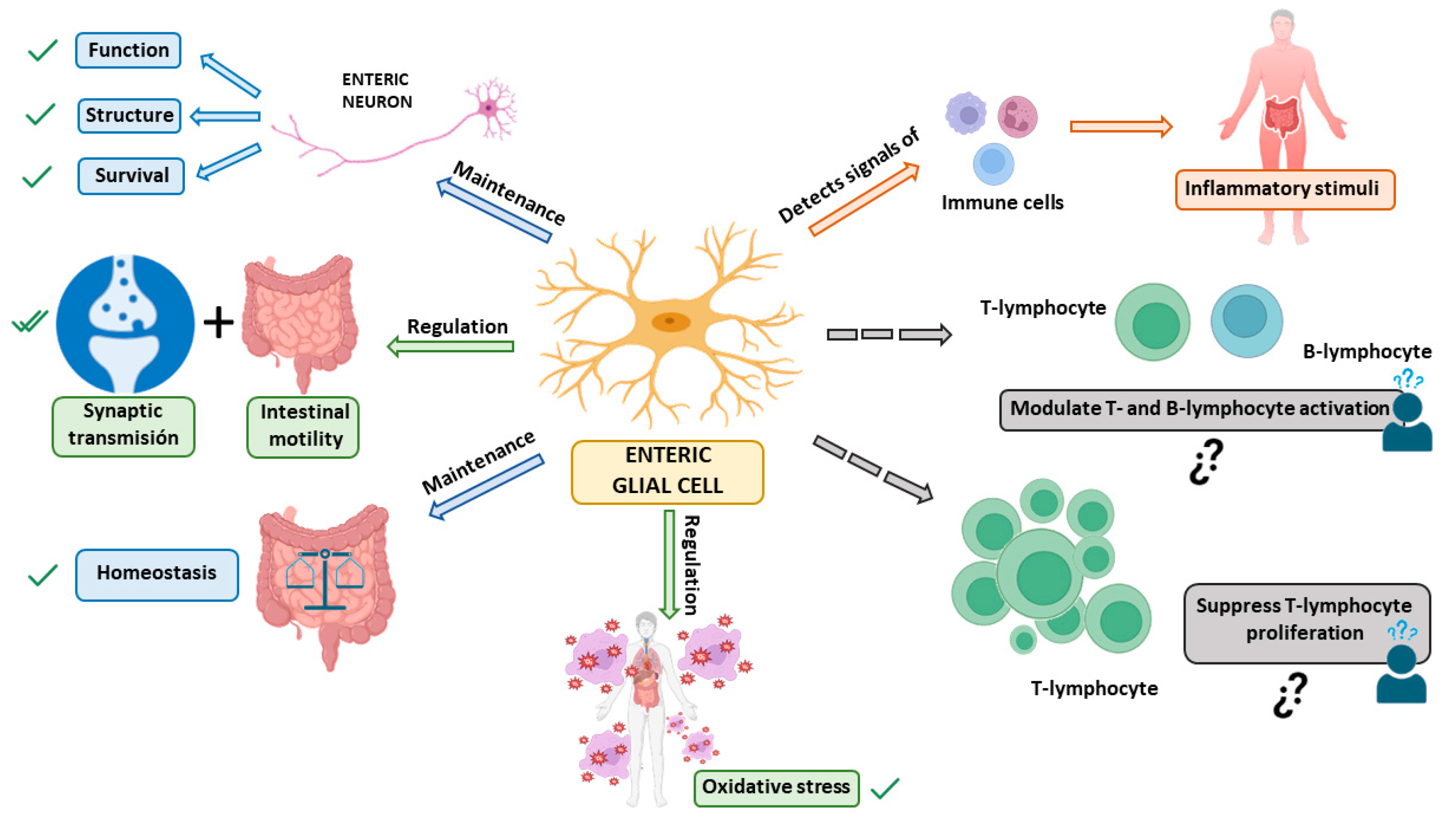
Figure 1. Functions of enteric glial cells (EGCs). The main functions of EGCs are: 1—maintenance of structure, function, and survival of enteric neurons [4][5][6][10]; 2—regulation of synaptic transmission and intestinal motility [5][6][10]; 3—maintenance of the gut and body’s homeostasis [4][5][6][10]; 4—ability to detect signals from immune cells to respond to inflammatory stimuli [21]. 3 5—regulation of oxidative stress to contribute to glutathione synthesis [5]. Additionally, functions currently under study are indicated by dashed arrows: 1—modulation of T and B-lymphocyte activation [21]; 2—ability to suppress T-lymphocyte proliferation [4]. All these functions may interfere with neurodegeneration in general, and the development of Parkinson’s disease in particular. ✓: Confirmed function of EGCs; ¿? need for confirmation of EGC function.
First, EGCs perform essential functions to support the survival and functions of neurons in the ENS [4]. Among their traditionally recognized functions, they have a crucial role in supporting enteric neurons and providing nutrition to them, thus maintaining the body’s homeostasis [4][5][6][10]. Specifically, EGCs may provide metabolic support to the neurons of the ENS and also give structural preservation for ENS neurons and their axons during intestinal motility [4]. They also regulate oxidative stress by contributing to glutathione synthesis, an antioxidant agent that plays an important role in detoxification processes [5].
Another function of EGCs is the regulation of synaptic transmission, mediating communication between the nervous system and the immune system [5][6]. Furthermore, they participate in neurotransmission in the ENS and regulate reflexes and intestinal processes underlying neuroinflammation in the gut [5]. A series of studies have shown that EGCs are fundamental for regulating intestinal motility [5][6][10] and they extend their influence on intestinal epithelial cells, where glial factors affect the maturation and differentiation of the intestinal epithelium [5]. Also, it has been observed that the immune system affects GI motility, so the communication between both systems modulates intestinal functions such as motility, ion transport, and mucosal permeability. When the ENS is subjected to certain conditions, EGCs can act as an extension of the immune system [22]. EGCs are capable of detecting microorganisms and signals from immune cells through cytokine receptors. Thanks to this, they mobilize a cellular cascade that leads to the recruitment and activation of immune cells capable of eliminating an infection and repairing tissue damage [21].
EGCs have also been shown to be essential for secretion and absorption in the intestinal epithelium [10]. Furthermore, EGCs maintain the integrity of the epithelial barrier in the intestines that regulates interactions between luminal contents with the underlying immune system, and the rest of the body. It is also important to highlight that the glial-derived neurotrophic factor (GDNF) released by EGCs has an anti-inflammatory effect in the intestines by inhibiting cell apoptosis and reducing the levels of proinflammatory cytokines. When there is mild tissue inflammation, GDNF is involved in the processes of epithelial reconstitution and maturation [7].
Given the variety of functions performed by EGCs, they are implicated in multiple GI pathologies. In various immune disorders, it has been observed that EGCs respond to proinflammatory cytokines thereby increasing local inflammation [23]. EGCs could also present disease-related antigens to modulate adaptive intestinal immunity and influence barrier integrity [24][25]. In inflammatory conditions such as IBD, ulcerative colitis, CD or inflammation due to viral infections, the role of EGCs has been deeply demonstrated [26][27][28][29][30][31]. Another important GI disorder with changes in EGCs is IBS, a pathology that involves alterations in the brain–gut axis due to various factors, from previous inflammation of the gut to stressful conditions that lead to peripheral and central hypersensitization [32][33]. There are also changes in the EGCs in other conditions that affect the digestive system, including acute inflammation and eating disorders [34][35][36]. As mentioned above, some of these intestinal dysfunctions, particularly those displaying overt or low-grade inflammation, have been related to PD development [24]. Furthermore, neurological and neurodegenerative conditions, like Alzheimer’s disease, have also been associated with changes in gut functions and EGCs [34]. Thus, EGCs may also play a prominent role in PD, as discussed next.
2. Parkinson’s Disease and Enteric Glia
As mentioned above, Braak’s hypothesis is supported by clinical observations indicating an association between PD and less frequent intestinal movements (constipation) that already occur years before diagnosis and aggravate with PD progression [34].
Indeed, in PD, GI symptoms appear before central symptoms and the components comprising the ENS are affected by this condition. Changes in intestinal microbiota can cause disruptions in the function of the epithelial barrier and intestinal permeability, affecting the epithelial cells of the digestive tract, the immune system, and the ENS, including both neurons and glial cells. Alterations in these two enteric cell populations may contribute to the initiation of the misfolding of α-syn protein, which can lead to neurological dysfunctions extending along the brain–gut axis and cause PD [37]. Thus, although in PD both enteric dopaminergic neurons and EGCs are affected, it is the latter that, when undergoing changes, trigger harmful consequences for the body.
Interestingly, it has been shown that EGCs respond to damage activation through Toll-like receptors (TLR) 2 and 4. For example, it was demonstrated how TLR-4 knockout mice could avoid PD symptoms by suppressing the immune response mediated by EGCs [38]. This is particularly important in the case of the gut dysbiosis that is associated to PD [39]. As a consequence of the alteration in the microbiota presented by patients, the intestinal epithelial barrier (IEB) is altered, facilitating the entry of pathogens [40][41]. The potentially harmful bacteria inside the gut alter the epithelial barrier even more through the production of endotoxins like LPS, a structural component of the outer membrane of Gram-negative bacteria with immunostimulatory characteristics, which may provoke systemic inflammation and even sepsis [42]. Thus, LPS is recognized by TLR-4 and stimulates the secretion of a variety of pro-inflammatory cytokines that further affect gut epithelial barrier integrity, enhancing bacterial translocation in turn. In this context, EGCs respond to this kind of harmful stimulations through TLR-2 and TLR-4, protecting the host against pathogens [43]. However, reactive EGCs also produce proinflammatory cytokines, growth factors and other immunomodulatory molecules, such as nitric oxide, contributing to amplify the proinflammatory environment and increasing the damage of the intestinal barrier. Moreover, intestinal microbiota may be involved in the modulation of intestinal α-syn aggregation and may further play a role in PD [41][44] since bacterial components like LPS could increase α-syn aggregation, further supporting the idea that the gut microbiome is involved in PD initiation [40].
Thus, already in the early stages of PD, EGCs may trigger neuroinflammation, causing synaptic dysfunction and abnormalities in intestinal motility, due to their key role in regulating motility, intestinal permeability, and immune responses. The increase in proinflammatory markers such as interleukin-6 (IL-6), interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), GFAP, and S100β in the intestinal mucosa of PD patients underlies the functional findings [45].
There are studies showing that the colon of PD patients has a higher presence of the glial markers GFAP, S100β, and Sox-10. Furthermore, other studies have shown that patients with PD experience not only inflammation but also oxidative stress in the intestine due to biochemical alterations that disrupt the neuro–glia relationship in their ENS [41][46][47].
References
- Capoccia, E.; Cirillo, C.; Gigli, S.; Pesce, M.; D’Alessandro, A.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. Enteric glia: A new player in inflammatory bowel diseases. Int. J. Immunopathol. Pharmacol. 2015, 28, 443–451.
- Coelho-Aguiar, J.d.M.; Bon-Frauches, A.C.; Gomes, A.L.; Veríssimo, C.P.; Aguiar, D.P.; Matias, D.; Thomasi, B.B.; Gomes, A.S.; Brito, G.A.; Moura-Neto, V. The enteric glia: Identity and functions. Glia 2015, 63, 921–935.
- Grubišić, V.; Verkhratsky, A.; Zorec, R.; Parpura, V. Enteric glia regulate gut motility in health and disease. Brain Res. Bull. 2018, 136, 109–117.
- Liu, C.; Yang, J. Enteric Glial Cells in Immunological Disorders of the Gut. Front. Cell Neurosci. 2022, 16, 895871.
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587.
- Boesmans, W.; Nash, A.; Tasnády, K.R.; Yang, W.; Stamp, L.; Hao, M.M. Development, Diversity, and Neurogenic Capacity of Enteric Glia. Front. Cell Dev. Biol. 2022, 19, 775102.
- López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. Nutraceuticals and Enteric Glial Cells. Molécules 2021, 26, 3762.
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925.
- Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Bernardini, N.; Blandizzi, C.; Fornai, M. Enteric Glia at the Crossroads between Intestinal Immune System and Epithelial Barrier: Implications for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 9199.
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241.
- Kim, J.; Lo, L.; Dormand, E.; Anderson, D.J. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 2003, 8, 17–31.
- Rao, M.; Nelms, B.D.; Dong, L.; Salinas-Rios, V.; Rutlin, M.; Gershon, M.D.; Corfas, G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 2015, 63, 2040–2057.
- Guillamón-Vivancos, T.; Gómez-Pinedo, U.; Matías-Guiu, J. Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia 2015, 30, 119–129.
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schäfer, K.H. Enteric Glia: S100, GFAP, and Beyond. Anat. Rec. 2019, 302, 1333–1344.
- López-Gómez, L.; Abalo, R. Modulation of Enteric Glial Cells by Nutraceuticals during Pathological Processes. In Natural Molecules in Neuroprotection and Neurotoxicity; De, O., Ed.; Academic Press: Cambridge, MA, USA, 2023.
- Rolle, U.; Nemeth, L.; Puri, P. Nitrergic innervation of the normal gut and in motility disorders of childhood. J. Pediatr. Surg. 2002, 37, 551–567.
- McCann, C.J.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e6.
- Nogueira, L.T.; Costa, D.V.; Gomes, A.S.; Martins, C.S.; Silva, A.M.; Coelho-Aguiar, J.M.; Castelucci, P.; Lima-Júnior, R.C.; Leitão, R.F.; Moura-Neto, V.; et al. The involvement of mast cells in the irinotecan-induced enteric neurons loss and reactive gliosis. J. Neuroinflamm. 2017, 14, 79.
- da Cunha Franceschi, R.; Nardin, P.; Machado, C.V.; Tortorelli, L.S.; Martinez-Pereira, M.A.; Zanotto, C.; Gonçalves, C.A.; Zancan, D.M. Enteric glial reactivity to systemic LPS administration: Changes in GFAP and S100B protein. Neurosci. Res. 2017, 119, 15–23.
- Thacker, M.; Rivera, L.R.; Cho, H.J.; Furness, J.B. The relationship between glial distortion and neuronal changes following intestinal ischemia and reperfusion. Neurogastroenterol. Motil. 2011, 23, e500–e509.
- Progatzky, F.; Pachnis, V. The role of enteric glia in intestinal immunity. Curr. Opin. Immunol. 2022, 77, 102183.
- Romero-Trujillo, J.O.; Frank-Márquez, N.; Cervantes-Bustamante, R.; Cadena-León, J.F.; Montijo-Barrios, E.; Zárate-Mondragón, F.; Cázares-Méndez, J.M.; Ramírez-Mayans, J. Enteric nervous system and gastrointestinal motility. Acta Pediátrica De México 2012, 33, 207–214.
- Ruhl, A.; Franzke, S.; Collins, S.M.; Stremmel, W. Interleukin-6 expression and regulation in rat enteric glial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1163–G1171.
- Li, Y.; Chen, Y.; Jiang, L.; Zhang, J.; Tong, X.; Chen, D.; Le, W. Intestinal Inflammation and Parkinson’s Disease. Aging Dis. 2021, 12, 2052–2068.
- Meir, M.; Kannapin, F.; Diefenbacher, M.; Ghoreishi, Y.; Kollmann, C.; Flemming, S.; Germer, C.T.; Waschke, J.; Leven, P.; Schneider, R.; et al. Intestinal Epithelial Barrier Maturation by Enteric Glial Cells Is GDNF-Dependent. Int. J. Mol. Sci. 2021, 22, 1887.
- Cornet, A.; Savidge, T.C.; Cabarrocas, J.; Deng, W.L.; Colombel, J.F.; Lassmann, H.; Desreumaux, P.; Liblau, R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. USA 2001, 98, 13306–13311.
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112.
- von Boyen, G.B.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3.
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11.
- Hagbom, M.; De Faria, F.M.; Winberg, M.E.; Westerberg, S.; Nordgren, J.; Sharma, S.; Keita, Å.V.; Loitto, V.; Magnusson, K.E.; Svensson, L. Neurotrophic factors protect the intestinal barrier from rotavirus insult in mice. MBio 2020, 11, e02834–e02919.
- Coquenlorge, S.; Van Landeghem, L.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Duchalais, E.; Neunlist, M.; Rolli-Derkinderen, M. The arachidonic acid metabolite 11β-ProstaglandinF2α controls intestinal epithelial healing: Deficiency in patients with Crohn’s disease. Sci. Rep. 2016, 6, 25203.
- Zeledón Corrales, N.; Serrano Suárez, J.A.; Fernández Agudelo, S. Irritable Bowel Syndrome. Rev. Méd. Sinerg. 2021, 6, e645.
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2020, 111, 104501.
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.H. Disorders of the enteric nervous system—A holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410.
- Rosenbaum, C.; Schick, M.A.; Wollborn, J.; Heider, A.; Scholz, C.J.; Cecil, A.; Niesler, B.; Hirrlinger, J.; Walles, H.; Metzger, M. Activation of Myenteric Glia during Acute Inflammation In Vitro and In Vivo. PLoS ONE 2016, 11, e0151335.
- Voss, U.; Sand, E.; Olde, B.; Ekblad, E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS ONE 2013, 8, e81413.
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620.
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Forsyth, C.B.; Huschens, A.M.; Shaikh, M.; Voigt, R.M.; Naqib, A.; Green, S.J.; Kordower, J.H.; et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 2019, 68, 829–843.
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61.
- Yang, H.; Li, S.; Le, W. Intestinal Permeability, Dysbiosis, Inflammation and Enteric Glia Cells: The Intestinal Etiology of Parkinson’s Disease. Aging Dis. 2022, 13, 1381–1390.
- Claudino Dos Santos, J.C.; Lima, M.P.P.; Brito, G.A.C.; Viana, G.S.B. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812.
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151.
- Chow, A.K.; Grubišić, V.; Gulbransen, B.D. Enteric Glia Regulate Lymphocyte Activation via Autophagy-Mediated MHC-II Expression. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1215–1237.
- Yemula, N.; Dietrich, C.; Dostal, V.; Hornberger, M. Parkinson’s Disease and the Gut: Symptoms, Nutrition, and Microbiota. J. Parkinsons Dis. 2021, 11, 1491–1505.
- Clairembault, T.; Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P. Enteric glial cells: New players in Parkinson’s disease? Mov. Disord. 2015, 30, 494–498.
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48.
- Trichka, J.; Zou, W.Q. Modulation of Neuroinflammation by the Gut Microbiota in Prion and Prion-Like Diseases. Pathogens 2021, 10, 887.
More
Information
Subjects:
Gastroenterology & Hepatology; Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
505
Revisions:
2 times
(View History)
Update Date:
26 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




