Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Delia DeBuc | -- | 3479 | 2024-01-25 13:24:56 | | | |
| 2 | Catherine Yang | -11 word(s) | 3468 | 2024-01-26 01:47:39 | | | | |
| 3 | Catherine Yang | + 11 word(s) | 3479 | 2024-01-29 02:06:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Acuña, K.; Sapahia, R.; Jiménez, I.N.; Antonietti, M.; Anzola, I.; Cruz, M.; García, M.T.; Krishnan, V.; Leveille, L.A.; Resch, M.D.; et al. Functional Near-Infrared Spectrometry. Encyclopedia. Available online: https://encyclopedia.pub/entry/54347 (accessed on 15 January 2026).
Acuña K, Sapahia R, Jiménez IN, Antonietti M, Anzola I, Cruz M, et al. Functional Near-Infrared Spectrometry. Encyclopedia. Available at: https://encyclopedia.pub/entry/54347. Accessed January 15, 2026.
Acuña, Kelly, Rishav Sapahia, Irene Newman Jiménez, Michael Antonietti, Ignacio Anzola, Marvin Cruz, Michael T. García, Varun Krishnan, Lynn A. Leveille, Miklós D. Resch, et al. "Functional Near-Infrared Spectrometry" Encyclopedia, https://encyclopedia.pub/entry/54347 (accessed January 15, 2026).
Acuña, K., Sapahia, R., Jiménez, I.N., Antonietti, M., Anzola, I., Cruz, M., García, M.T., Krishnan, V., Leveille, L.A., Resch, M.D., Galor, A., Habash, R., & Debuc, D.C. (2024, January 25). Functional Near-Infrared Spectrometry. In Encyclopedia. https://encyclopedia.pub/entry/54347
Acuña, Kelly, et al. "Functional Near-Infrared Spectrometry." Encyclopedia. Web. 25 January, 2024.
Copy Citation
Functional Near-Infrared Spectroscopy (fNIRS) has emerged as a promising tool for understanding the human brain’s complex workings due to its ability to measure changes in oxygenated and deoxygenated hemoglobin levels, thus providing insights into neural activity and functional connectivity. fNIRS technology offers a novel approach to studying brain function, especially visual processing and perception. fNIRS offers unique advantages, such as portability, cost-effectiveness and safety, making it suitable for clinical and research applications. Additionally, the combination of fNIRS with emerging technologies like virtual reality (VR), augmented reality (AR) and artificial intelligence (AI) opens new avenues for immersive investigations into brain function.
fNIRS
eye
virtual reality
artificial intelligence
augmented reality
1. Application to the Visual System
1.1. Understanding the Eye
Recent work has begun understanding the eye using fNIRS technology. A recent review paper published by a group from the Bascom Palmer Eye Institute at the University of Miami delves into the early design studies and promising findings that underscore the potential of NIRS as a valuable monitoring tool, offering insights into visual disorders and uncovering physiological responses during specific eye movements and visual stimuli [https://www.mdpi.com/2077-0383/13/1/282- cite our review paper here]. In an early design study of NIRS technology, oxygenated and deoxygenated hemoglobin results of saccadic eye movements were compared against fMRI measurements of the visual cortex [16]. These preliminary findings introduced the NIRS design as a useful monitoring tool when supplemented with fMRI in patients with visual disorders [16]. Another study found that patients with an upward gaze and strong trigeminal proprioceptive evocation seemed to induce rapid oxygen consumption in the ventromedial prefrontal cortex [17]. This upward gaze activates sweat glands, causing deoxyhemoglobin production that regulates this physiological arousal [17].
fNIRS has additionally provided assessment measures for treating amblyopia [1]. In a study of 10 subjects, Iwata et al. found that, when patients were treated with both eyes open compared to one eye occluded, there was significantly greater activation of the visual cortex observed through oxygenated hemoglobin levels [1]. fNIRS also illustrates the potential to study the primary visual cortex of migraine patients, as Yamakawa et al. found fNIRS measurements that showed statistical significance when visual stimulation intensity was applied to intrinsically photosensitive retinal ganglion cells projecting to the primary visual cortex [2].
A recent study compared the degree of change in oxygenated hemoglobin caused by visual stimulation in emmetropic and myopic participants [3]. When comparing emmetropic and uncorrected myopic participants, the degree of change was higher in emmetropic participants. A higher degree of change in oxygenated hemoglobin was observed in corrected myopic patients than in uncorrected myopic patients. Myopia-induced emmetropic participants had a decline in functional activity; however, this was recovered when the myopia lens was removed. These results indicate that myopic defocus tends to reduce the level of oxygenated hemoglobin and functional activity in the visual cortex. However, visual cortical function can be restored with optical correction, as measured by fNIRS [3]. This literature illustrates the usefulness of fNIRS in establishing a further understanding of the eye generally.
1.2. Eye Function
fNIRS has additionally been used to comprehend eye function. Wijeakumar et al. utilized fNIRS technology to quantify the extent of occipito-parietal activation when high-contrast checkerboard visual stimuli were presented [4]. This study found that dynamic pattern presentation produced a more significant hemodynamic response correlated to the activation of V1 neurons compared to static checkerboard stimuli [4]. This difference in the response indicates the recruitment of many temporal frequency-selective neurons and neurons sensitive to spatial frequencies and contrast [4]. This functional usage of fNIRS provides a basis for using this technology to assess eye function.
Additional work has confirmed the reliability of fNIRS for functional mapping by verifying the vertical asymmetry of the visual cortex using fNIRS measurements [5]. By presenting black and white wedge checkerboard stimuli to the four visual field quadrants, oxygenated hemoglobin concentrations were consistently higher in the contralateral hemisphere of the simulation, reflecting the retinotopic organization of the visual cortex [5]. These results from fNIRS measurements corroborate with findings from previous neuroimaging techniques such as fMRI or MEG, confirming the reliability of fNIRS for retinotopic mapping and investigations of vertical/horizontal functional asymmetries within the visual cortex [5].
When performing a simultaneous fNIRS–EEG study, fNIRS illustrated good sensitivity to low-level sensory processing in visual and auditory stimulation [6]. fNIRS results clearly distinguish between visual and auditory modalities, with high area specificity and stimulus selectivity [6]. Yarmouth et al. assessed the reliability of fNIRS to evaluate visual cortex activation within frontal eye fields during vergence eye movements using intraclass correlation coefficient (ICC) values [7]. Oxygenated hemoglobin (HbO) signals had higher ICC values compared to deoxygenated hemoglobin (HbR) signals, indicating increased reliability for only oxygenated hemoglobin signals [7]. This result may be due to a noisier HbR signal resulting from a visible cardiac wave in the raw data signal acquisition, as well as the fNIRS instrumentation manufacturer having stated that HbR has a lower signal-to-noise ratio compared to that of HbO [7]. These studies demonstrate the capability of fNIRS for comprehending eye function.
1.3. Eye Disease
Other literature has also examined the utility of fNIRS for various eye diseases. One study used fNIRS to delve into cortical neural correlates of visual fatigue during binocular depth perception for different disparities [8]. fNIRS measurements revealed an increase in oxygenated hemoglobin in the occipital cortex during binocular depth perception, especially in particular regions of V1 with overlap in V1 and V2, confirming that the parieto-occipital cortices are spatially correlated with binocular depth perception [8]. The study also revealed that, once stereovision is established, hemodynamic responses approach a peak with an amplitude correlated with and determined by stereopsis [8]. This trend indicates that visual fatigue may result from generating but not sustaining stereovision [8].
Treatment for visual vertigo patients often includes habituation exercises using optic flow to address symptoms of dizziness, disorientation or impaired balance [9]. Hoppes et al. investigated individuals with visual vertigo. They utilized fNIRS measurements to explore cerebral activation during optic flow to determine if visual fixation modulates brain activity. This study found greater cortical activation in the bilateral fronto-temporo-parietal lobes when the optic flow was viewed with fixation, providing preliminary evidence of the use of a fixation target during these habituation exercises [9].
Primary open-angle glaucoma (POAG) is a neurodegenerative disease associated with stress and quality of life [10]. One study investigated the effects of meditation on subjective quality of life and brain oxygenation in patients with POAG [10]. This short-term meditation course was associated with significant improvements in changes in HbO levels in the intervention prefrontal cortex, indicating meditation’s usefulness alongside standard treatment in patients with POAG [10]. Additionally, this study illustrates how fNIRS measurements can be used alongside previously established measurements to assess various eye diseases.
In recent work on glaucoma, another study investigated reductions in occipital hemodynamic responses following visual stimulation in healthy controls compared to glaucoma patients using time-domain fNIRS (TD-fNIRS) [11]. A statistical analysis of fNIRS measurements of 98 total participants showed lower amplitudes of oxygenated and deoxygenated hemoglobin concentrations in glaucomatous eyes compared to healthy control groups [11]. This result indicates multi-level degeneration of visual pathways in glaucomic individuals, demonstrating the usefulness of TD-fNIRS measurements as a supporting tool for evaluating glaucoma pathology [11]. This work illustrates how fNIRS may be used to evaluate the progression of various eye diseases and assess treatment for these conditions.
1.4. Visual Cortex
Various techniques combining fNIRS with other statistical models or brain imaging methods have illustrated the validity of fNIRS measurements in measuring visual cortex activity. In 2004, Schroeter et al. applied the general linear model, previously used to analyze fMRI data, to fNIRS optical imaging techniques, as these methods allow frequency domain analyses whose results can be used to generate a map with a network structure of various brain regions [12]. The general linear model results found that, when a visual stimulus was presented compared to no visual stimulation, the concentration of HbO increased, whereas the concentration of HbR decreased [12]. This study also found higher activity in the occipitotemporal region when participants were presented with rotating colored figures, which likely corresponded to the visual cortex region that processes motion, V5 [12]. Total and oxygenated hemoglobin increased in areas corresponding to V1–V3 when the checkerboard stimulus was presented [12]. These works confirm the validity of fNIRS measurements for visual cortex monitoring through gathered oxygenated and deoxygenated hemoglobin concentrations.
In combination with functional network analysis, fNIRS has been used to understand character recognition in the occipitotemporal cortex [13]. Hu et al. gathered data on behavioral performance, fNIRS measurements and functional brain connectivity [13]. The results revealed that most participants could more accurately identify a real Chinese character when compared to an artificially produced pseudo-Chinese character. This change in accuracy positively correlated to higher oxygenated hemoglobin concentrations in the bilateral occipital, temporal cortex and left fusiform gyrus. There was a much faster reaction time and accuracy for checkerboard stimuli compared to pseudo and real character cases, possibly due to the lack of involvement of orthographic, phonological or semantic processing present during character cases [13].
Additional work investigated how fNIRS and EEG combined can measure visual cortex function generally [14]. The results revealed stronger contralateral motor and visual cortex activations when comparing left and right contrast and frontal region activation during the Stroop task [14]. This paper confirmed that the hemodynamic responses measured through fNIRS significantly correlate with the electrical activity of an EEG across both motor and visual tasks, illustrating the validity of fNIRS measurements as a form of visual cortex monitoring [14]. These findings generally indicate the validity of fNIRS signals in visual cortex activation measurements, consistent with previous literature and expectations.
1.5. Visual Stimuli
Various studies have also found correlations between differing visual stimuli and hemodynamic responses to these stimuli. Wijeakumar et al. used fNIRS and visual evoked potential to investigate the correlation between neural and hemodynamic responses to stereoscopic stimuli [4]. This study observed increased oxygenated and total hemoglobin concentrations across the N1–P2 complex [4]. fNIRS, compared to visual evoked potentials (VEPs), measured slow vascular changes due to neural activation with several seconds of latency [4]. However, there was a correlation between VEP amplitudes and HbO concentration for complex stimuli [4]. In concurrence with previous literature, this study suggested that the N1–P2 complex could be a marker of stereopsis in V1 [4].
Another study used EEG and fNIRS measurements to examine whether lower visual cortex activation for visual processing could be due to more efficient visual sensory encoding in cochlear implant users compared to controls [15]. These results found more prominent decreased responses to repeated visual stimuli in cochlear implant users and enhanced visual adaptation and lower visual cortex activation in these participants [15]. These fNIRS data support the hypothesis that cochlear implant users more efficiently process visual stimuli than controls [15].
Repetition suppression is a well-characterized response to a recent experience found in the perceptual cortices of adult brains [16]. One study using fNIRS aimed to determine if infants exhibit similar neural responses to repetition as adults and found little evidence of auditory repetition suppression in the temporal cortex and no evidence of visual repetition suppression in the occipital lobe [16]. However, repeated visual stimuli elicited consistent responses in the occipital lobe, suggesting that asymmetry in repetition suppression may be due to visual repetition suppression’s later development [16].
Hoppes et al. used fNIRS to examine cerebral activation during the optic flow of individuals with and without visual vertigo [9]. The primary finding of this study was decreased activation in the bilateral middle frontal regions of visual vertigo participants during optic flow, which may represent a different form of control for these individuals over the usually reciprocal inhibitory visual–vestibular interaction present in individuals without visual vertigo [9]. These preliminary findings may allow for additional fNIRS application in monitoring changes in cerebral activation in individuals with complaints of dizziness and disorientation symptoms of visual vertigo [9].
More recent work investigated the influence of flickering frequency on the amplitude of changes in the concentration of HbO and HbR [17]. The findings demonstrate that the checkerboard flickering frequency significantly affects HbO concentration changes, with the highest amplitude of HbO concentration changes observed at a checkerboard flickering frequency of 8 Hz [17]. This study allows for a further understanding of concentrations of hemoglobin changes at differing flickering frequencies of visual stimuli [17]. Overall, the above studies illustrate the usefulness of fNIRS technology in understanding the cortical activation of various visual stimuli.
2. Future of fNIRS Technology
2.1. Virtual Reality
Though fNIRS has been applied previously to the visual system, this non-invasive imaging technique has been integrated with VR technologies. This integration has shown the potential to assist medical professionals in making clinical decisions and offers promising future applications [18]. fNIRS, combined with adaptive visuomotor tasks, has shown potential in enhancing neural activity within the brain’s sensorimotor regions and hand motor function [19]. Two possible functions have been proposed for using fNIRS in VR therapy. One involves monitoring and delivering enhanced feedback on cortical activation areas during treatment. Moreover, the other function involves incorporating fNIRS into a brain–computer interface paradigm for therapy [20].
Building on previous research that established a link between high-intensity intermittent aerobic exercise (HIE) and increased neural activity [21], a new study employed fNIRS and immersive VR. In cases where patients may have been unable to engage in physical activity due to a physical condition, a study conducted by Burin et al. utilized the illusion of immersive VR to increase their heart rates successfully [22]. In these VR spaces, patients subjectively reported feeling as though they were moving themselves when they had only moved their virtual avatars and not their physical bodies [22]. The study demonstrated that this virtual training positively affected the patient’s cognitive functioning and corresponding neurologic substrates, even without physical movement [22]. These findings indicate that a sense of ownership can modulate body and brain reactions regardless of physical movement. By utilizing the immersive VR platform and measuring neural function with fNIRS, sedentary patients with neurological disorders and motor impairment can benefit from this phenomenon in clinical treatment [22].
The increased use of VR in medical applications has been combined with fNIRS to monitor the effects of the VR system. According to the simulation hypothesis, the neural networks of an action–observation system located in the motor cortices of the brain are activated during overt motor execution and observation or imagery of the same motor action on platforms like VR [23]. A study by Holper et al. aimed to demonstrate the effectiveness of using a VR system in neurorehabilitation by assessing its impact on brain activation [24]. This work confirmed that fNIRS recording does not hinder the use of the VR environment, a crucial development for applying VR-fNIRS technology in neurorehabilitation. Nevertheless, two factors could limit this technology’s accuracy and potential usefulness. First, intersubject variability is evident at the group level and more so at the individual level [24]. This variability may arise from differences in motor imagery ability and anatomical and physiological variations among subjects and warrants further investigation. Second, the recording side (uni- or bilateral hemisphere) and hand side (left or right hand used during motor or imagery tasks) need to be taken into consideration to understand the combined effect of these factors better [24].
In a separate exploratory investigation, Seraglia et al. aimed to assess the feasibility of incorporating fNIRS brain imaging in a study of immersive VR experiences [25]. These researchers utilized a virtual line bisection task and identified cerebral activation in the parietal and occipital lobes [25]. This study underscored the advantages of fNIRS’s non-invasive nature, which allows patients to maintain their natural position during the procedure [25]. These results add to the growing evidence that combining fNIRS and VR is viable for examining conventional neuropsychological disorders.
2.2. Augmented Reality
In contrast to VR systems, augmented reality (AR) enriches the user’s surrounding environment by superimposing virtual objects onto the real world as overlays. By visually exploring the statistical distribution of fNIRS patients who underwent dimensionality reduction with principal component analysis, Galati et al. identified common and unique interaction patterns associated with different states of cognitive load [26]. Hemodynamic activity from fNIRS (HbO, HbR) exhibited a similar pattern, suggesting that the users’ neural activity was linked to their interactions [26]. This evidence supports the combination of AR and fNIRS, as it can be adapted to support and investigate a variety of complex decision-making and problem-solving tasks applicable to numerous real-world uses, including teaching and learning applications.
Researchers have utilized an fNIRS-based augmented reality brain–computer interface (BCI) in simulated real-time settings to assist medical professionals in identifying the location and timing of pain in patients. Hu et al. positioned fNIRS optodes over the patients’ prefrontal cortex and primary somatosensory area to observe cortical activity, and individuals with sensitive teeth underwent thermal stimulation [27]. This cortical activity was then overlaid onto a participant’s head in real time through an optical see-through head-mounted display (HoloLens) device worn by the clinician [27]. Another group demonstrated the effective integration of fNIRS-based BCIs and AR technology to tackle a six-class issue using only one mental task and an fNIRS channel [28] (Figure 4). This approach capitalizes on the potential of AR technology to enable smooth interaction with the real world, which warrants further investigation in future research.
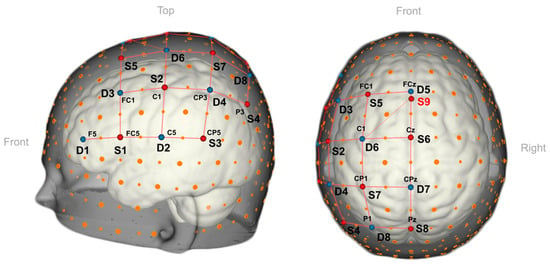
Figure 4. A 3D view of the fNIRS optode arrangement used in fNIRS-based BCIs integrated with AR technology [28]. The nine sources (S1–S9, red dots), eight detectors (D1–D8, blue dots) are placed over the left-hemispheric motor and premotor regions.
McKendrick et al. conducted a study to compare the cognitive effects of using an AR display (Google Glass) versus a handheld smartphone display during an outdoor navigation task [29]. They employed the fNIRS system to measure prefrontal cortex activity and incorporated two additional tasks to evaluate differences in mental workload and situational awareness while navigating [29]. The researchers determined that using an AR wearable display resulted in the least workload during specific working memory tasks and showed enhanced situational awareness based on their assessment of prefrontal hemodynamics [29].
Finally, one study examined the prefrontal cortex (PFC) oxygenation response when twenty-two male subjects were asked to maintain equilibrium while semi-immersed in a VR environment on a virtual tilt board (VTB) balancing over a pilot at a ±35° angle [30]. It was demonstrated that there was a bilateral increase in the oxygenation response of the PFC, which differed depending on the task’s difficulty [30]. These results could be useful for diagnostic testing and functional neurorehabilitation given its adaptability in the elderly and patients with movement disorders [30]. The progress made in combining AR with fNIRS highlights the potential of this technology, but more research is needed to bridge the divide between its use in research settings and real-world applications.
2.3. Artificial Intelligence
Along with the latest cutting-edge technologies like VR and AR, integrating machine learning (ML) and deep learning algorithm data with fNIRS offers unprecedented opportunities. Current breakthroughs in ML have been used to explore complex and voluminous fNIRS data. Tanveer et al. explored the use of convolutional neural networks (CNNs) to classify the drowsy and alert states of thirteen healthy subjects while driving a car simulator. The CNN architecture resulted in an average accuracy of 99.3% while detecting drowsy and alert states [31].
Although fNIRS has gained popularity for its portability, among other advantages, data collection remains challenging in certain populations or cases, such as infants. This difficulty complicates the task for machine learning algorithms, which require large datasets to generalize effectively. Here, generative adversarial networks have been utilized to solve the data scarcity problem. Wickramaratne et al. used a generative adversarial network to augment the data obtained for the classification of a tapping task, i.e., whether a subject’s task is a left finger tap, right finger tap or foot tap based on the fNIRS signal [32]. The classifier obtained an accuracy of 80% using actual data and 96% using augmented data [32].
Recently, the neuro-imaging field has experienced a paradigm shift due to breakthroughs in deep learning. Although these advances have primarily shown results in fMRI research rather than fNIRS, they signal exciting prospects for mapping brain activity across various neuro-imaging modalities. In a recent study, Takagi et al. successfully used latent diffusion models—initially developed by Rombach et al. [33]—to reconstruct high-resolution images of 512 × 512 pixels from human brain activity, all without the need for fine-tuning their deep generative networks [34] (Figure 5). Similarly, Perpetuini et al. developed a model for a generalizable retinotopy classification using acquired optical brain signals from the visual cortex during visual stimulation consisting of a rotating checkerboard wedge, flickering at 5 Hz [35]. Their results could encourage the use of optical fNIRS signals in real-time BCI applications.

Figure 5. Images reconstructed from fMRI data from one subject: top row shows presented images, bottom row shows obtained images [34].
Remarkably, the scope of reconstruction extends beyond images to include auditory stimuli. Utilizing non-linear decoding models applied to intracranial electroencephalography (iEEG) data obtained from 29 epilepsy patients, Bellier et al. successfully reconstructed Pink Floyd’s ‘Another Brick in the Wall’ from individuals who were passively listening to the track [36]. Similarly, Denk et al. accomplished music reconstruction using functional magnetic resonance imaging (fMRI) data [37].
In conclusion, the synergistic amalgamation of sophisticated data analysis techniques, such as machine learning algorithms, with fNIRS has inaugurated an expansive array of unattainable opportunities merely a few years prior.
References
- Kashou, N.H.; Xu, R.; Roberts, C.J.; Leguire, L.E. Using FMRI and FNIRS for localization and monitoring of visual cortex activities. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2007, 2007, 2634–2638.
- Matsuo, K.; Ban, R.; Hama, Y.; Yuzuriha, S. Eyelid Opening with Trigeminal Proprioceptive Activation Regulates a Brainstem Arousal Mechanism. PLoS ONE 2015, 10, e0134659.
- Iwata, Y.; Handa, T.; Ishikawa, H.; Shoji, N.; Shimizu, K. Efficacy of an Amblyopia Treatment Program with Both Eyes Open: A Functional Near-Infrared Spectroscopy Study. Am. Orthopt. J. 2016, 66, 87–91.
- Yamakawa, M.; Tachibana, A.; Tatsumoto, M.; Okajima, K.; Ueda, S.; Hirata, K. Hemodynamic responses related to intrinsically photosensitive retinal ganglion cells in migraine. Neurosci. Res. 2020, 160, 57–64.
- Zhang, Y.; Lin, X.; Bi, A.; Cao, N.; Zhang, T.; Wang, S.; Wen, Y.; Bi, H. Changes in visual cortical function in moderately myopic patients: A functional near-infrared spectroscopy study. Ophthalmic Physiol. Opt. 2022, 42, 36–47.
- Wijeakumar, S.; Shahani, U.; Simpson, W.A.; McCulloch, D.L. Localization of hemodynamic responses to simple visual stimulation: An fNIRS study. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2266–2273.
- Bastien, D.; Gallagher, A.; Tremblay, J.; Vannasing, P.; Thériault, M.; Lassonde, M.; Lepore, F. Specific functional asymmetries of the human visual cortex revealed by functional near-infrared spectroscopy. Brain Res. 2012, 1431, 62–68.
- Chen, L.C.; Sandmann, P.; Thorne, J.D.; Herrmann, C.S.; Debener, S. Association of Concurrent fNIRS and EEG Signatures in Response to Auditory and Visual Stimuli. Brain Topogr. 2015, 28, 710–725.
- Yaramothu, C.; Li, X.; Morales, C.; Alvarez, T.L. Reliability of Frontal Eye Fields Activation and Very Low-Frequency Oscillations Observed during Vergence Eye Movements: An fNIRS Study. Sci. Rep. 2020, 10, 712.
- Cai, T.; Zhu, H.; Xu, J.; Wu, S.; Li, X.; He, S. Human cortical neural correlates of visual fatigue during binocular depth perception: An fNIRS study. PLoS ONE 2017, 12, e0172426.
- Hoppes, C.W.; Sparto, P.J.; Whitney, S.L.; Furman, J.M.; Huppert, T.J. Changes in cerebral activation in individuals with and without visual vertigo during optic flow: A functional near-infrared spectroscopy study. Neuroimage Clin. 2018, 20, 655–663.
- Gagrani, M.; Faiq, M.A.; Sidhu, T.; Dada, R.; Yadav, R.K.; Sihota, R.; Kochhar, K.P.; Verma, R.; Dada, T. Meditation enhances brain oxygenation, upregulates BDNF and improves quality of life in patients with primary open angle glaucoma: A randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 741–753.
- Re, R.; Messenio, D.; Marano, G.; Spinelli, L.; Pirovano, I.; Contini, D.; Colombo, R.; Boracchi, P.; Biganzoli, E.; Cubeddu, R.; et al. Monitoring the haemodynamic response to visual stimulation in glaucoma patients. Sci. Rep. 2021, 11, 13567.
- Schroeter, M.L.; Bücheler, M.M.; Müller, K.; Uludağ, K.; Obrig, H.; Lohmann, G.; Tittgemeyer, M.; Villringer, A.; von Cramon, D.Y. Towards a standard analysis for functional near-infrared imaging. Neuroimage 2004, 21, 283–290.
- Hu, Z.; Zhang, J.; Couto, T.A.; Xu, S.; Luan, P.; Yuan, Z. Optical Mapping of Brain Activation and Connectivity in Occipitotemporal Cortex During Chinese Character Recognition. Brain Topogr. 2018, 31, 1014–1028.
- Chiarelli, A.M.; Perpetuini, D.; Croce, P.; Greco, G.; Mistretta, L.; Rizzo, R.; Vinciguerra, V.; Romeo, M.F.; Zappasodi, F.; Merla, A.; et al. Fiberless, Multi-Channel fNIRS-EEG System Based on Silicon Photomultipliers: Towards Sensitive and Ecological Mapping of Brain Activity and Neurovascular Coupling. Sensors 2020, 20, 2831.
- Chen, L.C.; Stropahl, M.; Schönwiesner, M.; Debener, S. Enhanced visual adaptation in cochlear implant users revealed by concurrent EEG-fNIRS. Neuroimage 2017, 146, 600–608.
- Emberson, L.L.; Cannon, G.; Palmeri, H.; Richards, J.E.; Aslin, R.N. Using fNIRS to examine occipital and temporal responses to stimulus repetition in young infants: Evidence of selective frontal cortex involvement. Dev. Cogn. Neurosci. 2017, 23, 26–38.
- Bejm, K.; Wojtkiewicz, S.; Sawosz, P.; Perdziak, M.; Pastuszak, Z.; Sudakou, A.; Guchek, P.; Liebert, A. Influence of contrast-reversing frequency on the amplitude and spatial distribution of visual cortex hemodynamic responses. Biomed. Opt. Express 2019, 10, 6296–6312.
- Rahman, M.A.; Siddik, A.B.; Ghosh, T.K.; Khanam, F.; Ahmad, M. A Narrative Review on Clinical Applications of fNIRS. J. Digit. Imaging 2020, 33, 1167–1184.
- Zheng, Y.; Tian, B.; Zhuang, Z.; Zhang, Y.; Wang, D. fNIRS-based adaptive visuomotor task improves sensorimotor cortical activation. J. Neural Eng. 2022, 19, 046023.
- Teo, W.P.; Muthalib, M.; Yamin, S.; Hendy, A.M.; Bramstedt, K.; Kotsopoulos, E.; Perrey, S.; Ayaz, H. Does a Combination of Virtual Reality, Neuromodulation and Neuroimaging Provide a Comprehensive Platform for Neurorehabilitation?—A Narrative Review of the Literature. Front. Hum. Neurosci. 2016, 10, 284.
- Kujach, S.; Byun, K.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Laskowski, R.; Dan, I.; Soya, H. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage 2018, 169, 117–125.
- Burin, D.; Liu, Y.; Yamaya, N.; Kawashima, R. Virtual training leads to physical, cognitive and neural benefits in healthy adults. Neuroimage 2020, 222, 117297.
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J. Cogn. Neurosci. 1999, 11, 491–501.
- Holper, L.; Muehlemann, T.; Scholkmann, F.; Eng, K.; Kiper, D.; Wolf, M. Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near-infrared spectroscopy (fNIRS). J. Neuroeng. Rehabil. 2010, 7, 57.
- Seraglia, B.; Gamberini, L.; Priftis, K.; Scatturin, P.; Martinelli, M.; Cutini, S. An exploratory fNIRS study with immersive virtual reality: A new method for technical implementation. Front. Hum. Neurosci. 2011, 5, 176.
- Galati, A.; Schoppa, R.; Lu, A. Exploring the SenseMaking Process through Interactions and fNIRS in Immersive Visualization. IEEE Trans. Vis. Comput. Graph. 2021, 27, 2714–2724.
- Hu, X.S.; Nascimento, T.D.; Bender, M.C.; Hall, T.; Petty, S.; O’Malley, S.; Ellwood, R.P.; Kaciroti, N.; Maslowski, E.; DaSilva, A.F. Feasibility of a Real-Time Clinical Augmented Reality and Artificial Intelligence Framework for Pain Detection and Localization From the Brain. J. Med. Internet Res. 2019, 21, e13594.
- Benitez-Andonegui, A.; Burden, R.; Benning, R.; Möckel, R.; Lührs, M.; Sorger, B. An Augmented-Reality fNIRS-Based Brain-Computer Interface: A Proof-of-Concept Study. Front. Neurosci. 2020, 14, 346.
- McKendrick, R.; Parasuraman, R.; Murtza, R.; Formwalt, A.; Baccus, W.; Paczynski, M.; Ayaz, H. Into the Wild: Neuroergonomic Differentiation of Hand-Held and Augmented Reality Wearable Displays during Outdoor Navigation with Functional Near Infrared Spectroscopy. Front. Hum. Neurosci. 2016, 10, 216.
- Ferrari, M.; Bisconti, S.; Spezialetti, M.; Basso Moro, S.; Di Palo, C.; Placidi, G.; Quaresima, V. Prefrontal cortex activated bilaterally by a tilt board balance task: A functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topogr. 2014, 27, 353–365.
- Tanveer, M.A.; Khan, M.J.; Qureshi, M.J.; Naseer, N.; Hong, K.S. Enhanced Drowsiness Detection Using Deep Learning: An fNIRS Study. IEEE Access 2019, 7, 137920–137929.
- Wickramaratne, S.D.; Mahmud, M.S. Conditional-GAN Based Data Augmentation for Deep Learning Task Classifier Improvement Using fNIRS Data. Front. Big Data 2021, 4, 659146.
- Rombach, R.; Blattmann, A.; Lorenz, D.; Esser, P.; Ommer, B. High-Resolution Image Synthesis with Latent Diffusion Models. In Proceedings of the 2022 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), New Orleans, LA, USA, 18–24 June 2022; pp. 10674–10685.
- Takagi, Y.; Nishimoto, S. High-resolution image reconstruction with latent diffusion models from human brain activity. BioRxiv 2022.
- Perpetuini, D.; Günal, M.; Chiou, N.; Koyejo, S.; Mathewson, K.; Low, K.A.; Fabiani, M.; Gratton, G.; Chiarelli, A.M. Fast Optical Signals for Real-Time Retinotopy and Brain Computer Interface. Bioengineering 2023, 10, 553.
- Bellier, L.; Llorens, A.; Marciano, D.; Gunduz, A.; Schalk, G.; Brunner, P.; Knight, R.T. Music can be reconstructed from human auditory cortex activity using nonlinear decoding models. PLoS Biol. 2023, 21, e3002176.
- Denk, T.I.; Takagi, Y.; Matsuyama, T.; Agostinelli, A.; Nakai, T.; Frank, C.H.; Nishimoto, S. Brain2Music: Reconstructing Music from Human Brain Activity. arXiv 2023, arXiv:2307.11078.
More
Information
Subjects:
Biophysics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
834
Revisions:
3 times
(View History)
Update Date:
29 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




