
Video Upload Options
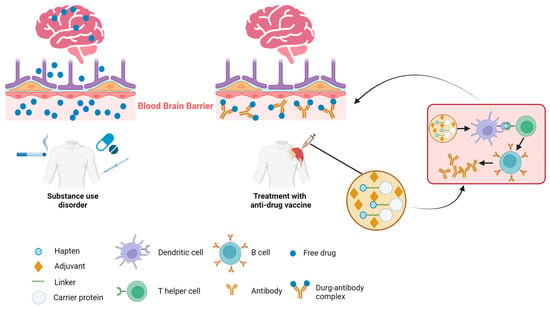
Addiction, particularly in relation to psychostimulants and opioids, persists as a global health crisis with profound social and economic ramifications. Traditional interventions, including medications and behavioral therapies, often encounter limited success due to the chronic and relapsing nature of addictive disorders. Consequently, there is significant interest in the development of innovative therapeutics to counteract the effects of abused substances. In recent years, vaccines have emerged as a novel and promising strategy to tackle addiction. Anti-drug vaccines are designed to stimulate the immune system to produce antibodies that bind to addictive compounds, such as nicotine, cocaine, morphine, methamphetamine, and heroin. These antibodies effectively neutralize the target molecules, preventing them from reaching the brain and eliciting their rewarding effects. By obstructing the rewarding sensations associated with substance use, vaccines aim to reduce cravings and the motivation to engage in drug use. Although anti-drug vaccines hold significant potential, challenges remain in their development and implementation. The reversibility of vaccination and the potential for combining vaccines with other addiction treatments offer promise for improving addiction outcomes.
1. The Mechanisms of Immunotherapies against SUDs
2. Recent Progress in Anti-Drug Vaccine Research
2.1. Opioid Vaccines
2.2. Nicotine Vaccines
2.3. Cocaine Vaccines
2.4. Methamphetamine Vaccine
References
- Xiaoshan, T.; Junjie, Y.; Wenqing, W.; Yunong, Z.; Jiaping, L.; Shanshan, L.; Kutty Selva, N.; Kui, C. Immunotherapy for treating methamphetamine, heroin and cocaine use disorders. Drug Discov. Today 2020, 25, 610–619.
- Hossain, M.; Davidson, M.; Kypreos, E.; Feehan, J.; Muir, J.; Nurgali, K.; Apostolopoulos, V. Immunotherapies for the treatment of drug addiction. Vaccines 2022, 10, 1778.
- Ciccarone, D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatry 2021, 34, 344–350.
- Rawson, R.; Erath, T.; Clark, H. The fourth wave of the overdose crisis: Examining the prominent role of psychomotor stimulants with and without fentanyl. Prev. Med. 2023, 176, 107625.
- Hu, T.; McCormack, D.; Juurlink, D.; Campbell, T.; Bayoumi, A.; Leece, P.; Kent, J.; Gomes, T. Initiation of opioid agonist therapy after hospital visits for opioid poisonings in Ontario. CMAJ Can. Med. Assoc. J. = J. De L’association Medicale Can. 2023, 195, E1709–E1717.
- Degenhardt, L.; Clark, B.; Macpherson, G.; Leppan, O.; Nielsen, S.; Zahra, E.; Larance, B.; Kimber, J.; Martino-Burke, D.; Hickman, M.; et al. Buprenorphine versus methadone for the treatment of opioid dependence: A systematic review and meta-analysis of randomised and observational studies. Lancet Psychiatry 2023, 10, 386–402.
- Volkow, N.; Blanco, C. Medications for opioid use disorders: Clinical and pharmacological considerations. J. Clin. Investig. 2020, 130, 10–13.
- Fairley, M.; Humphreys, K.; Joyce, V.; Bounthavong, M.; Trafton, J.; Combs, A.; Oliva, E.; Goldhaber-Fiebert, J.; Asch, S.; Brandeau, M.; et al. Cost-effectiveness of treatments for opioid use disorder. JAMA Psychiatry 2021, 78, 767–777.
- Kiluk, B.; Kleykamp, B.; Comer, S.; Griffiths, R.; Huhn, A.; Johnson, M.; Kampman, K.; Pravetoni, M.; Preston, K.; Vandrey, R.; et al. Clinical trial design challenges and opportunities for emerging treatments for opioid use disorder: A review. JAMA Psychiatry 2023, 80, 84–92.
- Raleigh, M.; Peterson, S.; Laudenbach, M.; Baruffaldi, F.; Carroll, F.; Comer, S.; Navarro, H.; Langston, T.; Runyon, S.; Winston, S.; et al. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS ONE 2017, 12, e0184876.
- Pravetoni, M.; Le Naour, M.; Tucker, A.; Harmon, T.; Hawley, T.; Portoghese, P.; Pentel, P. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem. 2013, 56, 915–923.
- Clinical Trials of Multivalent Opioid Vaccine Components. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04458545 (accessed on 4 August 2023).
- Pravetoni, M.; Vervacke, J.; Distefano, M.; Tucker, A.; Laudenbach, M.; Pentel, P. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS ONE 2014, 9, e96547.
- Kimishima, A.; Wenthur, C.; Zhou, B.; Janda, K. An advance in prescription opioid vaccines: Overdose mortality reduction and extraordinary alteration of drug half-life. ACS Chem. Biol. 2017, 12, 36–40.
- Ciccarone, D. Fentanyl in the US heroin supply: A rapidly changing risk environment. Int. J. Drug Policy 2017, 46, 107–111.
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.; Yuan, K.; Lu, L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl. Psychiatry 2019, 9, 282.
- Bremer, P.; Kimishima, A.; Schlosburg, J.; Zhou, B.; Collins, K.; Janda, K. Combatting synthetic designer opioids: A conjugate vaccine ablates lethal doses of fentanyl class drugs. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 3772–3775.
- Tenney, R.; Blake, S.; Bremer, P.; Zhou, B.; Hwang, C.; Poklis, J.; Janda, K.; Banks, M. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 2019, 158, 107730.
- Townsend, E.; Blake, S.; Faunce, K.; Hwang, C.; Natori, Y.; Zhou, B.; Bremer, P.; Janda, K.; Banks, M. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1681–1689.
- Haile, C.; Baker, M.; Sanchez, S.; Lopez Arteaga, C.; Duddupudi, A.; Cuny, G.; Norton, E.; Kosten, T.; Kosten, T. An immunconjugate vaccine alters distribution and reduces the antinociceptive, behavioral and physiological effects of fentanyl in male and female rats. Pharmaceutics 2022, 14, 2290.
- Eubanks, L.; Blake, S.; Natori, Y.; Ellis, B.; Bremer, P.; Janda, K. A highly efficacious carfentanil vaccine that blunts opioid-induced antinociception and respiratory depression. ACS Chem. Biol. 2021, 16, 277–282.
- Crouse, B.; Miller, S.; Muelken, P.; Hicks, L.; Vigliaturo, J.; Marker, C.; Guedes, A.; Pentel, P.; Evans, J.; LeSage, M.; et al. A TLR7/8 agonist increases efficacy of anti-fentanyl vaccines in rodent and porcine models. NPJ Vaccines 2023, 8, 107.
- Miller, S.; Crouse, B.; Hicks, L.; Amin, H.; Cole, S.; Bazin, H.; Burkhart, D.; Pravetoni, M.; Evans, J. A lipidated TLR7/8 adjuvant enhances the efficacy of a vaccine against fentanyl in mice. NPJ Vaccines 2023, 8, 97.
- Krendl, A.; Perry, B. Stigma toward substance dependence: Causes, consequences, and potential interventions. Psychol. Sci. Public Interest A J. Am. Psychol. Soc. 2023, 24, 90–126.
- Bonese, K.; Wainer, B.; Fitch, F.; Rothberg, R.; Schuster, C. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974, 252, 708–710.
- Méndez, S.; Matus-Ortega, M.; Miramontes, R.; Salazar-Juárez, A. Effect of the morphine/heroin vaccine on opioid and non-opioid drug-induced antinociception in mice. Eur. J. Pharmacol. 2021, 891, 173718.
- Ma, L.; Zhou, Q.; Zheng, H.; Li, S. Preparation and characterization of anti-morphine vaccine antibody. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2006, 22, 368–370.
- Raleigh, M.; Pravetoni, M.; Harris, A.; Birnbaum, A.; Pentel, P. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J. Pharmacol. Exp. Ther. 2013, 344, 397–406.
- Raleigh, M.; Laudenbach, M.; Baruffaldi, F.; Peterson, S.; Roslawski, M.; Birnbaum, A.; Carroll, F.; Runyon, S.; Winston, S.; Pentel, P.; et al. Opioid dose- and route-dependent efficacy of oxycodone and heroin vaccines in rats. J. Pharmacol. Exp. Ther. 2018, 365, 346–353.
- Raleigh, M.; Pentel, P.; LeSage, M. Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS ONE 2014, 9, e115696.
- Stowe, G.; Vendruscolo, L.; Edwards, S.; Schlosburg, J.; Misra, K.; Schulteis, G.; Mayorov, A.; Zakhari, J.; Koob, G.; Janda, K. A vaccine strategy that induces protective immunity against heroin. J. Med. Chem. 2011, 54, 5195–5204.
- Kosten, T.; Shen, X.; O’Malley, P.; Kinsey, B.; Lykissa, E.; Orson, F.; Kosten, T. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 223–229.
- Bremer, P.; Schlosburg, J.; Banks, M.; Steele, F.; Zhou, B.; Poklis, J.; Janda, K. Development of a clinically viable heroin vaccine. J. Am. Chem. Soc. 2017, 139, 8601–8611.
- Houston, T.; Chen, J.; Amante, D.; Blok, A.; Nagawa, C.; Wijesundara, J.; Kamberi, A.; Allison, J.; Person, S.; Flahive, J.; et al. Effect of technology-assisted brief abstinence game on long-term smoking cessation in individuals not yet ready to quit: A randomized clinical trial. JAMA Intern. Med. 2022, 182, 303–312.
- Keyler, D.; Hieda, Y.; St Peter, J.; Pentel, P. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 1999, 1, 241–249.
- Cornuz, J.; Zwahlen, S.; Jungi, W.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J.; et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS ONE 2008, 3, e2547.
- Hatsukami, D.; Jorenby, D.; Gonzales, D.; Rigotti, N.; Glover, E.; Oncken, C.; Tashkin, D.; Reus, V.; Akhavain, R.; Fahim, R.; et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011, 89, 392–399.
- Esterlis, I.; Hannestad, J.; Perkins, E.; Bois, F.; D’Souza, D.; Tyndale, R.; Seibyl, J.; Hatsukami, D.; Cosgrove, K.; O’Malley, S. Effect of a nicotine vaccine on nicotine binding to β2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am. J. Psychiatry 2013, 170, 399–407.
- Hartmann-Boyce, J.; Stead, L.; Cahill, K.; Lancaster, T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction 2013, 108, 1711–1721.
- Hoogsteder, P.; Kotz, D.; van Spiegel, P.; Viechtbauer, W.; van Schayck, O. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: A randomized placebo-controlled trial. Addiction 2014, 109, 1252–1259.
- Kallupi, M.; Xue, S.; Zhou, B.; Janda, K.; George, O. An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse. Sci. Adv. 2018, 4, eaat4751.
- Hu, Y.; Smith, D.; Frazier, E.; Hoerle, R.; Ehrich, M.; Zhang, C. The next-generation nicotine vaccine: A novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016, 106, 228–239.
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials 2017, 138, 46–56.
- Zhao, Z.; Powers, K.; Hu, Y.; Raleigh, M.; Pentel, P.; Zhang, C. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 2017, 123, 107–117.
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175.
- Hu, Y.; Zhao, Z.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Paradox of PEGylation in fabricating hybrid nanoparticle-based nicotine vaccines. Biomaterials 2018, 182, 72–81.
- Zhao, Y.; Li, Z.; Zhu, X.; Cao, Y.; Chen, X. Improving immunogenicity and safety of flagellin as vaccine carrier by high-density display on virus-like particle surface. Biomaterials 2020, 249, 120030.
- Hu, Y.; Zhao, Z.; Ehrich, M.; Zhang, C. Formulation of nanovaccines toward an extended immunity against nicotine. ACS Appl. Mater. Interfaces 2021, 13, 27972–27982.
- Alzhrani, R.; Xu, H.; Valdes, S.; Cui, Z. Intranasal delivery of a nicotine vaccine candidate induces antibodies in mouse blood and lung mucosal secretions that specifically neutralize nicotine. Drug Dev. Ind. Pharm. 2020, 46, 1656–1664.
- Scendoni, R.; Bury, E.; Ribeiro, I.; Cameriere, R.; Cingolani, M. Vaccines as a preventive tool for substance use disorder: A systematic review including a meta-analysis on nicotine vaccines’ immunogenicity. Hum. Vaccines Immunother. 2022, 18, 2140552.
- Fraleigh, N.; Lewicky, J.; Martel, A.; Diaz-Mitoma, F.; Le, H. Assessing neutralized nicotine distribution using mice vaccinated with the mucosal conjugate nicotine vaccine. Vaccines 2021, 9, 118.
- Tonstad, S.; Heggen, E.; Giljam, H.; Lagerbäck, P.; Tønnesen, P.; Wikingsson, L.; Lindblom, N.; de Villiers, S.; Svensson, T.; Fagerström, K. Niccine®, a nicotine vaccine, for relapse prevention: A phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2013, 15, 1492–1501.
- Fahim, R.; Kessler, P.; Kalnik, M. Therapeutic vaccines against tobacco addiction. Expert Rev. Vaccines 2013, 12, 333–342.
- Stephenson, R.; Toth, I. Anti-cocaine vaccine development: Where are we now and where are we going? J. Med. Chem. 2023, 66, 7086–7100.
- Bagasra, O.; Forman, L.; Howeedy, A.; Whittle, P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology 1992, 23, 173–179.
- Koetzner, L.; Deng, S.; Sumpter, T.; Weisslitz, M.; Abner, R.; Landry, D.; Woods, J. Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. J. Pharmacol. Exp. Ther. 2001, 296, 789–796.
- St John, A.; Choi, H.; Walker, Q.; Blough, B.; Kuhn, C.; Abraham, S.; Staats, H. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. NPJ Vaccines 2020, 5, 12.
- Moreno, A.; Mayorov, A.; Janda, K. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595.
- Miller, M.; Moreno, A.; Aarde, S.; Creehan, K.; Vandewater, S.; Vaillancourt, B.; Wright, M.; Janda, K.; Taffe, M. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol. Psychiatry 2013, 73, 721–728.
- Rüedi-Bettschen, D.; Wood, S.; Gunnell, M.; West, C.; Pidaparthi, R.; Carroll, F.; Blough, B.; Owens, S. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 2013, 31, 4596–4602.
- Shen, X.; Kosten, T.; Lopez, A.; Kinsey, B.; Kosten, T.; Orson, F. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013, 129, 41–48.
- Haile, C.; Kosten, T.; Shen, X.; O’Malley, P.; Winoske, K.; Kinsey, B.; Wu, Y.; Huang, Z.; Lykissa, E.; Naidu, N.; et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am. J. Addict. 2015, 24, 748–755.
- Haile, C.; Varner, K.; Huijing, X.; Arora, R.; Orson, F.; Kosten, T.; Kosten, T. Active and passive immunization with an anti-methamphetamine vaccine attenuates the behavioral and cardiovascular effects of methamphetamine. Vaccines 2022, 10, 1508.




