Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xuan, Y.; Wang, C.; Ghatak, S.; Sen, C.K. Tissue Nanotransfection Chips for In Vivo Tissue Reprogramming. Encyclopedia. Available online: https://encyclopedia.pub/entry/54309 (accessed on 08 February 2026).
Xuan Y, Wang C, Ghatak S, Sen CK. Tissue Nanotransfection Chips for In Vivo Tissue Reprogramming. Encyclopedia. Available at: https://encyclopedia.pub/entry/54309. Accessed February 08, 2026.
Xuan, Yi, Cong Wang, Subhadip Ghatak, Chandan K. Sen. "Tissue Nanotransfection Chips for In Vivo Tissue Reprogramming" Encyclopedia, https://encyclopedia.pub/entry/54309 (accessed February 08, 2026).
Xuan, Y., Wang, C., Ghatak, S., & Sen, C.K. (2024, January 24). Tissue Nanotransfection Chips for In Vivo Tissue Reprogramming. In Encyclopedia. https://encyclopedia.pub/entry/54309
Xuan, Yi, et al. "Tissue Nanotransfection Chips for In Vivo Tissue Reprogramming." Encyclopedia. Web. 24 January, 2024.
Copy Citation
Tissue nanotransfection (TNT), a cutting-edge technique of in vivo gene therapy, has gained substantial attention in various applications ranging from in vivo tissue reprogramming in regenerative medicine, and wound healing to cancer treatment. This technique harnesses the advancements in the semiconductor processes, facilitating the integration of conventional transdermal gene delivery methods—nanoelectroporation and microneedle technologies. TNT silicon chips have demonstrated considerable promise in reprogramming fibroblast cells of skin in vivo into vascular or neural cells in preclinical studies to assist in the recovery of injured limbs and damaged brain tissue.
tissue nanotransfection
electroporation
gene transfer
cell reprogramming
1. Microneedle-Based Electroporation for In Vivo Gene Transfer and Tissue Reprogramming
1.1. Microneedle-Type Electrodes-Based Bulk Electroporation for In Vivo Gene Transfer
Microneedles have been explored in various range of applications, including drug delivery, biosensing, and diagnostics [1][2][3]. It enables the penetration of the dense skin barrier, the stratum corneum layer, to effectively deliver drugs into the epidermis and dermis. However, the delivery efficacy of genes is limited because the cell membrane only allows small, nonpolar, or lipophilic molecules to pass through, serving as a barrier for hydrophilic and polar molecules like DNA and RNA [4]. These genes can enter cells via natural endocytosis, but the transfect efficiency is low. To address this challenge, recent innovations have combined microneedle technology and bulk electroporation, achieving higher transfection efficacy by overcoming both barriers of the stratum corneum and the cellular membrane. This approach demonstrates significant enhancement of gene transfer efficiency in vivo [5][6].
The MNE-BEP approach uses conductive microneedles as electrodes to apply electric pulses in vivo as illustrated in Figure 1A,B. The conductive microneedle array can be inserted into the skin to bypass the high resistance of the stratum corneum barrier. In addition, the required voltage compared to the traditional BEP is reduced, due to the smaller distances between electrodes (typically less than 1 mm). In this technique, the generic molecules are coated on the microneedles before the electroporation (Figure 1A or injected into the skin (Figure 1B). Due to the microneedles not reaching the deep dermis, the nerve-rich layer, the pain can be dramatically reduced, and the delivery depth can be precisely controlled with insertion depth.
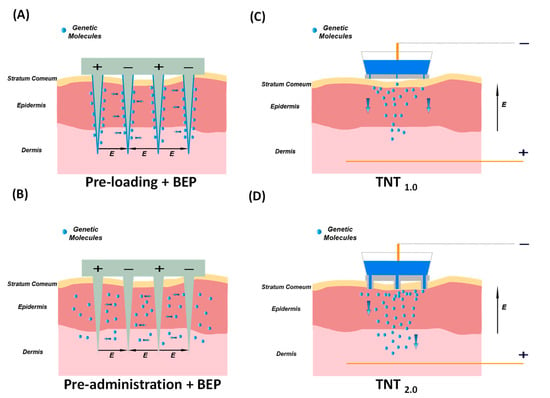
Figure 1. Microneedle-based Electroporation for In Vivo Gene Transfer Systems. Left: Microneedle-type electrodes. (A) The genetic molecules are pre-loaded onto the surface of solid microneedles before punching the skin. (B) The genetic molecules are pre-injected intradermally, followed by electroporation. Right: TNT-based in vivo gene transfer systems. (C) The first generation of TNT1.0 chip with nanochannels. (D) The second generation of TNT2.0 chip features a hollow microneedle array.
1.2. TNT for In Vivo Tissue Reprogramming
TNT is an innovative in vivo gene transfer technology, that employs the mechanism of HMN-LEP as illustrated in Figure 1E. TNT technique comprises a silicon (Si) chip, metal electrodes, and microsecond electric pulses to directly transfect target cells within the tissue. TNT successfully demonstrated transdermal gene therapy for skin repair [7], tumor regression [8], ischemic stroke recovery [9], and extreme chronic wound healing [10]. The first generation of the TNT1.0 chip utilizes the mechanism of nanoelectroporation via nanochannels as illustrated in Figure 1E and Figure 1C. Fibroblast cells of skin in vivo have been successfully reprogrammed into neuronal and endothelial cells by delivering specific gene cocktails in mice models [11][12][13]. The second generation of TNT2.0 chips that feature a hollow microneedle array is shown in Figure 1D. This modification is aimed at enhancing the physical contact between the TNT chip and skin to accommodate the nonuniform topography across the skin, thereby improving gene delivery efficiency. The TNT silicon chip exhibits advantages in terms of precise targeting, controlled release, and higher gene transfection, making it an invaluable tool in gene therapy and research. As this technology continues to evolve, it holds great potential for clinical applications in the fields of tissue engineering, regenerative medicine, and wound healing.
2. Fabrication of TNT Si Chip
Unlike the MNE-BEP, the microneedles in TNT are not used as electrodes. The TNT setup is comprised of three primary hardware components: a TNT silicon chip, electrodes, and an electroporator. The pivotal component among these is the TNT silicon chip, a hollow channel array, especially a hollow microneedle array fabricated on a Si substrate (Figure 2). In the TNT process, plasmid DNA is delivered to the tissue through these hollow channels with electrophoretic force [12][14][15].
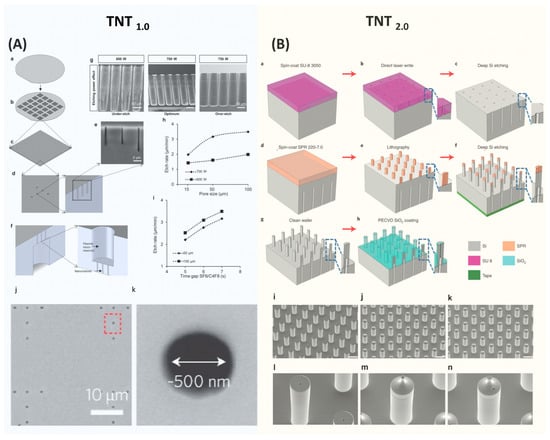
Figure 2. Semiconductor Process for Fabricating TNT Si Chip. (A) The device fabrication process of TNT1.0 chip. Figures adapted from [12] with permission. Copyright 2017, Springer Nature Limited. Published by Nature Nanotechnology, Springer Nature Limited. (B) The device fabrication process of TNT2.0 Si chip. Figures adapted from [15] with permission. Copyright 2021, The Author(s), Published by Nature Protocols, Springer Nature Limited. Figure 2 is not subject to the open access terms of this article and may not be reproduced without additional permission from the original copyright holder.
Two types of TNT chips have been developed [12][15]. The initial version of the TNT1.0 chip features nanochannels on a flat surface [12]. Subsequent enhancements led to the development of TNT2.0, which is a hollow microneedle array [15]. This design aimed to improve the contact between the chip and the skin surface, thereby enhancing delivery efficiency. In addition, the bore size increased from several hundred nanometers to a few microns. Both TNT chips were fabricated using Si because of their superior mechanical properties, favorable biocompatibility, and mass producibility using the standard semiconductor process.
The fabrication process of Si microneedle arrays has been optimized for particular shapes, inner structures, materials, and functionalities by combining CMOS and MEMS technologies. Dry and wet etching methods are the most common techniques to fabricate microneedles, but molding and electroplating are also common too [16][17][18][19][20].
TNT silicon chips are fabricated using the standard semiconductor process, primarily involving dry etching and optical lithography processes [12][15]. The brief fabrication process of TNT1.0 is shown in Figure 2A [12]. It starts with a 4-inch double-side-polished Si wafer with a thickness of ~200 µm. First, a thin photoresist was coated on the front side of the wafer, followed by patterning a nanohole array with a diameter of ~500 nm using optical lithography. These nanohole patterns were then etched into the Si wafer using a plasma etcher, forming nanochannels with ~10 µm deep. After removing the residue photoresist, the wafer was then flipped and a microhole array was patterned on the backside and etched to form micro reservoirs. These microreservoirs are directly connected to the nanochannels, enabling the unimpeded flow of gene solutions from the microreservoirs to the nanochannels, and eventually entering tissues via the electrophoretic force during the TNT process. The Si etching recipes were optimized to deliver the desired etching profiles and qualities. In the final step of the process, a thin silicon nitride film was deposited over the TNT chip to electrically insulate it.
The fabrication approach employed for TNT2.0 is similar to TNT1.0, yet it is more complicated as detailed in Figure 2B [15]. The process begins with spin coating a thick photoresist on one side of a silicon wafer, which was then patterned to define a microhole array using optical lithography. Subsequently, this pattern was etched to a specific depth using deep Si etching to form micro reservoirs. Next, a photoresist was spin-coated on the opposite side of the wafer and patterned to outline hollow microneedles with precise alignment. This is a critical step to ensure that the hollow microchannels and microreservoirs are well aligned. Employing the etching process again, the hollow microchannels were etched until they connected to the previously etched backside micro reservoirs.
The fabrication of hollow-type Si microneedle arrays is quite challenging, and the key process is to develop a high-aspect ratio of Si etching. A specialized etching technique of deep Si etching called the Bosch process, plays a crucial role [21]. This process alternates between isotropic SF6 etching and C4F8 passivation, enabling rapid Si etching with the vertical sidewalls. A distinctive feature of the Bosch process is its high etching selectivity [22]. Through fine-tuning of process parameters, the Bosch process enables the etching of Si to create deep trenches using relatively thin layers of photoresist or silicon dioxide as etching masks. The etching selectivity can reach over 100 with careful optimization of etching parameters. This high selectivity is particularly useful in the fabrication of complex 3D structures. However, in the standard two-step Bosch process, the achievable aspect ratio is typically 15:1 [22]. This restriction primarily results from the increased difficulty in the diffusion of etching gas into the deep and narrow microstructures and the re-deposition of etching byproducts at the bottom of the etched areas, which prevents further etching [23][24][25].
The aspect ratio of the TNT Si chips is over 15:1. To fabricate this high aspect ratio structure, a three-step Bosch process is employed [15][26]. This Bosch process incorporates passivation, clearing (involving the removal of passivated polymers using active argon), and etching. As a result, a high aspect ratio of over 30:1 can be achieved. In addition, using ramped process parameters, such as modulation of plasma energy, etching time, and pressure over time, demonstrated improved aspect ratios [27].
The diameters of hollow channels significantly impact the distribution of the electric field across the tissue, which is one of the factors influencing the delivery depth and the amount of transferring genetic molecules [14]. Therefore, after initial fabrication, the tailoring of the bore sizes of hollow needles is necessitated with thermal oxidation, chemical vapor deposition (CVD), or plasma-enhanced chemical vapor deposition (PECVD). Deposited materials, such as silicon dioxide or silicon nitride, effectively reduce the bore sizes to the target sizes. Additionally, these coated layers serve as an insulator between the skin and the TNT Si chip, ensuring the electric field only passes through the hollow microchannels to generate a localized electric field.
The prospective advancements in the domain of TNT device fabrication technology will focus on further enhancing the gene transfer efficacy in various circumstances such as deep tissue. This will involve refining the microneedle with shaper tips to ensure penetrates the stratum corneum layer to transfect the deeper tissue. Additionally, the focus will be placed on the system-level integration, which assembles various engineering components such as an electroporator and electrodes into the Si chip to minimize the footprint of setup.
Furthermore, the scalability of TNT manufacturing processes will be a critical task when transitioning from laboratory-scale production to large-volume manufacturing. This shift is vital for meeting the demands of clinical applications, ensuring cost-effective and wide accessibility for patients.
3. Clinical Applications
The integration of microneedle technology and electroporation opens a new era in the field of gene transfer. The unique strengths of both microscale resolution devices and widely suitable electroporation benefit a wide range of applications across diverse fields. The development of MNE-BEP has significantly enhanced the aspects of pain management, reduced voltage, and delivery efficacy. Furthermore, the clinical studies showed a promising future in cancer treatment [28][29], skin disease treatment [30], and DNA vaccination [6][31].
TNT takes additional measures to use a localized electric field, allowing for precise targeting, controllable delivery, and excellent transfection efficacy. For its application, the TNT technique can be classified into in vivo tissue reprogramming and non-reprogramming types based on whether the gene transfer induces cell-type changes in the tissues. In the treatment of diseases and repair of damaged tissue, patients often lose some capability to generate specific functional cells. This deficiency significantly affects the treatment outcomes and prevents the healing process. To improve this situation, in vivo tissue reprogramming via TNT presents a groundbreaking method for directly converting a type of cell (such as fibroblast) into desired functional cells in vivo [13]. This innovative approach bypasses the intermediate stem cell stage to increase efficiency and reduce the risk of tumorigenesis [12]. In the current research, the TNT silicon chip-based cell reprogramming has been demonstrated in the field of wound healing, diabetes, ischemic diseases, and cancer treatment. Non-reprogramming TNT applications, on the other hand, result in no change in the cell type while increasing the gene expression efficacy in the disease treatment process or exosome labeling.
In the realm of wound healing, the application of electroporation-based in vivo gene transfer has been proven to be a transformative approach. This direct method yields a high delivery efficiency of therapeutic genes into target cells at the wound site within a second. This approach can be especially beneficial for chronic wounds, a condition often resistant to traditional healing methods [10], and counteracting the underlying issues in these wounds, such as inflammation [32][33] or insufficient blood circulation [34]. This method can effectively treat patients with complex wounds.
Microchip-electroporation-based in vivo gene transfer also shows great promise in the field of cancer treatment. Microchip technology enables the precise delivery of cancer-fighting genes directly into tumor cells or surrounding tissues. Unlike conventional treatments that often affect both healthy and cancerous cells, TNT allows for the delivery of therapeutic genes in a highly localized manner, ensuring maximum impact on the tumor while minimizing side effects. Except for the in vivo gene delivery methods [8][28][35][36] mentioned, irreversible electroporation is also widely used to induce tumor cell death for cancer treatment [37].
Effective treatment for diabetic ischemic limbs is crucial in diabetes management, as it significantly impacts the patient’s daily life. The electroporation-based gene transfer can notably improve the ischemic conditions in diabetic mice using TNT [38].
References
- Abrbekoh, F.N.; Salimi, L.; Saghati, S.; Amini, H.; Karkan, S.F.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390.
- Liu, G.S.; Kong, Y.F.; Wang, Y.S.; Luo, Y.H.; Fan, X.D.; Xie, X.; Yang, B.R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740.
- Erdem, Ö.; Es, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent Advances in Microneedle-Based Sensors for Sampling, Diagnosis and Monitoring of Chronic Diseases. Biosensors 2021, 11, 296.
- Sachdev, S.; Potocnik, T.; Rems, L.; Miklavcic, D. Revisiting the role of pulsed electric fields in overcoming the barriers to gene electrotransfer. Bioelectrochemistry 2022, 144, 107994.
- Choi, S.O.; Kim, Y.C.; Lee, J.W.; Park, J.H.; Prausnitz, M.R.; Allen, M.G. Intracellular Protein Delivery and Gene Transfection by Electroporation Using a Microneedle Electrode Array. Small 2012, 8, 1081–1091.
- Xia, D.N.; Jin, R.; Byagathvalli, G.; Yu, H.; Ye, L.; Lu, C.Y.; Bhamla, M.S.; Yang, C.L.; Prausnitz, M.R. An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2110817118.
- Ghatak, S.; Khanna, S.; Roy, S.; Thirunavukkarasu, M.; Pradeep, S.R.; Wulff, B.C.; El Masry, M.S.; Sharma, A.; Palakurti, R.; Ghosh, N.; et al. Driving adult tissue repair via re-engagement of a pathway required for fetal healing. Mol. Ther. 2023, 31, 454–470.
- Gordillo, G.M.; Guda, P.R.; Singh, K.; Biswas, A.; Abouhashem, A.S.; Rustagi, Y.; Sen, A.; Kumar, M.; Das, A.; Ghatak, S.; et al. Tissue nanotransfection causes tumor regression by its effect on nanovesicle cargo that alters microenvironmental macrophage state. Mol. Ther. 2023, 31, 1402–1417.
- Lemmerman, L.R.; Balch, M.H.H.; Moore, J.T.; Alzate-Correa, D.; Rincon-Benavides, M.A.; Salazar-Puerta, A.; Gnyawali, S.; Harris, H.N.; Lawrence, W.; Ortega-Pineda, L.; et al. Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Sci. Adv. 2021, 7, abd4735.
- Singh, K.; Rustagi, Y.; Abouhashem, A.S.; Tabasum, S.; Verma, P.; Hernandez, E.; Pal, D.; Khona, D.K.; Mohanty, S.K.; Kumar, M.; et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J. Clin. Investig. 2022, 132, 157279.
- Roy, S.; Sen, C.K.; Ghatak, S.; Higuita-Castro, N.; Palakurti, R.; Nalluri, N.; Clark, A.; Stewart, R.; Gallego-Perez, D.; Prater, D.N.; et al. Neurogenic tissue nanotransfection in the management of cutaneous diabetic polyneuropathy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102220.
- Gallego-Perez, D.; Pal, D.; Ghatak, S.; Malkoc, V.; Higuita-Castro, N.; Gnyawali, S.; Chang, L.Q.; Liao, W.C.; Shi, J.F.; Sinha, M.; et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 2017, 12, 974–979.
- Pal, D.; Ghatak, S.; Singh, K.; Abouhashem, A.S.; Kumar, M.; El Masry, M.S.; Mohanty, S.K.; Palakurti, R.; Rustagi, Y.; Tabasum, S.; et al. Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat. Commun. 2023, 14, 1129.
- Li, Z.; Xuan, Y.; Ghatak, S.; Guda, P.R.; Roy, S.; Sen, C.K. Modeling the gene delivery process of the needle array-based tissue nanotransfection. Nano Res. 2022, 15, 3409–3421.
- Xuan, Y.; Ghatak, S.; Clark, A.; Li, Z.G.; Khanna, S.; Pak, D.; Agarwal, M.; Roy, S.; Duda, P.; Sen, C.K. Fabrication and use of silicon hollow-needle arrays to achieve tissue nanotransfection in mouse tissue in vivo. Nat. Protoc. 2021, 16, 5707–5738.
- Wilke, N.; Mulcahy, A.; Ye, S.R.; Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 2005, 36, 650–656.
- Held, J.; Gaspar, J.; Ruther, P.; Hagner, M.; Cismak, A.; Heilmann, A.; Paul, O. Design of experiment characterization of microneedle fabrication processes based on dry silicon etching. J. Micromech. Microeng. 2010, 20, 025024.
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207.
- Badnikar, K.; Jayadevi, S.N.; Pahal, S.; Sripada, S.; Nayak, M.M.; Vemula, P.K.; Subrahmanyam, D.N. Generic Molding Platform for Simple, Low-Cost Fabrication of Polymeric Microneedles. Macromol. Mater. Eng. 2020, 305, 2000072.
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotech. 2021, 12, 1034–1046.
- Ji, J.; Tay, F.E.; Miao, J.; Iliescu, C. Microfabricated Silicon Microneedle Array for Transdermal Drug Delivery; IOP Publishing: Bristol, UK, 2006.
- Huff, M. Recent Advances in Reactive Ion Etching and Applications of High-Aspect-Ratio Microfabrication. Micromachines 2021, 12, 991.
- Gottscho, R.A.; Jurgensen, C.W.; Vitkavage, D.J. Microscopic uniformity in plasma etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1992, 10, 2133–2147.
- Blauw, M.A.; Zijlstra, T.; Bakker, R.A.; van der Drift, E. Kinetics and crystal orientation dependence in high aspect ratio silicon dry etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2000, 18, 3453–3461.
- Coburn, J.W.; Winters, H.F. Conductance considerations in the reactive ion etching of high aspect ratio features. Appl. Phys. Lett. 1989, 55, 2730–2732.
- Lai, S.L.; Johnson, D.; Westerman, R. Aspect ratio dependent etching lag reduction in deep silicon etch processes. J. Vac. Sci. Technol. A 2006, 24, 1283–1288.
- Tang, Y.; Sandoughsaz, A.; Owen, K.J.; Najafi, K. Ultra deep reactive ion etching of high aspect-ratio and thick silicon using a ramped-parameter process. J. Microelectromech. Syst. 2018, 27, 686–697.
- Yang, T.R.; Huang, D.; Li, C.H.; Zhao, D.Y.; Li, J.S.; Zhang, M.J.; Chen, Y.F.; Wang, Q.N.; Liang, Z.C.; Liang, X.J.; et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today 2021, 36, 101017.
- Wilke, N.; Hibert, C.; O’Brien, J.; Morrissey, A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens. Actuators A Phys. 2005, 123–124, 319–325.
- Huang, D.; Zhao, D.Y.; Wang, X.X.; Li, C.H.; Yang, T.R.; Du, L.L.; Wei, Z.W.; Cheng, Q.; Cao, H.Q.; Liang, Z.C.; et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics 2018, 8, 2361–2376.
- Choi, S.O.; Kim, Y.C.; Park, J.H.; Hutcheson, J.; Gill, H.S.; Yoon, Y.K.; Prausnitz, M.R.; Allen, M.G. An electrically active microneedle array for electroporation. Biomed. Microdevices 2010, 12, 263–273.
- Moore, J.T.; Wier, C.G.; Lemmerman, L.R.; Ortega-Pineda, L.; Dodd, D.J.; Lawrence, W.R.; Duarte-Sanmiguel, S.; Dathathreya, K.; Diaz-Starokozheva, L.; Harris, H.N.; et al. Nanochannel-Based Poration Drives Benign and Effective Nonviral Gene Delivery to Peripheral Nerve Tissue. Adv. Biosyst. 2020, 4, e2000157.
- Hassanein, A.H.; Sinha, M.; Neumann, C.R.; Mohan, G.; Khan, I.; Sen, C.K. A Murine Tail Lymphedema Model. J. Vis. Exp. 2021, 168, e61848.
- Diaz-Starokozheva, L.; Das, D.; Gu, X.; Moore, J.T.; Lemmerman, L.R.; Valerio, I.; Powell, H.M.; Higuita-Castro, N.; Go, M.R.; Palmer, A.F.; et al. Early Intervention in Ischemic Tissue with Oxygen Nanocarriers Enables Successful Implementation of Restorative Cell Therapies. Cell Mol. Bioeng. 2020, 13, 435–446.
- Briz, P.; López-Alonso, B.; Sarnago, H.; Burdío, J.M.; Lucía, O. Tumor location on electroporation therapies by means of multi-electrode structures and machine learning. Bioelectrochemistry 2023, 154, 108510.
- Duarte-Sanmiguel, S.; Salazar-Puerta, A.I.; Panic, A.; Dodd, D.; Francis, C.; Alzate-Correa, D.; Ortega-Pineda, L.; Lemmerman, L.; Rincon-Benavides, M.A.; Dathathreya, K.; et al. ICAM-1-decorated extracellular vesicles loaded with miR-146a and Glut1 drive immunomodulation and hinder tumor progression in a murine model of breast cancer. Biomater. Sci. 2023, 11, 6834–6847.
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231.
- Rustagi, Y.; Abouhashem, A.S.; Verma, P.; Verma, S.S.; Hernandez, E.; Liu, S.; Kumar, M.; Guda, P.R.; Srivastava, R.; Mohanty, S.K.; et al. Endothelial Phospholipase Cgamma2 Improves Outcomes of Diabetic Ischemic Limb Rescue Following VEGF Therapy. Diabetes 2022, 71, 1149–1165.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
697
Revisions:
2 times
(View History)
Update Date:
25 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




