Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bing Wang | -- | 1698 | 2024-01-24 15:55:18 | | | |
| 2 | Peter Tang | Meta information modification | 1698 | 2024-01-25 03:46:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, B.; Deng, J.; Donati, V.; Merali, N.; Frampton, A.E.; Giovannetti, E.; Deng, D. Porphyromonas gingivalis in Cancer Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/54301 (accessed on 16 January 2026).
Wang B, Deng J, Donati V, Merali N, Frampton AE, Giovannetti E, et al. Porphyromonas gingivalis in Cancer Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/54301. Accessed January 16, 2026.
Wang, Bing, Juan Deng, Valentina Donati, Nabeel Merali, Adam E. Frampton, Elisa Giovannetti, Dongmei Deng. "Porphyromonas gingivalis in Cancer Development" Encyclopedia, https://encyclopedia.pub/entry/54301 (accessed January 16, 2026).
Wang, B., Deng, J., Donati, V., Merali, N., Frampton, A.E., Giovannetti, E., & Deng, D. (2024, January 24). Porphyromonas gingivalis in Cancer Development. In Encyclopedia. https://encyclopedia.pub/entry/54301
Wang, Bing, et al. "Porphyromonas gingivalis in Cancer Development." Encyclopedia. Web. 24 January, 2024.
Copy Citation
A periodontal pathogen, namely, Porphyromonas gingivalis, was found to be associated with all three types of cancers: oral squamous cell carcinoma (OSCC), colorectal cancer (CRC), and pancreatic ductal adenocarcinoma (PDAC).
periodontitis
oral squamous cell carcinomas
colorectal cancers
pancreatic ductal carcinomas
bateria–host interactions
microbial interaction
1. Introduction
The human microbiome consists of a diverse range of bacteria that play a vital role in maintaining the equilibrium between health and diseases [1][2]. Among them, the oral microbiome stands as the second most diverse and intricate ecosystem [3] residing in the oral cavity, which is the primary site of entrance into both the digestive and respiratory systems. The enlarged Human Oral Microbiome Database reports the presence of over 700 bacterial species involved in dynamic and intricate microbial interactions [4]. The oral microbiome has garnered increasing attention in recent years due to its potential implication for various health/disease conditions, not only inside the oral cavity but also at distant body sites [5][6].
The significant advancements in next-generation sequencing technology and bioinformatic tools have facilitated the exploration of the harmonious equilibrium among the microbiome, the host, and the environment. It has been revealed that the microbial community as a whole, rather than a few single microbes, maintains this equilibrium. In the healthy state, the microbiome and host establish a symbiotic relationship; whereas in the disease state, a dysbiosis environment promotes the prevalence of pathogenic species in a microbiome, ultimately contributing to the development of illnesses [7]. It was shown that the dysbiotic oral microbiome not only leads to oral infectious diseases, such as caries and periodontitis, but also plays an important role in the development of multiple systemic diseases, including cardiovascular disease, rheumatoid arthritis, Alzheimer’s disease, pulmonary disease, and cancer [8][9][10][11][12].
Cancer, which is characterized by uncontrolled cell growth and potential metastasis, remains a leading cause of mortality globally [13]. Traditionally, the etiological factors of cancer have been attributed to intrinsic factors, like genetic, environmental, and lifestyle components [14]. Recent discoveries in cancer research revealed that tumor growth might be affected by the dynamic interactions between all constituents inside the tumor microenvironment (TME) as well [15][16]. Within the TME, there are not only intricate signaling contacts between cellular and non-cellular factors but also reciprocal interactions involving microbial components [16][17]. It was believed that the microbiome could be operated as a powerful regulator inside the TME, thereby influencing the host (immune) responses [7][18].
Oral squamous cell carcinoma (OSCC) and gastrointestinal cancers, including colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC), are among the malignancies that have been linked to the oral microbiome in an intriguing way [19]. It was hypothesized that oral microbes reach distant body sites via the circulatory system after processes such as mastication and routine oral hygiene practices, like teeth brushing and flossing [20]. The concept of the “oral-gut axis” was proposed to illustrate this connection [21]. For example, Fusobacterium nucleatum, which is a well-known Gram-negative pathogen for periodontal infections, has been implicated in the development of systemic disorders, including premature birth, inflammatory bowel disease, and CRC. Another periodontal pathogen, namely, Porphyromonas gingivalis, was found to be associated with all three types of cancers: OSCC, CRC, and PDAC [22][23]. Until now, ample studies have explored the role of oral microbes in the onset and development of cancers [6][19][24]. However, it is noteworthy that most studies focused on the role of a single bacterial species. It has been acknowledged that tumor tissues do not harbor a single bacterial species, but a multi-species microbial community that accommodates active bacterial interactions [25][26]. These bacterial interactions could change the formation of tumors. For example, Pustelny et al. demonstrated in a murine tumor model where mice were coinfected with the cystic fibrosis pathogen Pseudomonas aeruginosa and a strictly anaerobic bacterium Veillonella parvula had worse survival rates due to higher P. aeruginosa loads in tumor tissues compared with those coinfected with either bacterial species alone [27]. Lertpirlyapong et al. showed that gastric colonization with multi-species microbiota and the carcinogenic pathogen Helicobacter pylori in male mice leads to more invasive gastrointestinal intraepithelial neoplasia than colonization with H. pylori alone [28]. Although both studies were conducted in mice, the experimental evidence hints at the potential importance of microbial interaction in cancer development. Thus, the existing knowledge on the contribution of a single bacterial species to cancer progression is insufficient without considering the complex dynamics arising from interactions within the microbial community.
The oral pathogen F. nucleatum is known as a bridging bacterium that is able to coaggregate with various bacterial species, such as P. gingivalis, Treponema denticola, and Prevotella intermedia [29][30]. Similarly, the fimbriae of P. gingivalis can mediate the coaggregation with Streptococcus gordonii, Veillonella sp., and Aggregatibacter actinomycetemcomitans [31][32]. It was shown that F. nucleatum can enhance the invasion of human gingival epithelial cells by P. gingivalis, which might increase the transmission of P. gingivalis to other body sites in periodontitis patients [33].
2. The Prevalence of P. gingivalis in OSCC, CRC, and PDAC
P. gingivalis is a well-known periodontal pathogen. It is a Gram-negative anaerobic bacterium associated with the onset and progression of periodontitis [34]. Previous studies showed that it can colonize malignant tissues in an oral cavity, such as OSCC, ESCC, and gingival carcinoma [35]. Sayehmiri et al. conducted a meta-analysis that revealed the presence of P. gingivalis is associated with a risk increase of more than 1.36-fold in the development of OSCC [36]. An excessive amount of P. gingivalis was identified as a potential risk factor for OSCC [37][38][39].
In addition to oral cancers, P. gingivalis was frequently related to cancers at other body sites, including esophageal cancer, lung cancer, CRC, and PDAC [40][41][42][43]. Ample clinical studies found high abundances of P. gingivalis in both tumor tissue and fecal samples of CRC patients, which were correlated with the onset of CRC and poor prognosis in patients. For example, in a cross-sectional study, Kerdreux et al. examined 247 CRC patients and 89 controls (stages I–IV). They found a significant increase in P. gingivalis levels in fecal samples of CRC patients compared with the healthy controls. P. gingivalis could be identified in the fecal samples of 2.6–5.3% of CRC patients [22]. In another cohort study, P. gingivalis was detectable in 10 out of 31 CRC tissue samples using quantitative polymerase chain reaction (qPCR). A higher prevalence of P. gingivalis was found in individuals with the latter phases of colonic carcinogenesis [44].
Similarly, a high prevalence of P. gingivalis in PDAC patients has been reported. A recent prospective cohort study of 361 individuals diagnosed with PDAC found that the presence of P. gingivalis was associated with a 59% rise in PDAC development. Results also show an imbalance in oral microbial composition occurred before the onset of the cancer [45]. Another prospective cohort study examined 405 individuals diagnosed with pancreatic cancer, together with 410 control subjects, and found that the individuals with high levels of antibodies against P. gingivalis had a greater than twofold increased risk of developing PDAC [46].
Overall, there is clear clinical evidence that a high level of P. gingivalis, either in an oral cavity, fecal samples, or tumor tissues, is associated with the development of all three types of cancers.
3. Mechanisms of P. gingivalis in Cancer Development
As a known periodontal pathogen, P. gingivalis employs many strategies to compromise tissue integrity and impair the host immune response. These strategies include the prevention of cell apoptosis, stimulation of cell proliferation, initiation of chronic inflammation, and generation of oncometabolites [47].
Previous studies indicate that P. gingivalis can promote tumorigenesis by influencing various signaling pathways (Figure 1): (1) Upon infection of the host by P. gingivalis, the B7-H1 receptor can be activated, facilitating the apoptosis of activated T cells. The increased expression of B7-H1 receptors in host cells may impact the persistence of inflammatory illnesses [48]. (2) Nucleoside Diphosphate Kinase (NDK), which is the effector protein produced by intracellular P. gingivalis, can block the signaling of extracellular adenosine triphosphate (ATP)/purinergic receptor (P2X7) on macrophages by consuming ATP. This prevents inflammasome activation and the secretion of interleukin-1β (IL-1β), consequently facilitating the process of tumorigenesis [49]. The NDK enzyme is also known to phosphorylate heat shock protein 27 (HSP27), and it is capable of triggering antiapoptotic processes upon phosphorylation [50]. (3) P. gingivalis activates antiapoptotic pathways, such as Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3) and phosphoinositide 3-kinase PI3K/protein kinase B (Akt) in oral epithelial cells, thus promoting OSCC [51]. Besides inhibiting the intrinsic apoptosis of the invaded epithelial cells, P. gingivalis can also enhance the progression of the S phase of the cell cycle by inhibiting the p53 tumor suppressor gene (TSG) through the FimA adhesin [52]. Expression of the aforementioned B7-H1 receptor can inhibit the effector T cells by inducing regulatory T cells, which is beneficial for the invaded cell survival [48][53]. Due to the induction of regulatory T cells by the B7-H1 receptor, the immune system is (partly) evaded. (4) The activation of extracellular signal-regulated kinase 1/2 (ERK1/2)-protein ETS1, p38/HSP27, and PAR2 (protease-activated receptor 2)/nuclear factor kappa B (NF-κB) pathways was observed in response to P gingivalis infection, leading to the induction of pro-matrix metalloproteinase-9 (pro-MMP-9) expression, hence the increasing levels of MMP-9 and enhanced cellular invasion [54][55][56].
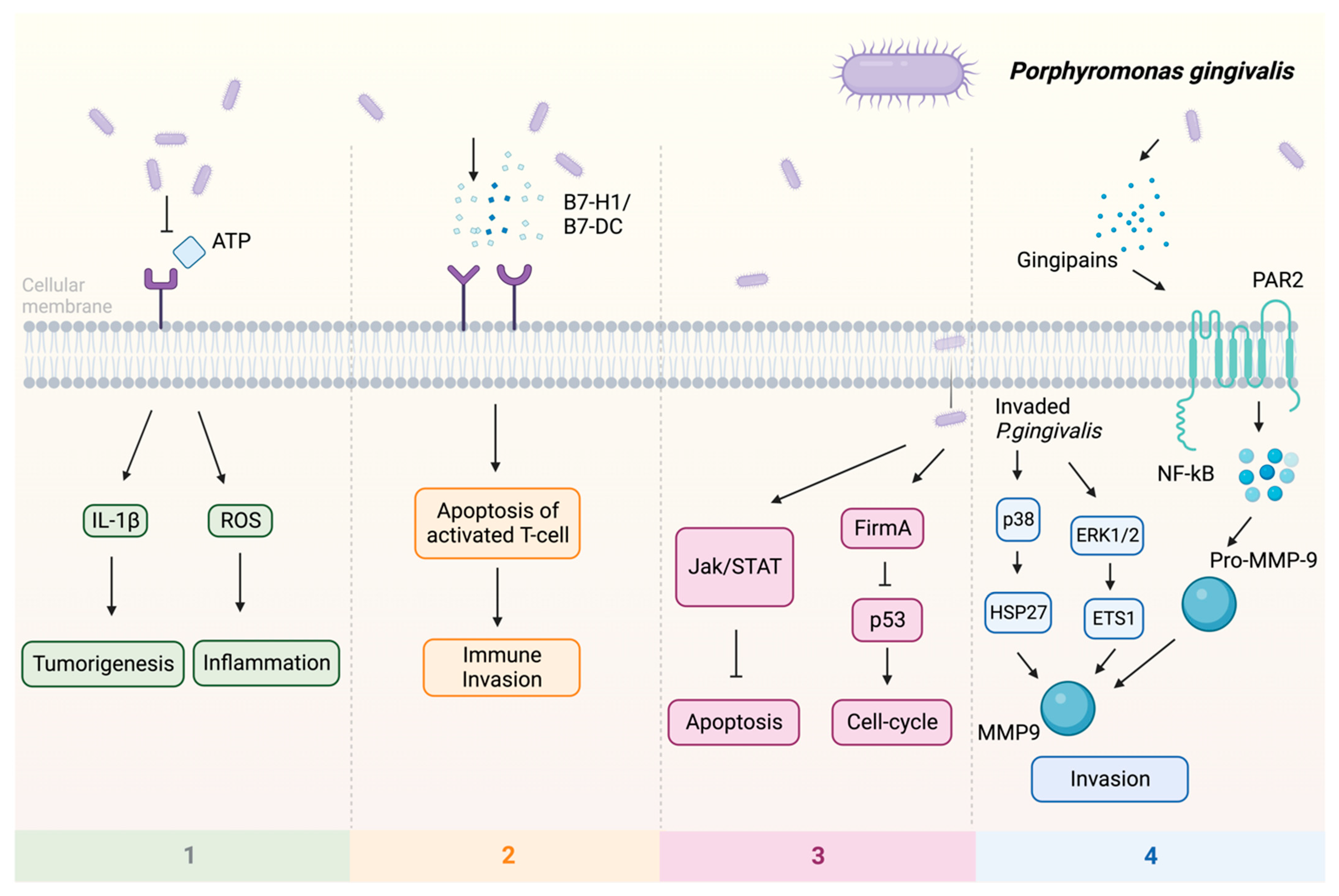
Figure 1. Multiple pathways employed by P. gingivalis in tumor induction: (1) P2X7 activation via ATP is blocked, leading to the stimulation of IL-1β, which promotes tumorigenesis, and the induction of ROS, which fosters a pro-inflammatory microenvironment. (2) Facilitation of immune evasion occurs through the activation of B7-H1 and B7-DC receptors, contributing to a (partial) circumvention of the immune system. Immune evasion is facilitated through the activation of B7-H1 and B7-DC receptors, contributing to a (partial) evasion of the immune system. (3) Activation of FimA results in the downregulation of p53, enhancing the host cell’s cell cycle while simultaneously suppressing apoptosis. The JAK/STAT axis is also implicated in the downregulation of apoptosis. (4) Additionally, P. gingivalis stimulates invasion through PAR2 activation via gingipains, activating NF-κB signaling, which leads to the formation of MMP-9, thereby enhancing P. gingivalis invasion. Upon invasion, pro-MMP-9 undergoes upregulation facilitated by ERK1/2 and ETS1, along with activation of p38 and HSP27. Abbreviations: ATP—adenosine triphosphate; ERK1/2—extracellular signal-regulated kinase 1/2; ETS1—protein; FimA—protein; HSP27—heat shock protein 27; IL-1β—interleukin-1β; JAK—Janus kinase; MMP-9—matrix metalloproteinase-9; NF-κB—nuclear factor kappa B; P2X7—purinergic receptor; pro-MMP-9—pro-matrix metalloproteinase-9; p38, p53—protein; ROS—reaction oxygen species; STAT—signal transducer and activator of transcription.
References
- Relman, D.A. The human microbiome and the future practice of medicine. JAMA 2015, 314, 1127–1128.
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646.
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143.
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18.
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318.
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral. Sci. 2022, 14, 14.
- Li, Z.; Liu, Y.; Zhang, L. Role of the microbiome in oral cancer occurrence, progression and therapy. Microb. Pathog. 2022, 169, 105638.
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500.
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2015, 60, 69–80.
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin, S.; Gai, Z.; Heng, X.; Zhang, C.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 8, 17126.
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738.
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475.
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695.
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046.
- Sevcikova, A.; Izoldova, N.; Stevurkova, V.; Kasperova, B.; Chovanec, M.; Ciernikova, S.; Mego, M. The Impact of the Microbiome on Resistance to Cancer Treatment with Chemotherapeutic Agents and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 488.
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596.
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925.
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor microbiome—An integral part of the tumor microenvironment. Front. Oncol. 2022, 12, 1063100.
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088.
- Choy, A.T.F.; Carnevale, I.; Coppola, S.; Meijer, L.L.; Kazemier, G.; Zaura, E.; Deng, D.; Giovannetti, E. The microbiome of pancreatic cancer: From molecular diagnostics to new therapeutic approaches to overcome chemoresistance caused by metabolic inactivation of gemcitabine. Expert. Rev. Mol. Diagn. 2018, 18, 1005–1009.
- Lam, G.A.; Albarrak, H.; McColl, C.J.; Pizarro, A.; Sanaka, H.; Gomez-Nguyen, A.; Cominelli, F.; Paes Batista da Silva, A. The Oral-Gut Axis: Periodontal Diseases and Gastrointestinal Disorders. Inflamm. Bowel Dis. 2023, 29, 1153–1164.
- Kerdreux, M.; Edin, S.; Löwenmark, T.; Bronnec, V.; Löfgren-Burström, A.; Zingmark, C.; Ljuslinder, I.; Palmqvist, R.; Ling, A. Porphyromonas gingivalis in Colorectal Cancer and its Association to Patient Prognosis. J. Cancer 2023, 14, 1479–1485.
- Gnanasekaran, J.; Binder Gallimidi, A.; Saba, E.; Pandi, K.; Eli Berchoer, L.; Hermano, E.; Angabo, S.; Makkawi, H.a.; Khashan, A.; Daoud, A. Intracellular Porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers 2020, 12, 2331.
- Reitano, E.; de’Angelis, N.; Gavriilidis, P.; Gaiani, F.; Memeo, R.; Inchingolo, R.; Bianchi, G.; de’Angelis, G.L.; Carra, M.C. Oral Bacterial Microbiota in Digestive Cancer Patients: A Systematic Review. Microorganisms 2021, 9, 2585.
- Hooper, S.J.; Crean, S.J.; Lewis, M.A.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006, 44, 1719–1725.
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012, 12, 144.
- Pustelny, C.; Komor, U.; Pawar, V.; Lorenz, A.; Bielecka, A.; Moter, A.; Gocht, B.; Eckweiler, D.; Müsken, M.; Grothe, C. Contribution of Veillonella parvula to Pseudomonas aeruginosa-mediated pathogenicity in a murine tumor model system. Infect. Immun. 2015, 83, 417–429.
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014, 63, 54–63.
- Coppenhagen-Glazer, S.; Sol, A.; Abed, J.; Naor, R.; Zhang, X.; Han, Y.W.; Bachrach, G. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 2015, 83, 1104–1113.
- Okuda, T.; Kokubu, E.; Kawana, T.; Saito, A.; Okuda, K.; Ishihara, K. Synergy in biofilm formation between Fusobacterium nucleatum and Prevotella species. Anaerobe 2012, 18, 110–116.
- Rosen, G.; Sela, M.N. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol. Lett. 2006, 256, 304–310.
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811.
- Saito, A.; Inagaki, S.; Kimizuka, R.; Okuda, K.; Hosaka, Y.; Nakagawa, T.; Ishihara, K. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2008, 54, 349–355.
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068.
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404.
- Sayehmiri, F.; Sayehmiri, K.; Asadollahi, K.; Soroush, S.; Bogdanovic, L.; Jalilian, F.A.; Emaneini, M.; Taherikalani, M. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol. 2015, 28, 160–167.
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral. Microbiol. 2019, 11, 1563410.
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797.
- Purcell, R.V.; Visnovska, M.; Biggs, P.J.; Schmeier, S.; Frizelle, F.A. Distinct gut microbiome patterns associate with consensus molecular subtypes of colorectal cancer. Sci. Rep. 2017, 7, 11590.
- Bellotti, R.; Speth, C.; Adolph, T.E.; Lass-Flörl, C.; Effenberger, M.; Öfner, D.; Maglione, M. Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development. Cancers 2021, 13, 3431.
- Malinowski, B.; Węsierska, A.; Zalewska, K.; Sokołowska, M.M.; Bursiewicz, W.; Socha, M.; Ozorowski, M.; Pawlak-Osińska, K.; Wiciński, M. The role of Tannerella forsythia and Porphyromonas gingivalis in pathogenesis of esophageal cancer. Infect. Agent. Cancer 2019, 14, 3.
- Liu, Y.; Yuan, X.; Chen, K.; Zhou, F.; Yang, H.; Yang, H.; Qi, Y.; Kong, J.; Sun, W.; Gao, S. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl. Oncol. 2021, 14, 100972.
- Kong, J.; Yuan, X.; Wang, J.; Liu, Y.; Sun, W.; Gu, B.; Lan, Z.; Gao, S. Frequencies of Porphyromonas gingivalis detection in oral-digestive tract tumors. Pathol. Oncol. Res. 2021, 27, 628942.
- Wang, X.; Jia, Y.; Wen, L.; Mu, W.; Wu, X.; Liu, T.; Liu, X.; Fang, J.; Luan, Y.; Chen, P.; et al. Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Res. 2021, 81, 2745–2759.
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127.
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770.
- Nasiri, K.; Amiri Moghaddam, M.; Etajuri, E.A.; Badkoobeh, A.; Tavakol, O.; Rafinejad, M.; Forutan Mirhosseini, A.; Fathi, A. Periodontitis and progression of gastrointestinal cancer: Current knowledge and future perspective. Clin. Transl. Oncol. 2023, 25, 2801–2811.
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310.
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903.
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601.
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354.
- Kuboniwa, M.; Hasegawa, Y.; Mao, S.; Shizukuishi, S.; Amano, A.; Lamont, R.J.; Yilmaz, O.P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008, 10, 122–128.
- Zhou, Y.; Luo, G.H. Porphyromonas gingivalis and digestive system cancers. World J. Clin. Cases 2019, 7, 819–829.
- Kuo, P.J.; Tu, H.P.; Chin, Y.T.; Lu, S.H.; Chiang, C.Y.; Chen, R.Y.; Fu, E. Cyclosporine-A inhibits MMP-2 and -9 activities in the presence of Porphyromonas gingivalis lipopolysaccharide: An experiment in human gingival fibroblast and U937 macrophage co-culture. J. Periodontal Res. 2012, 47, 431–438.
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014, 16, 131–145.
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
626
Revisions:
2 times
(View History)
Update Date:
25 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




