
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dante Cantini | -- | 3827 | 2024-01-24 13:29:24 | | | |
| 2 | Mona Zou | Meta information modification | 3827 | 2024-01-25 10:00:23 | | |
Video Upload Options
The risk of parasitic infection has a major influence on animal behaviour. Organisms must adjust their behaviour to avoid various modes of parasitic infection and pathogen acquisition. Social species are at an increased risk of parasitic transmission as they spend more time in the proximity of others that may carry parasites. The detection of parasitic risk is also critical in mate assessment and choice. Perceptual systems and behavioural responses have evolved to detect individuals who are parasitized and pose the risk of parasitic transmission. This includes the integration of inputs from various sensory modalities (e.g., olfaction), brain regions and networks, and neuromodulatory systems. Understanding the neurobiological systems involved in detecting the parasite infection risk and the expression of disgust will allow us to better understand the evolution and regulation of pathogen avoidance and mate choice.
1. Introduction
2. Brain Regions
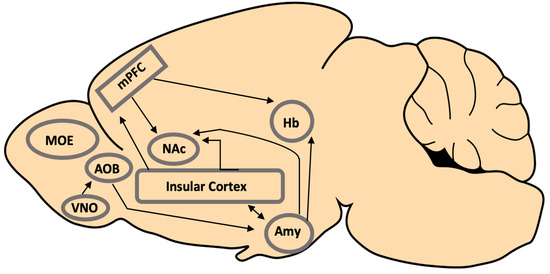
2.1. Medial Amygdala
2.2. Insular Cortex
2.3. Habenula
2.4. Nucleus Accumbens
2.5. Concluding Statement on Brain Regions
2.6. Immune–Brain Interactions
2.7. Microbiome–Immune–Brain Interactions
3. Neuropeptides and Hormones Involved in Pathogen Avoidance
3.1. Oxytocin
3.2. Vasopressin
3.3. Estrogens
3.4. Progesterone
3.5. Concluding Statement on Neuropeptides and Hormones
References
- Schmid-Hempel, P. Parasites and Their Social Hosts. Trends Parasitol. 2017, 33, 453–462.
- Favreau, F.-R.; Goldizen, A.W.; Pays, O. Interactions among social monitoring, anti-predator vigilance and group size in eastern grey kangaroos. Proc. R. Soc. B Biol. Sci. 2010, 277, 2089–2095.
- Gersick, A.S.; Snyder-Mackler, N.; White, D.J. Ontogeny of social skills: Social complexity improves mating and competitive strategies in male brown-headed cowbirds. Anim. Behav. 2012, 83, 1171–1177.
- Nöbel, S.; Wang, X.; Talvard, L.; Tariel, J.; Lille, M.; Cucherousset, J.; Roussigné, M.; Danchin, E. The importance of population heterogeneities in detecting social learning as the foundation of animal cultural transmission. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220431.
- Hofmann, H.A.; Beery, A.K.; Blumstein, D.T.; Couzin, I.D.; Earley, R.L.; Hayes, L.D.; Hurd, P.L.; Lacey, E.A.; Phelps, S.M.; Solomon, N.G.; et al. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 2014, 29, 581–589.
- Sah, P.; Mann, J.; Bansal, S.; Farine, D. Disease implications of animal social network structure: A synthesis across social systems. J. Anim. Ecol. 2018, 87, 546–558.
- Rifkin, J.L.; Nunn, C.L.; Garamszegi, L.Z. Do Animals Living in Larger Groups Experience Greater Parasitism? A Meta-Analysis. Am. Nat. 2012, 180, 70–82.
- Patterson, J.E.H.; Ruckstuhl, K.E. Parasite infection and host group size: A meta-analytical review. Parasitology 2013, 140, 803–813.
- Altizer, S.; Harvell, D.; Friedle, E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol. Evol. 2003, 18, 589–596.
- Kappeler, P.M.; Cremer, S.; Nunn, C.L. Sociality and health: Impacts of sociality on disease susceptibility and transmission in animal and human societies. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140116.
- Freeland, W.J. Pathogens and the Evolution of Primate Sociality. Biotropica 1976, 8, 12–24.
- Ezenwa, V.O.; Ghai, R.R.; McKay, A.F.; Williams, A.E. Group living and pathogen infection revisited. Curr. Opin. Behav. Sci. 2016, 12, 66–72.
- Rogers-Carter, M.M.; Christianson, J.P. An insular view of the social decision-making network. Neurosci. Biobehav. Rev. 2019, 103, 119–132.
- Soutschek, A. Neural Circuits Regulating Social Behavior: Highlighting the Causal Contribution of the Lateral Habenula. Biol. Psychiatry 2018, 83, 546–547.
- Kang, N.; McCarthy, E.; Cherry, J.; Baum, M. A sex comparison of the anatomy and function of the main olfactory bulb–medial amygdala projection in mice. Neuroscience 2011, 172, 196–204.
- Curtis, J.T.; Wang, Z. Forebrain c- fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster). Physiol. Behav. 2003, 80, 95–101.
- Brennan, P.A.; Keverne, E.B. Something in the Air? New Insights into Mammalian Pheromones. Curr. Biol. 2004, 14, R81–R89.
- Bergan, J.F.; Ben-Shaul, Y.; Dulac, C. Sex-specific processing of social cues in the medial amygdala. eLife 2014, 3, e02743.
- Ben-Shaul, Y.; Katz, L.; Mooney, R.; Dulac, C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc. Natl. Acad. Sci. USA 2010, 107, 5172–5177.
- Raam, T.; Hong, W. Organization of neural circuits underlying social behavior: A consideration of the medial amygdala. Curr. Opin. Neurobiol. 2021, 68, 124–136.
- Choi, G.B.; Dong, H.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Swanson, L.W.; Anderson, D.J. Lhx6 Delineates a Pathway Mediating Innate Reproductive Behaviors from the Amygdala to the Hypothalamus. Neuron 2005, 46, 647–660.
- Miller, S.M.; Marcotulli, D.; Shen, A.; Zweifel, L.S. Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat. Neurosci. 2019, 22, 565–575.
- Martinez, R.; Carvalho-Netto, E.; Ribeiro-Barbosa, É.; Baldo, M.V.; Canteras, N. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience 2011, 172, 314–328.
- Dielenberg, R.; Hunt, G.; McGregor, I. “When a rat smells a cat”: The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 2001, 104, 1085–1097.
- Arakawa, H.; Arakawa, K.; Deak, T. Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience 2010, 171, 1141–1151.
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586.
- Rogers-Carter, M.M.; Varela, J.A.; Gribbons, K.B.; Pierce, A.F.; McGoey, M.T.; Ritchey, M.; Christianson, J.P. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci. 2018, 21, 404–414.
- Butti, C.; Hof, P.R. The insular cortex: A comparative perspective. Brain Struct. Funct. 2010, 214, 477–493.
- Gehrlach, D.A.; Weiand, C.; Gaitanos, T.N.; Cho, E.; Klein, A.S.; Hennrich, A.A.; Conzelmann, K.-K.; Gogolla, N. A whole-brain connectivity map of mouse insular cortex. eLife 2020, 9, e55585.
- Min, J.-Y.; Park, S.; Cho, J.; Huh, Y. The anterior insular cortex processes social recognition memory. Sci. Rep. 2023, 13, 10853.
- Rieger, N.S.; Worley, N.B.; Ng, A.J.; Christianson, J.P. Insular cortex modulates social avoidance of sick rats. Behav. Brain Res. 2022, 416, 113541.
- Dolensek, N.; Gehrlach, D.A.; Klein, A.S.; Gogolla, N. Facial expressions of emotion states and their neuronal correlates in mice. Science 2020, 368, 89–94.
- Pardo-Bellver, C.; Cádiz-Moretti, B.; Novejarque, A.; Martínez-García, F.; Lanuza, E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front. Neuroanat. 2012, 6, 33.
- Kargl, D.; Kaczanowska, J.; Ulonska, S.; Groessl, F.; Piszczek, L.; Lazovic, J.; Buehler, K.; Haubensak, W. The amygdala instructs insular feedback for affective learning. eLife 2020, 9, e60336.
- Kayyal, H.; Yiannakas, A.; Kolatt Chandran, S.; Khamaisy, M.; Sharma, V.; Rosenblum, K. Activity of Insula to Basolateral Amygdala Projecting Neurons is Necessary and Sufficient for Taste Valence Representation. J. Neurosci. 2019, 39, 9369–9382.
- Namboodiri, V.M.K.; Rodriguez-Romaguera, J.; Stuber, G.D. The habenula. Curr. Biol. 2016, 26, R873–R877.
- Flanigan, M.; Aleyasin, H.; Takahashi, A.; Golden, S.A.; Russo, S.J. An emerging role for the lateral habenula in aggressive behavior. Pharmacol. Biochem. Behav. 2017, 162, 79–86.
- Wang, D.; Li, Y.; Feng, Q.; Guo, Q.; Zhou, J.; Luo, M. Learning shapes the aversion and reward responses of lateral habenula neurons. eLife 2017, 6, e23045.
- Benekareddy, M.; Stachniak, T.J.; Bruns, A.; Knoflach, F.; von Kienlin, M.; Künnecke, B.; Ghosh, A. Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biol. Psychiatry 2018, 83, 607–617.
- Weiss, J.; Vacher, H.; Trouillet, A.-C.; Leinders-Zufall, T.; Zufall, F.; Chamero, P. Sensing and avoiding sick conspecifics requires Gαi2 + vomeronasal neurons. BMC Biol. 2023, 21, 152.
- He, Z.-X.; Xi, K.; Liu, K.-J.; Yue, M.-H.; Wang, Y.; Yin, Y.-Y.; Liu, L.; He, X.-X.; Yu, H.-L.; Xing, Z.-K.; et al. A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli. Int. J. Mol. Sci. 2023, 24, 4346.
- Klawonn, A.M.; Malenka, R.C. Nucleus accumbens modulation in reward and aversion. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2018; Volume 83, pp. 119–129.
- Britt, J.P.; Benaliouad, F.; McDevitt, R.A.; Stuber, G.D.; Wise, R.A.; Bonci, A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 2012, 76, 790–803.
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625.
- de Jong, J.W.; Afjei, S.A.; Dorocic, I.P.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 2019, 101, 133–151.
- Zhou, K.; Xu, H.; Lu, S.; Jiang, S.; Hou, G.; Deng, X.; He, M.; Zhu, Y. Reward and aversion processing by input-defined parallel nucleus accumbens circuits in mice. Nat. Commun. 2022, 13, 6244.
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184.
- Damsma, G.; Pfaus, J.G.; Wenkstern, D.; Phillips, A.G.; Fibiger, H.C. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: Comparison with novelty and locomotion. Behav. Neurosci. 1992, 106, 181.
- Guadarrama-Bazante, I.L.; Rodríguez-Manzo, G. Nucleus accumbens dopamine increases sexual motivation in sexually satiated male rats. Psychopharmacology 2019, 236, 1303–1312.
- Dai, B.; Sun, F.; Tong, X.; Ding, Y.; Kuang, A.; Osakada, T.; Li, Y.; Lin, D. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 2022, 40, 111246.
- Cheng, J.Y.; Zhang, S.W.; Tai, F.D. Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behav. Pharmacol. 2016, 27, 672–680.
- Dölen, G.; Malenka, R.C. The emerging role of nucleus accumbens oxytocin in social cognition. Biol. Psychiatry 2014, 76, 354–355.
- Liu, Y.; Wang, Z.X. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003, 121, 537–544.
- Ågmo, A. Sexual motivation—An inquiry into events determining the occurrence of sexual behavior. Behav. Brain Res. 1999, 105, 129–150.
- Quintana, G.R.; Mac Cionnaith, C.E.; Pfaus, J.G. Behavioral, neural, and molecular mechanisms of conditioned mate preference: The role of opioids and first experiences of sexual reward. Int. J. Mol. Sci. 2022, 23, 8928.
- Pitchers, K.K.; Frohmader, K.S.; Vialou, V.; Mouzon, E.; Nestler, E.J.; Lehman, M.N.; Coolen, L.M. ΔFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010, 9, 831–840.
- Tseng, Y.T.; Schaefke, B.; Wei, P.; Wang, L. Defensive responses: Behaviour, the brain and the body. Nat. Rev. Neurosci. 2023, 24, 655–671.
- Martin-Fernandez, M.; Menegolla, A.P.; Lopez-Fernandez, G.; Winke, N.; Jercog, D.; Kim, H.-R.; Girard, D.; Dejean, C.; Herry, C. Prefrontal circuits encode both general danger and specific threat representations. Nat. Neurosci. 2023, 26, 2147–2157.
- Leschak, C.J.; Hornstein, E.A.; Haltom, K.E.B.; Johnson, K.L.; Breen, E.C.; Irwin, M.R.; Eisenberger, N.I. Ventromedial prefrontal cortex activity differentiates sick from healthy faces: Associations with inflammatory responses and disease avoidance motivation. Brain Behav. Immun. 2022, 100, 48–54.
- Hamasato, E.K.; Lovelock, D.; Palermo-Neto, J.; Deak, T. Assessment of social behavior directed toward sick partners and its relation to central cytokine expression in rats. Physiol. Behav. 2017, 182, 128–136.
- Keller, J.K.; Wülfing, C.; Wahl, J.; Diekhof, E.K. Disease-related disgust promotes antibody release in human saliva. Brain Behav. Immun. Health 2022, 24, 100489.
- Schaller, M.; Miller, G.E.; Gervais, W.M.; Yager, S.; Chen, E. Mere Visual Perception of Other People’s Disease Symptoms Facilitates a More Aggressive Immune Response. Psychol. Sci. 2010, 21, 649–652.
- Truby, N.L.; Kim, R.K.; Silva, G.M.; Qu, X.; Picone, J.A.; Alemu, R.; Neve, R.L.; Cui, X.; Liu, J.; Hamilton, P.J. A zinc finger transcription factor tunes social behaviors by controlling transposable elements and immune response in prefrontal cortex. bioRxiv 2023.
- Filiano, A.J.; Xu, Y.; Tustison, N.J.; Marsh, R.L.; Baker, W.; Smirnov, I.; Overall, C.C.; Gadani, S.P.; Turner, S.D.; Weng, Z.; et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 2016, 535, 425–429.
- Archie, E.A.; Tung, J. Social behavior and the microbiome. Curr. Opin. Behav. Sci. 2015, 6, 28–34.
- Bleich, A.; Hansen, A.K. Time to include the gut microbiota in the hygienic standardisation of laboratory rodents. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 81–92.
- Hansen, A.K.; Krych, Ł.; Nielsen, D.S.; Hansen, C.H.F. A Review of Applied Aspects of Dealing with Gut Microbiota Impact on Rodent Models. ILAR J. 2015, 56, 250–264.
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959.
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, 587.
- Lavrinienko, A.; Tukalenko, E.; Mappes, T.; Watts, P.C. Skin and gut microbiomes of a wild mammal respond to different environmental cues. Microbiome 2018, 6, 209.
- Archie, E.A.; Theis, K.R. Animal behaviour meets microbial ecology. Anim. Behav. 2011, 82, 425–436.
- Ezenwa, V.O.; Williams, A.E. Microbes and animal olfactory communication: Where do we go from here? BioEssays 2014, 36, 847–854.
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E.; Collier, R.J. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056.
- Li, Q.; Korzan, W.J.; Ferrero, D.M.; Chang, R.B.; Roy, D.S.; Buchi, M.; Lemon, J.K.; Kaur, A.W.; Stowers, L.; Fendt, M.; et al. Synchronous Evolution of an Odor Biosynthesis Pathway and Behavioral Response. Curr. Biol. 2013, 23, 11–20.
- Yi, X.; Cha, M. Gut Dysbiosis Has the Potential to Reduce the Sexual Attractiveness of Mouse Female. Front. Microbiol. 2022, 13, 916766.
- Ueyama, J.; Nadai, M.; Kanazawa, H.; Iwase, M.; Nakayama, H.; Hashimoto, K.; Yokoi, T.; Baba, K.; Takagi, K.; Takagi, K.; et al. Endotoxin from various gram-negative bacteria has differential effects on function of hepatic cytochrome P450 and drug transporters. Eur. J. Pharmacol. 2005, 510, 127–134.
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53.
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205.
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322.
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412.
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088.
- Zeibich, L.; Koebele, S.V.; Bernaud, V.E.; Ilhan, Z.E.; Dirks, B.; Northup-Smith, S.N.; Neeley, R.; Maldonado, J.; Nirmalkar, K.; Files, J.A.; et al. Surgical Menopause and Estrogen Therapy Modulate the Gut Microbiota, Obesity Markers, and Spatial Memory in Rats. Front. Cell. Infect. Microbiol. 2021, 11, 702628.
- Acharya, K.D.; Noh, H.L.; Graham, M.E.; Suk, S.; Friedline, R.H.; Gomez, C.C.; Parakoyi, A.E.R.; Chen, J.; Kim, J.K.; Tetel, M.J. Distinct Changes in Gut Microbiota Are Associated with Estradiol-Mediated Protection from Diet-Induced Obesity in Female Mice. Metabolites 2021, 11, 499.
- de Catanzaro, D. Sex steroids as pheromones in mammals: The exceptional role of estradiol. Horm. Behav. 2015, 68, 103–116.
- Pietras, R.J. Sex pheromone production by preputial gland: The regulatory role of estrogen. Chem. Senses 1981, 6, 391–408.
- Bouilloud, M.; Galan, M.; Dubois, A.; Diagne, C.; Marianneau, P.; Roche, B.; Charbonnel, N. Three-way relationships between gut microbiota, helminth assemblages and bacterial infections in wild rodent populations. Peer Community J. 2023, 3, e18.
- Zhao, Y.; Yang, S.; Li, B.; Li, W.; Wang, J.; Chen, Z.; Yang, J.; Tan, H.; Li, J. Alterations of the Mice Gut Microbiome via Schistosoma japonicum Ova-Induced Granuloma. Front. Microbiol. 2019, 10, 352.
- Pandian, J. Analysis of Wild Rodent Gut Microbiota as a Function of Exposure to Ticks and Tick-borne Pathogens. Unbuplished Honor. Theses 2023, 1683. Available online: https://scholarship.richmond.edu/honors-theses/1683 (accessed on 3 January 2024).
- Aivelo, T.; Norberg, A. Parasite–microbiota interactions potentially affect intestinal communities in wild mammals. J. Anim. Ecol. 2018, 87, 438–447.
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Finlay, B.B.; Maizels, R.M. Commensal-pathogen interactions in the intestinal tract: Lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014, 5, 522–532.
- Su, C.; Su, L.; Li, Y.; Long, S.R.; Chang, J.; Zhang, W.; Walker, W.A.; Xavier, R.J.; Cherayil, B.J.; Shi, H.N. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018, 11, 144–157.
- Delbare, S.Y.N.; Ahmed-Braimah, Y.H.; Wolfner, M.F.; Clark, A.G. Interactions between the microbiome and mating influence the female’s transcriptional profile in Drosophila melanogaster. Sci. Rep. 2020, 10, 18168.
- Arbuthnott, D.; Levin, T.C.; Promislow, D.E.L. Impacts of Wolbachia and the microbiome on mate choice in Drosophila melanogaster. J. Evol. Biol. 2016, 29, 461–468.
- Winslow, J.T.; Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288.
- Young, L.J. The neurobiology of social recognition, approach, and avoidance. Biol. Psychiatry 2002, 51, 18–26.
- Kavaliers, M.; Ossenkopp, K.; Choleris, E. Social neuroscience of disgust. Genes Brain Behav. 2019, 18, e12508.
- Goodson, J.L. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 2013, 38, 465–478.
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017, 77, 169–189.
- Kavaliers, M.; Choleris, E.; Ågmo, A.; Pfaff, D.W. Olfactory-mediated parasite recognition and avoidance: Linking genes to behavior. Horm. Behav. 2004, 46, 272–283.
- Kavaliers, M.; Colwell, D.D.; Wah, D.T.; Bishnoi, I.R.; Ossenkopp, K.P.; Choleris, E. Conspecific infection threat rapidly biases the social responses of female mice: Involvement of oxytocin. Horm. Behav. 2019, 113, 67–75.
- Boulet, N.P.; Cloutier, C.J.; Ossenkopp, K.-P.; Kavaliers, M. Oxytocin, social factors, and the expression of conditioned disgust (Anticipatory nausea) in male rats. Behav. Pharmacol. 2016, 27, 718–725.
- Paletta, P.; Bass, N.; Kavaliers, M.; Choleris, E. The role of oxytocin in shaping complex social behaviours: Possible interactions with other neuromodulators. Philos. Trans. R. Soc. B 2022, 377, 20210058.
- Lynch, K.S.; Ryan, M.J. Understanding the Role of Incentive Salience in Sexual Decision-Making. Integr. Comp. Biol. 2020, 60, 712–721.
- Yoest, K.E.; Cummings, J.A.; Becker, J.B. Ovarian Hormones Mediate Changes in Adaptive Choice and Motivation in Female Rats. Front. Behav. Neurosci. 2019, 13, 250.
- Thompson, T.L.; Moss, R.L. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci. Lett. 1997, 229, 145–148.
- Choleris, E.; Clipperton-Allen, A.E.; Phan, A.; Valsecchi, P.; Kavaliers, M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front. Neuroendocrinol. 2012, 33, 140–159.
- Kavaliers, M.; Choleris, E.; Tenk, C.M.; Pfaff, D.W.; Ogawa, S. Estrogen receptor α and β involvement in the mediation of the aversive responses of female mice to the volatile and involatile odors of parasitized males. Soc. Behav. Neuroendocrinol. Abstr. 2008, 33, 634–642.
- Johnston, R.E. Chemical communication in rodents: From pheromones to individual recognition. J. Mammal 2003, 84, 1141–1162.
- Lymer, J.M.; Sheppard, P.A.S.; Kuun, T.; Blackman, A.; Jani, N.; Mahbub, S.; Choleris, E. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 2018, 89, 30–38.
- Sexton, C. Effects of an Oxytocin Receptor Antagonist on Estrogens’ Facilitative Effects on Social Recognition in the Medial Amygdala on Female Mice. Master’s Thesis, Department of Psychology, University of Guelph, Guelph, ON, Canada, 2022.
- Arakawa, H.; Cruz, S.; Deak, T. Attractiveness of illness-associated odorant cues in female rats is modulated by ovarian hormones, but not associated with pro-inflammatory cytokine levels. Brain Behav. Immun. 2012, 26, 40–49.
- Brown, C.M.; Mulcahey, T.A.; Filipek, N.C.; Wise, P.M. Production of Proinflammatory Cytokines and Chemokines During Neuroinflammation: Novel Roles for Estrogen Receptors α and β. Endocrinology 2010, 151, 4916–4925.
- Seredynski, A.L.; Balthazart, J.; Christophe, V.J.; Ball, G.F.; Cornil, C.A. Neuroestrogens rapidly regulate sexual motivation but not performance. J. Neurosci. 2013, 33, 164–174.
- Ogawa, S.; Eng, V.; Taylor, J.; Lubahn, D.B.; Korach, K.S.; Pfaff, D.W. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 1998, 139, 5070–5081.
- Fleischman, D.S.; Fessler, D.M.T. Progesterone’s effects on the psychology of disease avoidance: Support for the compensatory behavioral prophylaxis hypothesis. Horm. Behav. 2011, 59, 271–275.
- Kavaliers, M.; Bishnoi, I.R.; Ossenkopp, K.-P.; Choleris, E. Differential effects of progesterone on social recognition and the avoidance of pathogen threat by female mice. Horm. Behav. 2021, 127, 104873.
- Bressan, P.; Kramer, P. Progesterone does raise disgust. Horm. Behav. 2022, 137, 104937.
- Lopes, P.C. Anticipating infection: How parasitism risk changes animal physiology. Funct. Ecol. 2023, 37, 821–830.




