
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco Marquez | -- | 6632 | 2024-01-24 10:34:31 | | | |
| 2 | Mona Zou | + 1 word(s) | 6633 | 2024-01-24 10:38:24 | | |
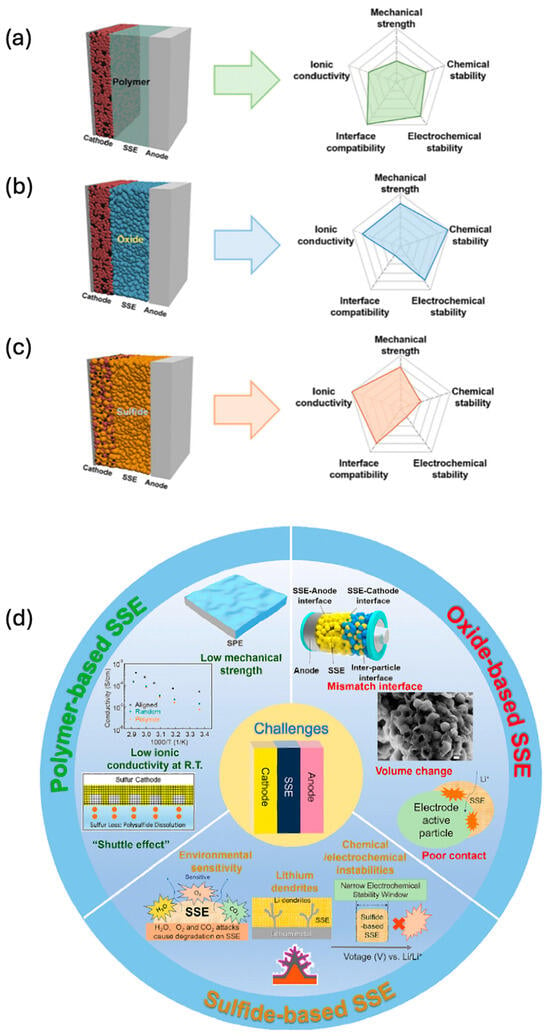
Video Upload Options
The evolution of energy storage technologies has been pivotal in advancing contemporary technological capabilities, significantly contributing to the development of sustainable energy systems. Historically, energy storage has undergone various phases of innovation, each enhancing the efficiency, safety, and environmental impact. A notable transition is occurring towards solid-state energy storage, exemplified by the development and implementation of solid-state batteries (SSBs). This shift is driven by two main factors: the recognition of the limitations in traditional energy storage systems, particularly those using liquid electrolytes, like in lithium-ion batteries (LE-LIBs), and substantial progress in materials science, introducing novel materials and fabrication techniques vital for solid-state energy storage systems
1. Introduction
- ○
-
Oxide Electrolytes: LIPON, NASICON, and Garnet Type;
- ○
-
Sulfide Electrolytes: LPS and Argyrodites;
- ○
-
Polymer Electrolytes;
- ○
-
Halide Electrolytes;
- ○
-
Composite Electrolytes;
- ○
-
Hybrid Solid–Liquid Electrolytes.
2. Oxide Electrolytes
2.1. LIPON
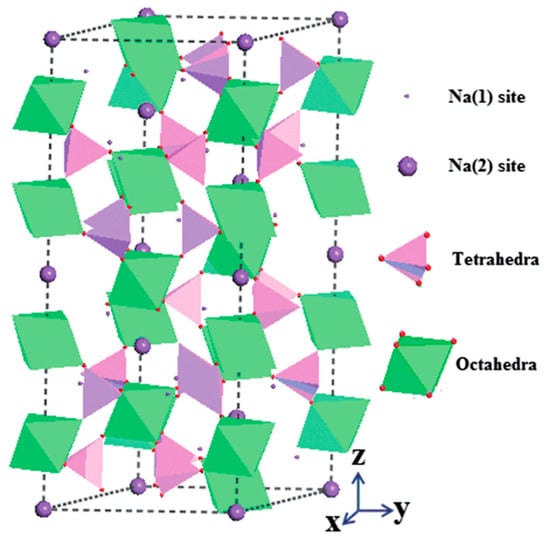
2.2. NASICON
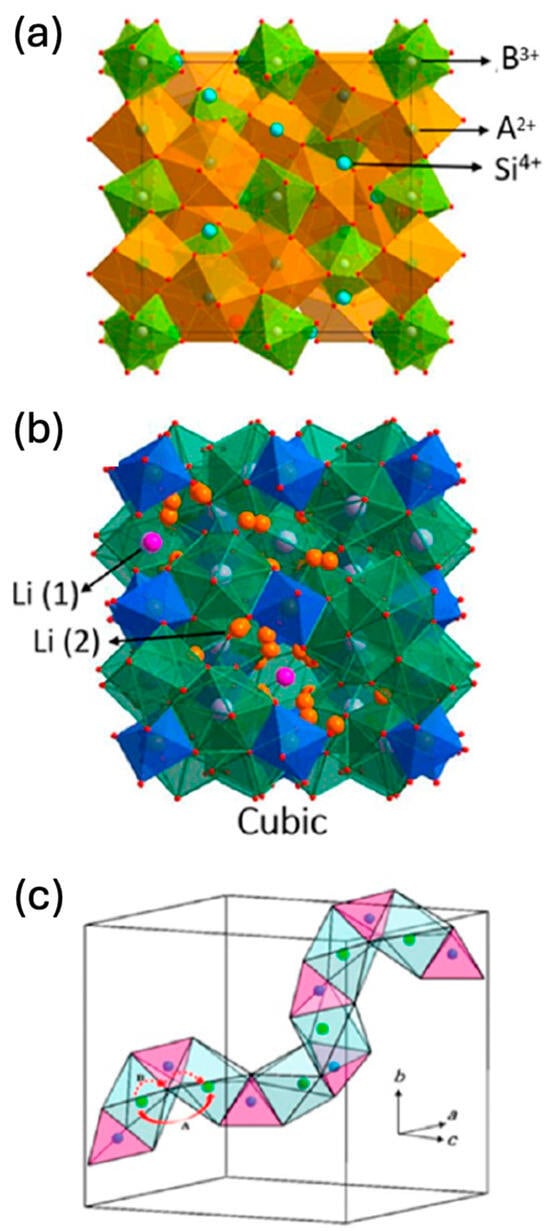
2.3. Garnet-Type
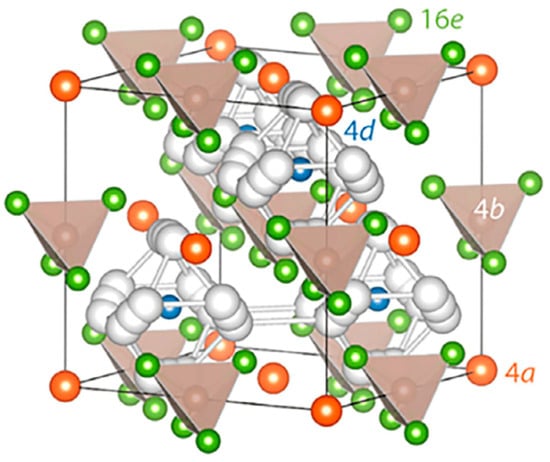
3. Sulfide Electrolytes
3.1. LPS
3.2. Argyrodites
4. Polymer Electrolytes
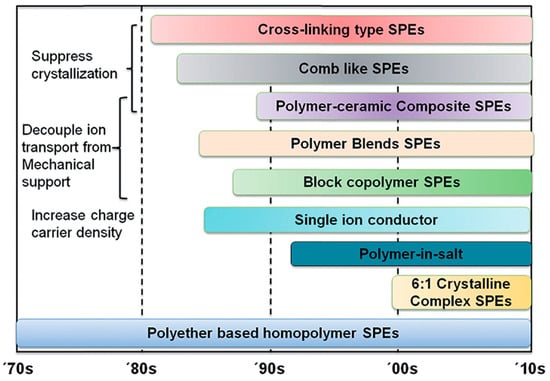
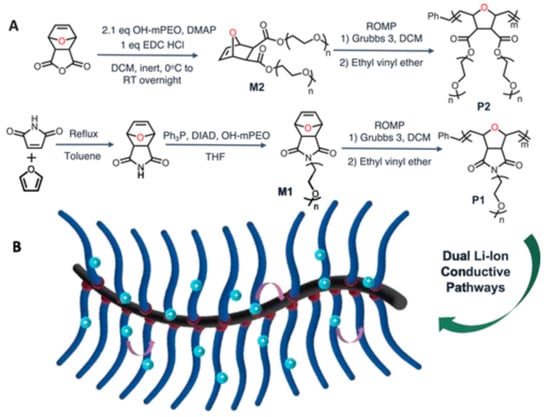
5. Halide Electrolytes
6. Composite Electrolytes
7. Hybrid Solid Electrolyte–Liquid Electrolyte
8. Progress, Challenges, and Prospects in Solid Electrolytes
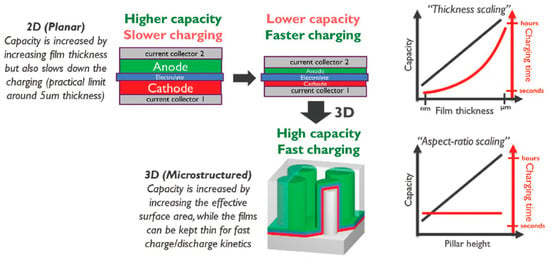
References
- Liang, X.; Wang, L.; Wu, X.; Feng, X.; Wu, Q.; Sun, Y.; Xiang, H.; Wang, J. Solid-State Electrolytes for Solid-State Lithium-Sulfur Batteries: Comparisons, Advances and Prospects. J. Energy Chem. 2022, 73, 370–386.
- Shannon, R.D.; Taylor, B.E.; English, A.D.; Berzins, T. New Li Solid Electrolytes. Electrochim. Acta 1977, 22, 783–796.
- Torres, V.M.; Kalnaus, S.; Martin, S.W.; Duggan, C.; Westover, A.S. Structure-mechanical Properties Correlation in Bulk LiPON Glass Produced by Nitridation of Metaphosphate Melts. J. Am. Ceram. Soc. 2023, 106, 6565–6576.
- De Souza, J.E.; Rojas De Souza, S.; Gebhardt, R.; Kmiec, S.; Whale, A.; Warthen Martin, S. LiPON and NaPON Glasses: A Study of the Ammonolysis of Lithium and Sodium Metaphosphate Melts. Int. J. Appl. Glass Sci. 2020, 11, 78–86.
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid Electrolyte: The Key for High-Voltage Lithium Batteries. Adv. Energy Mater. 2015, 5, 1401408.
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable Inorganic Thin-Film Battery for Fully Flexible Electronic Systems. Nano Lett. 2012, 12, 4810–4816.
- Kalnaus, S.; Westover, A.S.; Kornbluth, M.; Herbert, E.; Dudney, N.J. Resistance to Fracture in the Glassy Solid Electrolyte Lipon. J. Mater. Res. 2021, 36, 787–796.
- Kim, Y.; Dudney, N.J.; Chi, M.; Martha, S.K.; Nanda, J.; Veith, G.M.; Liang, C. A Perspective on Coatings to Stabilize High-Voltage Cathodes: LiMn1.5Ni0.5O4 with Sub-Nanometer Lipon Cycled with LiPF6 Electrolyte. J. Electrochem. Soc. 2013, 160, A3113–A3125.
- Martha, S.K.; Nanda, J.; Kim, Y.; Unocic, R.R.; Pannala, S.; Dudney, N.J. Solid Electrolyte Coated High Voltage Layered–Layered Lithium-Rich Composite Cathode: Li1.2Mn0.525Ni0.175Co0.1O2. J. Mater. Chem. A 2013, 1, 5587.
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yang, Y.; Chen, J.; Jing, M.; Li, F.; Banks, C.E. First Exploration of Na-Ion Migration Pathways in the NASICON Structure Na3V2(PO4)3. J. Mater. Chem. A 2014, 2, 5358.
- Passerini, S.; Bresser, D.; Moretti, A.; Varzi, A. Batteries: Present and Future Energy Storage Challenges; Wiley-VCH: Hoboken, NJ, USA, 2020; ISBN 978-3-527-82731-2.
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic Conductivity of Solid Electrolytes Based on Lithium Titanium Phosphate. J. Electrochem. Soc. 1990, 137, 1023–1027.
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High Ionic Conductivity in Lithium Lanthanum Titanate. Solid State Commun. 1993, 86, 689–693.
- Tolganbek, N.; Serikkazyyeva, A.; Kalybekkyzy, S.; Sarsembina, M.; Kanamura, K.; Bakenov, Z.; Mentbayeva, A. Interface Modification of NASICON-Type Li-Ion Conducting Ceramic Electrolytes: A Critical Evaluation. Mater. Adv. 2022, 3, 3055–3069.
- Lu, J.; Li, Y. Perovskite-type Li-ion Solid Electrolytes: A Review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754.
- Turney, D.E.; Yadav, G.G.; Gallaway, J.W.; Kolhekar, S.; Huang, J.; D’Ambrose, M.J.; Banerjee, S. Aqueous Mn-Zn and Ni-Zn Batteries for Sustainable Energy Storage. In Energy-Sustainable Advanced Materials; Alston, M., Lambert, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–26. ISBN 978-3-030-57491-8.
- Tolganbek, N.; Yerkinbekova, Y.; Khairullin, A.; Bakenov, Z.; Kanamura, K.; Mentbayeva, A. Enhancing Purity and Ionic Conductivity of NASICON-Typed Li1.3Al0.3Ti1.7(PO4)3 Solid Electrolyte. Ceram. Int. 2021, 47, 18188–18195.
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Li, Y.; Liu, S.; Xie, K. New Class of LAGP-Based Solid Polymer Composite Electrolyte for Efficient and Safe Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 41837–41844.
- Hamao, N.; Yamaguchi, Y.; Hamamoto, K. Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process. Materials 2021, 14, 4737.
- Grady, Z.M.; Tsuji, K.; Ndayishimiye, A.; Hwan-Seo, J.; Randall, C.A. Densification of a Solid-State NASICON Sodium-Ion Electrolyte Below 400 °C by Cold Sintering With a Fused Hydroxide Solvent. ACS Appl. Energy Mater. 2020, 3, 4356–4366.
- Zhao, E.; Ma, F.; Guo, Y.; Jin, Y. Stable LATP/LAGP Double-Layer Solid Electrolyte Prepared via a Simple Dry-Pressing Method for Solid State Lithium Ion Batteries. RSC Adv. 2016, 6, 92579–92585.
- Knauth, P. Inorganic Solid Li Ion Conductors: An Overview. Solid State Ion. 2009, 180, 911–916.
- Chen, R.; Nolan, A.M.; Lu, J.; Wang, J.; Yu, X.; Mo, Y.; Chen, L.; Huang, X.; Li, H. The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium. Joule 2020, 4, 812–821.
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440.
- Abouali, S.; Yim, C.-H.; Merati, A.; Abu-Lebdeh, Y.; Thangadurai, V. Garnet-Based Solid-State Li Batteries: From Materials Design to Battery Architecture. ACS Energy Lett. 2021, 6, 1920–1941.
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781.
- Baral, A.K.; Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Evaluation of Fundamental Transport Properties of Li-Excess Garnet-Type Li5+2xLa3Ta2−xYxO12 (x = 0.25, 0.5 and 0.75) Electrolytes Using AC Impedance and Dielectric Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 11356.
- Xie, H.; Alonso, J.A.; Li, Y.; Fernández-Díaz, M.T.; Goodenough, J.B. Lithium Distribution in Aluminum-Free Cubic Li7La3Zr2O12. Chem. Mater. 2011, 23, 3587–3589.
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal Chemistry and Stability of “Li7La3Zr2O12” Garnet: A Fast Lithium-Ion Conductor. Inorg. Chem. 2011, 50, 1089–1097.
- Jin, Y.; McGinn, P.J. Al-Doped Li7La3Zr2O12 Synthesized by a Polymerized Complex Method. J. Power Sources 2011, 196, 8683–8687.
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300.
- Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A = Sr, Ba): Novel Garnet-Like Oxides for Fast Lithium Ion Conduction. Adv. Funct. Mater. 2005, 15, 107–112.
- Rettenwander, D.; Wagner, R.; Reyer, A.; Bonta, M.; Cheng, L.; Doeff, M.M.; Limbeck, A.; Wilkening, M.; Amthauer, G. Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal. J. Phys. Chem. C 2018, 122, 3780–3785.
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li7La3Zr2O12 Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 157, A1076.
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396.
- Ni, J.E.; Case, E.D.; Sakamoto, J.S.; Rangasamy, E.; Wolfenstine, J.B. Room Temperature Elastic Moduli and Vickers Hardness of Hot-Pressed LLZO Cubic Garnet. J. Mater. Sci. 2012, 47, 7978–7985.
- Shen, F.; Dixit, M.B.; Xiao, X.; Hatzell, K.B. Effect of Pore Connectivity on Li Dendrite Propagation within LLZO Electrolytes Observed with Synchrotron X-ray Tomography. ACS Energy Lett. 2018, 3, 1056–1061.
- Tsai, C.-L.; Roddatis, V.; Chandran, C.V.; Ma, Q.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626.
- Cheng, E.J.; Sharafi, A.; Sakamoto, J. Intergranular Li Metal Propagation through Polycrystalline Li6.25Al0.25La3Zr2O12 Ceramic Electrolyte. Electrochim. Acta 2017, 223, 85–91.
- Shen, X.; Zhang, Q.; Ning, T.; Liu, T.; Luo, Y.; He, X.; Luo, Z.; Lu, A. Critical Challenges and Progress of Solid Garnet Electrolytes for All-Solid-State Batteries. Mater. Today Chem. 2020, 18, 100368.
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030.
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural Change of Li2S–P2S5 Sulfide Solid Electrolytes in the Atmosphere. Solid State Ion. 2011, 182, 116–119.
- Tatsumisago, M.; Hayashi, A. Sulfide Glass-Ceramic Electrolytes for All-Solid-State Lithium and Sodium Batteries. Int. J. Appl. Glass Sci. 2014, 5, 226–235.
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium Superionic Conductor. Nat. Mater. 2011, 10, 682–686.
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131.
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A Sulphide Lithium Super Ion Conductor Is Superior to Liquid Ion Conductors for Use in Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 627–631.
- Dietrich, C.; Weber, D.A.; Sedlmaier, S.J.; Indris, S.; Culver, S.P.; Walter, D.; Janek, J.; Zeier, W.G. Lithium Ion Conductivity in Li2S–P2S5 Glasses—Building Units and Local Structure Evolution during the Crystallization of Superionic Conductors Li3PS4, Li7P3S11 and Li4P2S7. J. Mater. Chem. A 2017, 5, 18111–18119.
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. High Lithium Ion Conducting Glass-Ceramics in the System Li2S–P2S5. Solid State Ion. 2006, 177, 2721–2725.
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74.
- Jung, Y.S.; Oh, D.Y.; Nam, Y.J.; Park, K.H. Issues and Challenges for Bulk-Type All-Solid-State Rechargeable Lithium Batteries Using Sulfide Solid Electrolytes. Isr. J. Chem. 2015, 55, 472–485.
- Tatsumisago, M.; Hayashi, A. Superionic Glasses and Glass–Ceramics in the Li2S–P2S5 System for All-Solid-State Lithium Secondary Batteries. Solid State Ion. 2012, 225, 342–345.
- Ohno, S.; Banik, A.; Dewald, G.F.; Kraft, M.A.; Krauskopf, T.; Minafra, N.; Till, P.; Weiss, M.; Zeier, W.G. Materials Design of Ionic Conductors for Solid State Batteries. Prog. Energy 2020, 2, 022001.
- Deiseroth, H.; Kong, S.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758.
- Zhou, L.; Park, K.-H.; Sun, X.; Lalère, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019, 4, 265–270.
- Bai, X.; Duan, Y.; Zhuang, W.; Yang, R.; Wang, J. Research Progress in Li-Argyrodite-Based Solid-State Electrolytes. J. Mater. Chem. A 2020, 8, 25663–25686.
- Zulfiqar, Z.; Zulfiqar, S.; Abbas, Q.; Mirzaeian, M.; Raza, R. Potential Electrolytes for Solid State Batteries and Its Electrochemical Analysis—A Review. Energy Storage 2023, est2.506.
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907.
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent Advances in Solid Polymer Electrolytes for Lithium Batteries. Nano Res. 2017, 10, 4139–4174.
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175.
- Iojoiu, C.; Paillard, E. Solid-State Batteries with Polymer Electrolytes; Bard, A.J., Ed.; Wiley: Hoboken, NJ, USA, 2007.
- Manuel Stephan, A.; Nahm, K.S. Review on Composite Polymer Electrolytes for Lithium Batteries. Polymer 2006, 47, 5952–5964.
- Benrabah, D.; Baril, D.; Sanchez, J.-Y.; Armand, M.; Gard, G.G. Comparative Electrochemical Study of New Poly(Oxyethylene)–Li Salt Complexes. J. Chem. Soc. Faraday Trans. 1993, 89, 355–359.
- Fang, Z.; Ma, Q.; Liu, P.; Ma, J.; Hu, Y.-S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. Novel Concentrated Li-Based Ether Electrolyte for Superior Stability of Metallic Lithium Anode. ACS Appl. Mater. Interfaces 2017, 9, 4282–4289.
- Chakrabarti, A.; Filler, R.; Mandal, B.K. Synthesis and Properties of a New Class of Fluorine-Containing Dilithium Salts for Lithium-Ion Batteries. Solid State Ion. 2010, 180, 1640–1645.
- Ganapatibhotla, L.V.N.R.; Maranas, J.K. Interplay of Surface Chemistry and Ion Content in Nanoparticle-Filled Solid Polymer Electrolytes. Macromolecules 2014, 47, 3625–3634.
- Appetecchi, G.B.; Passerini, S. PEO-Carbon Composite Lithium Polymer Electrolyte. Electrochim. Acta 2000, 45, 2139–2145.
- Scrosati, B. Progress in Lithium Polymer Battery R&D. J. Power Sources 2001, 100, 93–100.
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513.
- Cheng, S.; Smith, D.M.; Pan, Q.; Wang, S.; Li, C.Y. Anisotropic Ion Transport in Nanostructured Solid Polymer Electrolytes. RSC Adv. 2015, 5, 48793–48810.
- Wang, M.; Zhang, H.; Li, Y.; Liu, R.; Yang, H. Accelerated Ion Transportation in Liquid Crystalline Polymer Networks for Superior Solid-State Lithium Metal Batteries. Chem. Eng. J. 2023, 476, 146658.
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051.
- An, S.Y.; Wu, X.; Zhao, Y.; Liu, T.; Yin, R.; Ahn, J.H.; Walker, L.M.; Whitacre, J.F.; Matyjaszewski, K. Highly Conductive Polyoxanorbornene-Based Polymer Electrolyte for Lithium-Metal Batteries. Adv. Sci. 2023, 10, 2302932.
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries. Adv. Mater. 2018, 30, 1803075.
- Wang, S.; Bai, Q.; Nolan, A.M.; Liu, Y.; Gong, S.; Sun, Q.; Mo, Y. Lithium Chlorides and Bromides as Promising Solid-State Chemistries for Fast Ion Conductors with Good Electrochemical Stability. Angew. Chem. Int. Ed. 2019, 58, 8039–8043.
- Li, C.; Gu, L.; Maier, J. Enhancement of the Li Conductivity in LiF by Introducing Glass/Crystal Interfaces. Adv. Funct. Mater. 2012, 22, 1145–1149.
- Wang, C.; Liang, J.; Kim, J.T.; Sun, X. Prospects of Halide-Based All-Solid-State Batteries: From Material Design to Practical Application. Sci. Adv. 2022, 8, eadc9516.
- Liang, J.; Li, X.; Adair, K.R.; Sun, X. Metal Halide Superionic Conductors for All-Solid-State Batteries. Acc. Chem. Res. 2021, 54, 1023–1033.
- Zhu, Y.; Mo, Y. Materials Design Principles for Air-Stable Lithium/Sodium Solid Electrolytes. Angew. Chem. Int. Ed. 2020, 59, 17472–17476.
- Wang, S.; Xu, X.; Cui, C.; Zeng, C.; Liang, J.; Fu, J.; Zhang, R.; Zhai, T.; Li, H. Air Sensitivity and Degradation Evolution of Halide Solid State Electrolytes upon Exposure. Adv. Funct. Mater. 2022, 32, 2108805.
- Li, W.; Liang, J.; Li, M.; Adair, K.R.; Li, X.; Hu, Y.; Xiao, Q.; Feng, R.; Li, R.; Zhang, L.; et al. Unraveling the Origin of Moisture Stability of Halide Solid-State Electrolytes by In Situ and Operando Synchrotron X-ray Analytical Techniques. Chem. Mater. 2020, 32, 7019–7027.
- Tao, B.; Zhong, D.; Li, H.; Wang, G.; Chang, H. Halide Solid-State Electrolytes for All-Solid-State Batteries: Structural Design, Synthesis, Environmental Stability, Interface Optimization and Challenges. Chem. Sci. 2023, 14, 8693–8722.
- Kwak, H.; Kim, J.-S.; Han, D.; Kim, J.S.; Park, J.; Kwon, G.; Bak, S.-M.; Heo, U.; Park, C.; Lee, H.-W.; et al. Boosting the Interfacial Superionic Conduction of Halide Solid Electrolytes for All-Solid-State Batteries. Nat. Commun. 2023, 14, 2459.
- Liu, J.; Wang, S.; Qie, Y.; Sun, Q. Identifying Lithium Fluorides for Promising Solid-State Electrolyte and Coating Material of High-Voltage Cathode. Mater. Today Energy 2021, 21, 100719.
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer Electrolytes for Lithium Polymer Batteries. J. Mater. Chem. A 2016, 4, 10038–10069.
- Grundish, N.S.; Goodenough, J.B.; Khani, H. Designing Composite Polymer Electrolytes for All-Solid-State Lithium Batteries. Curr. Opin. Electrochem. 2021, 30, 100828.
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46.
- Pitawala, H.M.J.C.; Dissanayake, M.A.K.L.; Seneviratne, V.A.; Mellander, B.-E.; Albinson, I. Effect of Plasticizers (EC or PC) on the Ionic Conductivity and Thermal Properties of the (PEO)9LiTf: Al2O3 Nanocomposite Polymer Electrolyte System. J. Solid State Electrochem. 2008, 12, 783–789.
- Shi, Y.; Tan, D.; Li, M.; Chen, Z. Nanohybrid Electrolytes for High-Energy Lithium-Ion Batteries: Recent Advances and Future Challenges. Nanotechnology 2019, 30, 302002.
- Hallinan, D.T.; Villaluenga, I.; Balsara, N.P. Polymer and Composite Electrolytes. MRS Bull. 2018, 43, 759–767.
- Wang, W.; Yi, E.; Fici, A.J.; Laine, R.M.; Kieffer, J. Lithium Ion Conducting Poly(Ethylene Oxide)-Based Solid Electrolytes Containing Active or Passive Ceramic Nanoparticles. J. Phys. Chem. C 2017, 121, 2563–2573.
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745.
- Fu, K.; Gong, Y.; Dai, J.; Gong, A.; Han, X.; Yao, Y.; Wang, C.; Wang, Y.; Chen, Y.; Yan, C.; et al. Flexible, Solid-State, Ion-Conducting Membrane with 3D Garnet Nanofiber Networks for Lithium Batteries. Proc. Natl. Acad. Sci. USA 2016, 113, 7094–7099.
- Vargas-Barbosa, N.M.; Roling, B. Dynamic Ion Correlations in Solid and Liquid Electrolytes: How Do They Affect Charge and Mass Transport? ChemElectroChem 2020, 7, 367–385.
- Park, K.; Yu, B.-C.; Jung, J.-W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical Nature of the Cathode Interface for a Solid-State Lithium-Ion Battery: Interface between LiCoO2 and Garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051–8059.
- Schwietert, T.K.; Arszelewska, V.A.; Wang, C.; Yu, C.; Vasileiadis, A.; De Klerk, N.J.J.; Hageman, J.; Hupfer, T.; Kerkamm, I.; Xu, Y.; et al. Clarifying the Relationship between Redox Activity and Electrochemical Stability in Solid Electrolytes. Nat. Mater. 2020, 19, 428–435.
- Woolley, H.M.; Vargas-Barbosa, N.M. Hybrid Solid Electrolyte-Liquid Electrolyte Systems for (Almost) Solid-State Batteries: Why, How, and Where To? J. Mater. Chem. A 2023, 11, 1083–1097.
- Vivek, J.P.; Meddings, N.; Garcia-Araez, N. Negating the Interfacial Resistance between Solid and Liquid Electrolytes for Next-Generation Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 633–646.
- Gupta, A.; Kazyak, E.; Dasgupta, N.P.; Sakamoto, J. Electrochemical and Surface Chemistry Analysis of Lithium Lanthanum Zirconium Tantalum Oxide (LLZTO)/Liquid Electrolyte (LE) Interfaces. J. Power Sources 2020, 474, 228598.
- Hatz, A.-K.; Calaminus, R.; Feijoo, J.; Treber, F.; Blahusch, J.; Lenz, T.; Reichel, M.; Karaghiosoff, K.; Vargas-Barbosa, N.M.; Lotsch, B.V. Chemical Stability and Ionic Conductivity of LGPS-Type Solid Electrolyte Tetra-Li7SiPS8 after Solvent Treatment. ACS Appl. Energy Mater. 2021, 4, 9932–9943.
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing Thin but Robust Electrolytes for Solid-State Batteries. Nat. Energy 2021, 6, 227–239.
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900805.
- Sahal, M.; Molloy, J.; Narayanan, V.R.; Ladani, L.; Lu, X.; Rolston, N. Robust and Manufacturable Lithium Lanthanum Titanate-Based Solid-State Electrolyte Thin Films Deposited in Open Air. ACS Omega 2023, 8, 28651–28662.
- Li, Y.; Sun, Z.; Yuan, X.; Jin, H.; Zhao, Y. NaBr-Assisted Sintering of Na3Zr2Si2PO12 Ceramic Electrolyte Stabilizes a Rechargeable Solid-State Sodium Metal Battery. ACS Appl. Mater. Interfaces 2023, 15, 49321–49328.
- Lin, C.; Ihrig, M.; Kung, K.; Chen, H.; Scheld, W.S.; Ye, R.; Finsterbusch, M.; Guillon, O.; Lin, S. Low-Temperature Sintering of Li0.33La0.55TiO3 Electrolyte for All-Solid-State Li Batteries. J. Eur. Ceram. Soc. 2023, 43, 7543–7552.
- Ramos, E.; Browar, A.; Roehling, J.; Ye, J. CO2 Laser Sintering of Garnet-Type Solid-State Electrolytes. ACS Energy Lett. 2022, 7, 3392–3400.
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing Strategies for Solid Electrolyte in Batteries. Front. Energy Res. 2020, 8, 571440.
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single Lithium-Ion Conducting Solid Polymer Electrolytes: Advances and Perspectives. Chem. Soc. Rev. 2017, 46, 797–815.
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A Review of Lithium and Non-Lithium Based Solid State Batteries. J. Power Sources 2015, 282, 299–322.
- Chen, A.-N.; Wu, J.-M.; Liu, K.; Chen, J.-Y.; Xiao, H.; Chen, P.; Li, C.-H.; Shi, Y.-S. High-Performance Ceramic Parts with Complex Shape Prepared by Selective Laser Sintering: A Review. Adv. Appl. Ceram. 2018, 117, 100–117.
- Gao, X.; Zheng, M.; Yang, X.; Sun, R.; Zhang, J.; Sun, X. Emerging Application of 3D-Printing Techniques in Lithium Batteries: From Liquid to Solid. Mater. Today 2022, 59, 161–181.
- Chen, C.; Zuo, Y.; Ye, W.; Li, X.; Deng, Z.; Ong, S.P. A Critical Review of Machine Learning of Energy Materials. Adv. Energy Mater. 2020, 10, 1903242.
- Xu, Z.; Xia, Y. Progress, Challenges and Perspectives of Computational Studies on Glassy Superionic Conductors for Solid-State Batteries. J. Mater. Chem. A 2022, 10, 11854–11880.
- Garcia-Mendez, R.; Smith, J.G.; Neuefeind, J.C.; Siegel, D.J.; Sakamoto, J. Correlating Macro and Atomic Structure with Elastic Properties and Ionic Transport of Glassy Li2S-P2S5 (LPS) Solid Electrolyte for Solid-State Li Metal Batteries. Adv. Energy Mater. 2020, 10, 2000335.
- Sadowski, M.; Albe, K. Computational Study of Crystalline and Glassy Lithium Thiophosphates: Structure, Thermodynamic Stability and Transport Properties. J. Power Sources 2020, 478, 229041.
- Smith, J.G.; Siegel, D.J. Low-Temperature Paddlewheel Effect in Glassy Solid Electrolytes. Nat. Commun. 2020, 11, 1483.





