Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ping Zeng | -- | 1590 | 2024-01-23 03:09:52 | | | |
| 2 | Fanny Huang | Meta information modification | 1590 | 2024-01-23 07:32:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. Application of Membrane Technology in the Pharmaceutical Industry. Encyclopedia. Available online: https://encyclopedia.pub/entry/54220 (accessed on 07 February 2026).
Ma R, Li J, Zeng P, Duan L, Dong J, Ma Y, et al. Application of Membrane Technology in the Pharmaceutical Industry. Encyclopedia. Available at: https://encyclopedia.pub/entry/54220. Accessed February 07, 2026.
Ma, Ruirui, Juan Li, Ping Zeng, Liang Duan, Jimin Dong, Yunxia Ma, Lingkong Yang. "Application of Membrane Technology in the Pharmaceutical Industry" Encyclopedia, https://encyclopedia.pub/entry/54220 (accessed February 07, 2026).
Ma, R., Li, J., Zeng, P., Duan, L., Dong, J., Ma, Y., & Yang, L. (2024, January 23). Application of Membrane Technology in the Pharmaceutical Industry. In Encyclopedia. https://encyclopedia.pub/entry/54220
Ma, Ruirui, et al. "Application of Membrane Technology in the Pharmaceutical Industry." Encyclopedia. Web. 23 January, 2024.
Copy Citation
With the advancement in membrane technology, membrane separation technology has been found increasingly widespread applications in the pharmaceutical industry. It is utilized in drug separation and purification, wastewater treatment, and the recycling of wastewater resources.
membrane technology application
pharmaceutical industry
1. Introduction
Membrane technology has undergone a long, historical development in laboratory research and achieved its first major industrial application in the 1960s [1]. Membrane is a kind of material with a selective separation function, and can transfer one component and restrict others because of the special properties of the components [2]. The main membrane technologies include microfiltration, ultrafiltration, nanofiltration, reverse osmosis, pervaporation, electrodialysis, and membrane bioreactors [3]. Membrane separation technology has unique advantages such as a high efficiency, wide applicability, and minimal environmental impact [2]. With a rapid development over the past few decades, membrane separation technology has become one of the emerging technologies and has been used in numerous industrial sectors.
With the rapid development of membrane technology, the application of membrane separation technology in the pharmaceutical industry is becoming increasingly widespread. Researchers have found extensive applications in pharmaceutical processes such as drug purification, wastewater treatment, and wastewater resource utilization [4][5]. The scientific and reasonable applications of membrane separation technology in the pharmaceutical field can improve production efficiency, ensure the progressiveness of pharmaceutical processes, reduce pollution, and promote the pharmaceutical industry’s development towards a greener, environmentally friendly, and highly efficient direction.
2. History of Membrane Technology Application in the Pharmaceutical Industry
The application of membrane technology in the pharmaceutical field originated with the preparation of pharmaceutical-grade water. In 1972, GOW from the UK used cellulose acetate reverse osmosis membranes to produce pyrogen-free water [6]. Following this, in 1974, the U.S. pharmaceutical company Upjohn employed Dupont-produced, aromatic polyamide hollow fiber, reverse osmosis membrane modules to manufacture injectable water, meeting regulatory standards for injection water [7]. The 19th edition of the U.S. Pharmacopeia in 1975 introduced membrane methods for injectable water production, challenging the traditional belief in the reliability of distillation alone [8]. In the 1980s, membrane technology gradually extended to the purification and production of pharmaceuticals. Millipore, in the early 1980s, applied a combination of membrane filtration techniques to separate and refine cephalosporin C from fermentation broth [9]. In 1983, an ultrafiltration system developed by Japan’s Asahi Chemical Industry was reported, which used hollow fiber membranes for drug purification [10]. Subsequently, membrane materials like hollow fiber, ultrafiltration, and reverse osmosis membranes were applied for the extraction of antibiotics, vitamins, and proteases [11][12][13][14].
Since entering the 21st century, membrane separation technology has been gradually used in pharmaceutical wastewater treatment. A sewage treatment plant in Queensland used microfiltration and reverse osmosis technology to treat eleven kinds of drugs and two kinds of endocrine disruptors from different treatment categories. The results showed that the microfiltration and reverse osmosis systems can reduce the concentration of pollutants by an order of magnitude, and the overall removal efficiency of the circulating water is higher than 97% [15]. In addition, researchers proposed combining membrane separation technology with biological treatment units for pharmaceutical wastewater treatment [16][17][18]. After 2011, membrane technology in the pharmaceutical industry underwent a rapid development, with a surge in applications post-2016, notably focusing on the removal of pharmaceuticals, hormones, endocrine disruptors, and antibiotic resistance genes from wastewater [19]. An overview of the international application history of membrane technology in the pharmaceutical industry is depicted in Figure 1. In the 1970s, membrane technology was primarily employed for pharmaceutical-grade water preparation. Moving into the 1980s and 1990s, this transitioned towards drug purification and production. After entering the 21st century, there was a notable shift towards utilizing membrane technology for pharmaceutical wastewater treatment.
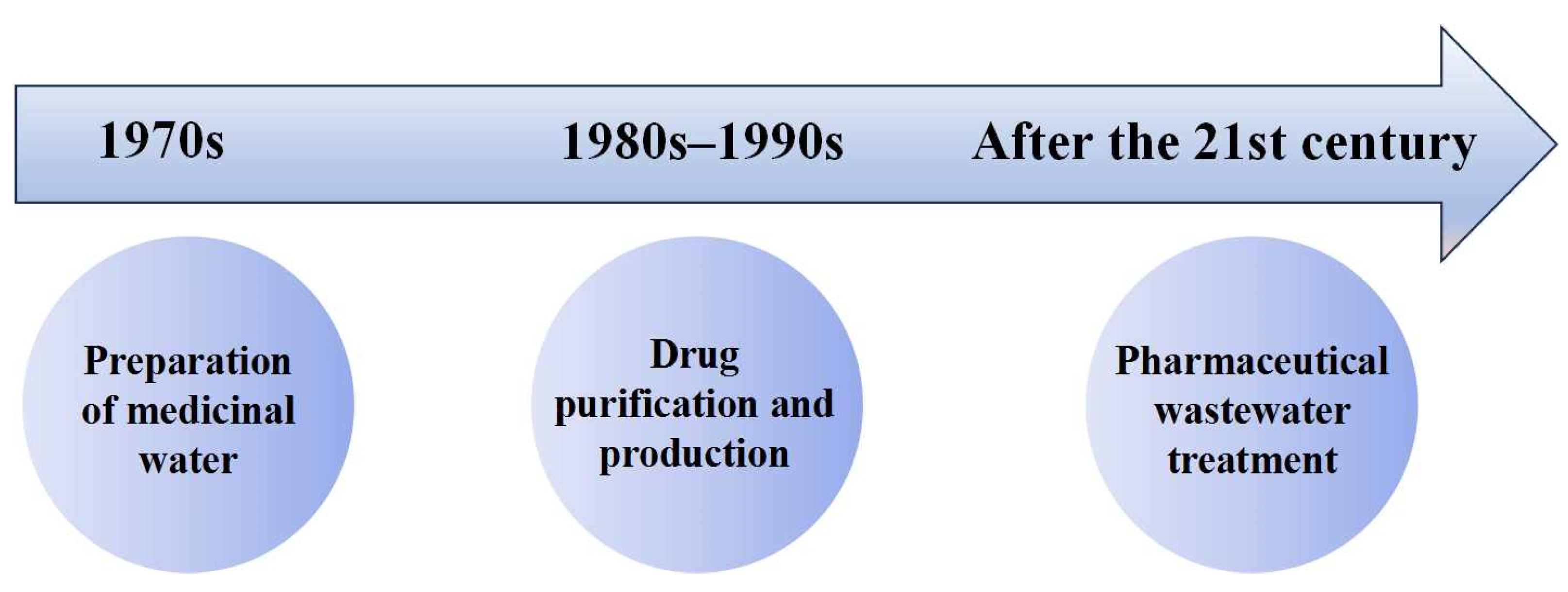
Figure 1. International application history of membrane technology in the pharmaceutical industry.
In China, the application of membrane technology in the pharmaceutical industry could be traced back to the 1960s. The Institute of Health Devices of the Academy of Military Medical Sciences (IHDAMMS) developed electrodialysis pure water equipment that uses multistage processes combined with pretreatment technologies of raw water to directly treat Tianjin tap water into injection water that meets pharmacopoeia standards [20]. In the late 1970s, with the advancement of membrane technology, the IHDAMMS began researching the use of reverse osmosis to produce pharmaceutical-grade water [21]. By the 1980s, membrane separation technology was also applied to the extraction of traditional Chinese medicines. In 1979, the pharmacy bureau of the PLA Air Force Beijing Hospital utilized membrane separation technology for the extraction of effective components from traditional Chinese medicines, demonstrating the efficacy of ultrafiltration in removing impurities while retaining the main constituents [22]. In 1981, they further utilized this method to prepare traditional Chinese medicine injections [23]. In the late 1990s, membrane technology found its application in antibiotic production. In 1989, Dalian Pharmaceutical Factory introduced Danish DDS sanitary reverse osmosis membrane equipment to concentrate the decolorization solution of streptomycin sulfate [9]. In 1994, the NFB series plate-type reverse osmosis device from the Penglai reverse osmosis equipment factory was successfully applied in streptomycin production at Jining Antibiotic Factory, demonstrating an improved quality, higher yield, and reduced energy and material consumption [24]. In 1999, Santar Membrane achieved a breakthrough in the crucial technology for cephalosporin production, developing a membrane separation-based process for 7-ACA production [25]. In 2000, Northeast Pharmaceutical General Factory utilized Ultra-Flo ultrafiltration membrane systems for the removal of impurities in a vitamin C fermentation broth, showing the potential of the membrane separation technology in shortening production processes, reducing costs, and increasing yields [26]. Subsequently, the application of membrane separation technology expanded to the production of other antibiotics like erythromycin and penicillin [27][28]. After entering the 21st century, with the rapid development of membrane technology, membrane separation techniques were further applied to the treatment of pharmaceutical wastewater.
The Chinese journey of membrane technology in the pharmaceutical industry, as illustrated in Figure 2, shows its evolution from the 1960s to 1970s primarily in pharmaceutical-grade water preparation to its gradual application in traditional Chinese medicine extraction in the 1980s, antibiotic production in the 1990s, and eventually, wastewater treatment in the 21st century.
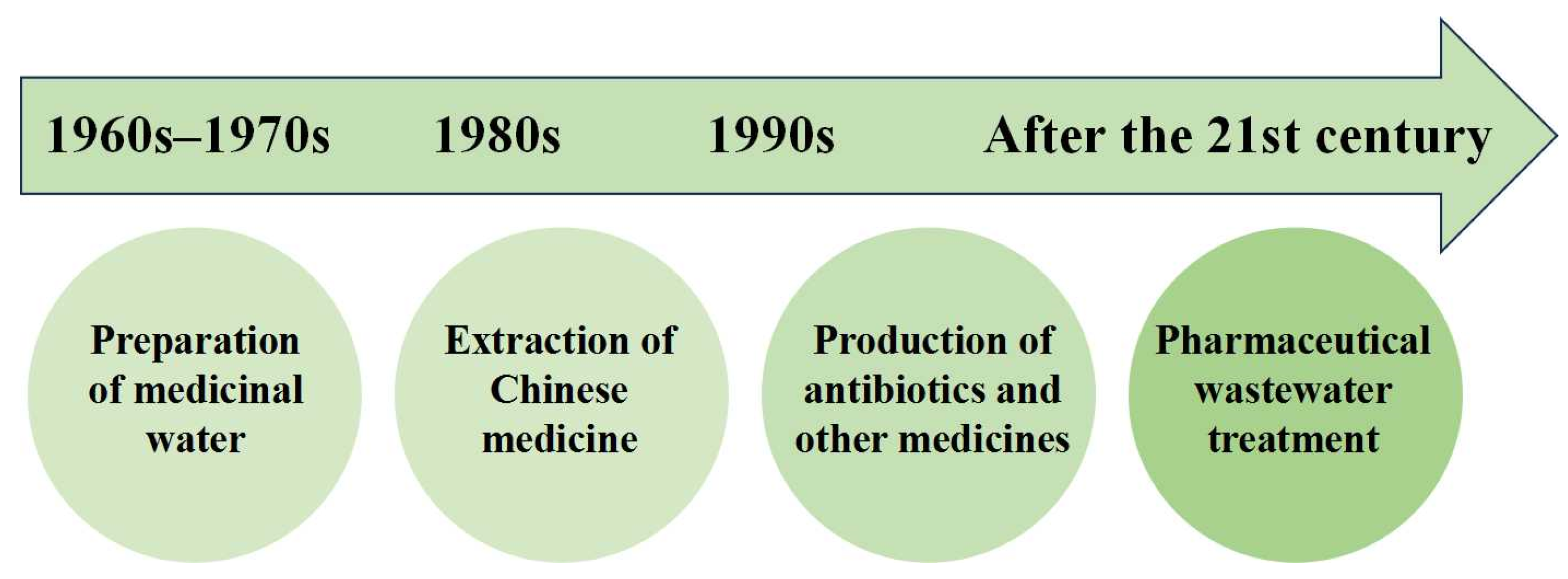
Figure 2. Chinese application history of membrane technology in the pharmaceutical industry.
3. Application of Membrane Technology in the Pharmaceutical Industry
The application of membrane technology in the pharmaceutical industry primarily encompasses microfiltration, ultrafiltration, nanofiltration, reverse osmosis, membrane bioreactors, electrodialysis, osmosis, osmotic vaporization, and combined processes. These membrane technologies are mainly used for pharmaceutical production, wastewater treatment, and wastewater product recovery, as shown in Table 1.
Microfiltration is the earliest membrane technology. The separation mechanism of microfiltration is sieving with a membrane pore size of 0.01–1 μm, which can allow macromolecular organic compounds and dissolved solids to pass through [2]. It is mainly used to intercept particles, bacteria, and pollutants from the liquid or gas phase to achieve the purposes of purification and bacteria removal [29]. In the pharmaceutical industry, microfiltration is mainly used for sterilization filtration, clarification of pharmaceutical solutions, removal of particles and viruses, purification of medical water, and pretreatment of ultrafiltration and reverse osmosis processes [30]. Among them, a microfiltration membrane with a pore size of 0.6–0.8 μm can be used for the removal of bacteria and the filtration of gases. A microfiltration membrane with a pore size of 0.45 μm is the most commonly used, often used for the purification of liquid material and water, and a microfiltration membrane with a pore size of 0.2 μm can be used for the sterilization filtration of liquid medicine [30].
Ultrafiltration is a kind of membrane filtration method with a molecular level membrane having a pore size of 10–100 nm. It uses the pressure difference on both sides of the membrane to selectively separate solutes with different molecular weights. In the pharmaceutical industry, ultrafiltration can not only be used to remove bacteria, viruses, and particles, but also pyrogens, hyphae, and proteins. It is often used for the fractionation and desalting concentration of macromolecular substances, the purification of small molecular substances, and the depyrogenation of pharmaceutical and biochemical preparations [31].
Nanofiltration is a membrane separation process between ultrafiltration and reverse osmosis, with a membrane pore size of 1–10 nm. The separation mechanism of nanofiltration is adsorption–diffusion. In the pharmaceutical industry, nanofiltration can be used for clarification, sterilization, filtration, protein removal, and the separation and purification of fermentation broths such as those for antibiotics, vitamins, amino acids, and enzymes [32]. It can also be used for the desalination and concentration of 6-APA, 7-ACA, 7-ADCA, and other semisynthetic antibiotics [30].
Reverse osmosis is also a membrane filtration process driven by pressure. The pore size of a reverse osmosis membrane is less than 1 nm, and its function is to intercept ionic substances that only pass through the solvent. The organic and inorganic molecules are separated from the feed solution through a solution diffusion process [33]. In the pharmaceutical industry, reverse osmosis is mainly used for drug concentration, purification and separation, desalination, preparation of water for preparation, water for injection, dialysis water, and sterile water [31].
Membrane bioreactor is a new wastewater treatment technology that combines membrane separation technology with biological treatment [34]. This technology adopts an immersion membrane component with a unique structure that is placed in an aeration tank. After aerobic aeration and biological treatment, the wastewater is pumped through a filter membrane and then pumped out [35]. In the pharmaceutical industry, membrane bioreactors are often combined with other technologies for the treatment of pharmaceutical wastewater [36].
Table 1. Application of different process types in the pharmaceutical industry.
| Membrane Technology Types | Application Areas | Earliest Application Time | References |
|---|---|---|---|
| Microfiltration | Pharmaceutical production | 1994 | [37] |
| Wastewater treatment | 2005 | [38] | |
| Wastewater product recovery | 2015 | [39] | |
| Ultrafiltration | Pharmaceutical production | 1983 | [10] |
| Wastewater treatment | 2004 | [40] | |
| Nanofiltration | Pharmaceutical production | 2003 | [41] |
| Wastewater treatment | 1993 | [42] | |
| Wastewater product recovery | 2003 | [43] | |
| Reverse Osmosis | Pharmaceutical production | 1984 | [44] |
| Wastewater treatment | 2003 | [45] | |
| Wastewater product recovery | 2017 | [46] | |
| Membrane Bioreactor | Wastewater treatment | 1995 | [47] |
| Pharmaceutical production | 2008 | [48] | |
| Electrodialysis | Pharmaceutical production | 2020 | [49] |
| Wastewater treatment | 2019 | [50] | |
| Wastewater product recovery | 2013 | [51] | |
| Osmosis | Wastewater treatment | 2011 | [52] |
| Wastewater product recovery | 2015 | [53] | |
| Osmotic Vaporization | Wastewater treatment | 2016 | [54] |
| Wastewater product recovery | 2006 | [55] | |
| Combined Processes | Wastewater treatment | 1987 | [56] |
| Pharmaceutical production | 2004 | [57] | |
| Reuse of reclaimed water | 2011 | [58] |
References
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.T., Shammas, N.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 13, pp. 1–45.
- Wang, C.; Wang, Y.; Qin, H.; Lin, H.; Chhuon, K. Application of Microfiltration membrane Technology in Water treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012158.
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516.
- Ankush; Mandal, M.K.; Sharma, M.; Khushboo, S.; Pandey, S.; Dubey, K.K. Membrane Technologies for the Treatment of Pharmaceutical Industry Wastewater. In Water and Wastewater Treatment Technologies. Energy, Environment and Sustainability; Bui, X.T., Chiemchaisri, C., Fujioka, T., Varjani, S., Eds.; Springer: Singapore, 2019; pp. 103–116.
- Liu, H.B.; Li, B.; Guo, L.W.; Pan, L.M.; Zhu, H.X.; Tang, Z.S.; Xing, W.H.; Cai, Y.Y.; Duan, J.A.; Wang, M.; et al. Current and Future Use of Membrane Technology in the Traditional Chinese Medicine Industry. Sep. Purif. Rev. 2022, 51, 484–502.
- Production of Solutions. British Patent 1450030, 22 September 1976. Available online: https://technology.matthey.com/article/20/1/33-36/ (accessed on 11 January 2023).
- Juberg, D.L.; Pauli, W.A.; Artiss, D.H. Application of reverse osmosis for the generation of water for injection. Bull. Parenter. Drug Assoc. 1977, 31, 70–77.
- United States Pharmacopeial Convention. The United States Pharmacopoeia XIX; United States Pharmacopeial Convention: North Bethesda, MD, USA, 1975.
- Hao, C.; Huang, X. Membrane separation technology and its application in pharmaceutical production. Chem. Pharm. Eng. 2004, 25, 1–4.
- Warashina, T.; Hashino, Y.; Kobayashi, T. Development of Hollow-Fiber Type Ultrafiltration System. Research and Development in Japan Awarded the Okochi Memorial Prize; Elsevier: Amsterdam, The Netherlands, 1983; pp. 51–54.
- Toussaint, G.; Ding, L.H.; Jaffrin, M.Y.; Hassairi, I.; Nonus, M. Recovery of α-Agarase Enzyme from Fermentation Broths by Membrane Crossflow Filtration. Sep. Sci. Technol. 2000, 35, 795–809.
- Manrong, J.; Pingli, L.; Qinghui, S.; Shichang, W. Composite hollow fiber membrane and its application in concentrating vitamine B12. Water Treat. 1995, 10, 215–219.
- Chaufer, B.; Rollin, M.; Grangeon, A.; Dulieu-Barton, J.M. Tetracycline Removal or Concentration with an Inorganic Ultrafiltration Membrane Modified by Quaternized Polyvinylimidazole Coating. Key Eng. Mater. 1992, 61–62, 249–254.
- Stremovskii, L.L.; Navashin, S.M. Purification and separation of benzylpenicillin and phenoxymethylpenicillin by ultrafiltration through a semipermeable membrane. Dokl. Chem. Technol. 1984, 274–276, 42–45.
- Al-Rifai, J.; Khabbaz, H.; Schäfer, A.I. Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol. 2011, 77, 60–67.
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci. Technol. 2007, 41, 3708–3714.
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654–2664.
- Clara, M.; Strenn, B.; Ausserleitner, M.; Kreuzinger, N. Comparison of the behaviour of selected micropollutants in a membrane bioreactor and a conventional wastewater treatment plant. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2004, 50, 29–36.
- Ren, S.; Boo, C.; Guo, N.; Wang, S.; Elimelech, M.; Wang, Y. Photocatalytic Reactive Ultrafiltration Membrane for Removal of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2018, 52, 8666–8673.
- Gong, C. Review and prospect of membrane production of medicinal water. Membr. Sci. Technol. 1999, 19, 8–13.
- Gong, C.; Li, M.; Dai, F.; Sun, X.; Sun, S.; Chen, W. Water for injection is prepared by reverse osmosis. Mil. Med. Sci. 1982, 17, 383–389.
- The Pharmacy Bureau of the PLA Air Force Beijing Hospital. Chinese herbal medicine injection was prepared by ultrafiltration technology. Chin. Tradit. Herb. Drugs 1979, 10, 12–13+49.
- The Pharmacy Bureau of the PLA Air Force Beijing Hospital. Experimental study on preparation of compound Chinese medicine injection by ultrafiltration method. Chin. Tradit. Herb. Drugs 1981, 12, 8–12.
- Zhang, Z.; Wang, S.; Jiang, Z.; Jing, Y.; Lin, Y.; Chu, Q. Application of plate reverse osmosis device in streptomycin production process. Technol. Water Treat. 1994, 6, 349–351.
- Li, C.; Fang, F.; He, X.; Xia, H.; Lan, W. Application of Ultrafiltration for Purification of Cephalosporin C. J. Fujian Med. Univ. 2001, 35, 53–56.
- Zhang, L.; Gao, Y.; Li, J. Application of ultrafiltration in improvement of the vitamin C production process. Membr. Sci. Technol. 2000, 5, 60–61.
- Wang, X.; Zhang, C.; Zhao, J. Separation mechanism of nanofiltration membranes and its applications in food and pharmaceutical industries. Membr. Sci. Technol. 2000, 1, 29–36.
- Li, X.; Luan, B.; Han, G. Application of ultrafiltration in the extraction of antibiotics. Technol. Water Treat. 1996, 4, 33–36.
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Chichester, UK; Hoboken, NJ, USA, 2012.
- Hao, C.; Huang, X. Application of membrane technology in pharmaceutical production. J. Filtr. Sep. 2004, 3, 34–37.
- Xue, G.; Hu, X.; Chen, X.; Zheng, X. Applications of Membrane Separation Technology in the Production of Medicine and Medical Treatment. Chem. Ind. Eng. 2009, 26, 183–188.
- Mallakpour, S.; Azadi, E. Nanofiltration membranes for food and pharmaceutical industries. Emerg. Mater. 2022, 5, 1329–1343.
- Peters, T. Membrane Technology for Water Treatment. Chem. Eng. Technol. 2010, 33, 1233–1240.
- Gu, Y.L.; Huang, J.H.; Zeng, G.M.; Shi, L.X.; Shi, Y.H.; Yi, K.X. Fate of pharmaceuticals during membrane bioreactor treatment: Status and perspectives. Bioresour. Technol. 2018, 268, 733–748.
- Yu, X.; Sun, Y. Application of membrane bioreactor technology in the transformation of biopharmaceutical wastewater treatment system. Chin. J. Biol. 2019, 32, 1169–1171, 1176.
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010.
- Goldner, H. Pharmaceutical Processes Benefit From New Filtration Systems. RD Mag. 1994, 36, 25.
- Doll, T.E.; Frimmel, F.H. Cross-flow microfiltration with periodical back-washing for photocatalytic degradation of pharmaceutical and diagnostic residues-evaluation of the long-term stability of the photocatalytic activity of TiO2. Water Res. 2005, 39, 847–854.
- Samaia, M.; Chikhi, M.; Bouzerara, F. Elimination of Penicillin V by Membrane Process. Chem. Eng. Trans. 2015, 43, 2467–2472.
- Li, S.Z.; Li, X.Y.; Wang, D.Z. Crystallization of oxytetracycline from fermentation waste liquor: Influence of biopolymer impurities. J. Colloid Interface Sci. 2004, 279, 100–108.
- Zhang, W.; He, G.H.; Gao, P.; Chen, G.H. Development and characterization of composite nanofiltration membranes and their application in concentration of antibiotics. Sep. Purif. Technol. 2003, 30, 27–35.
- Treffry-Goatley, K.; Gilron, J. The application of nanofiltration membranes to the treatment of industrial effluent and process streams. Filtr. Sep. 1993, 30, 63–66.
- Zhu, A.; Zhu, W.P.; Wu, Z.; Jing, Y.F. Recovery of clindamycin from fermentation wastewater with nanofiltration membranes. Water Res. 2003, 37, 3718–3732.
- McBain, D. Reverse-Osmosis Offers Many Advantages. Water Serv. 1984, 88, 144–145, 147.
- Reinhard, M.; Montgomery-Brown, J.; Louie, J.S.; Gross, B. From effluent to new water: Performance evaluation and quality assurance. Chimia 2003, 57, 561–566.
- Hsieh, D.S.; Lindrud, M.; Lu, X.J.; Zordan, C.; Tang, L.Y.; Davies, M. A Process for Active Pharmaceutical Ingredient Recovery from Tablets Using Green Engineering Technology. Org. Process Res. Dev. 2017, 21, 1272–1285.
- Benitez, J.; Rodriguez, A.; Malaver, R. Stabilization and dewatering of wastewater using hollow fiber membranes. Water Res. 1995, 29, 2281–2286.
- Ong, A.L.; Kamaruddin, A.H.; Bhatia, S.; Aboul-Enein, H.Y. Enantioseparation of (R,S)-ketoprofen using Candida antarctica lipase B in an enzymatic membrane reactor. J. Sep. Sci. 2008, 31, 2476–2485.
- Gao, W.T.; Chen, Q.; Du, M.G.; Zhang, W.M.; Cao, C.Y.; Song, W.G. Enabling an atom-economic production of chiral amino alcohols by electrodialysis with bipolar membranes. Green Chem. 2020, 22, 2213–2224.
- Arola, K.; Ward, A.; Mänttäri, M.; Kallioinen, M.; Batstone, D. Transport of pharmaceuticals during electrodialysis treatment of wastewater. Water Res. 2019, 161, 496–504.
- Ravikumar, Y.V.L.; Sridhar, S.; Satyanarayana, S.V. Development of an electrodialysis-distillation integrated process for separation of hazardous sodium azide to recover valuable DMSO solvent from pharmaceutical effluent. Sep. Purif. Technol. 2013, 110, 20–30.
- Hancock, N.T.; Xu, P.; Heil, D.M.; Bellona, C.; Cath, T.Y. Comprehensive Bench- and Pilot-Scale Investigation of Trace Organic Compounds Rejection by Forward Osmosis. Environ. Sci. Technol. 2011, 45, 8483–8490.
- Pan, S.F.; Zhu, M.P.; Chen, J.P.; Yuan, Z.H.; Zhong, L.B.; Zheng, Y.M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane—Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83.
- Woldemariam, D.; Kullab, A.; Fortkamp, U.; Magner, J.; Royen, H.; Martin, A. Membrane distillation pilot plant trials with pharmaceutical residues and energy demand analysis. Chem. Eng. J. 2016, 306, 471–483.
- Kreis, P.; Górak, A. Process analysis of hybrid separation processes: Combination of distillation and pervaporation. Chem. Eng. Res. Des. 2006, 84, 595–600.
- Cai, B.X.; Lang, K.M.; Liu, Y.R.; Chen, Y.M. The refinement and concentration of agricultural antibiotic A in aqueous solution using membrane processes. Desalination 1987, 62, 341–351.
- Li, S.Z.; Li, X.Y.; Wang, D.M. Membrane (RO-UF) filtration for antibiotic wastewater treatment and recovery of antibiotics. Sep. Purif. Technol. 2004, 34, 109–114.
- Joss, A.; Baenninger, C.; Foa, P.; Koepke, S.; Krauss, M.; McArdell, C.S.; Rottermann, K.; Wei, Y.; Zapata, A.; Siegrist, H. Water reuse: >90% water yield in MBR/RO through concentrate recycling and CO2 addition as scaling control. Water Res. 2011, 45, 6141–6151.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
23 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




