
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Azhar Ansari | -- | 3389 | 2024-01-22 22:23:01 | | | |
| 2 | Catherine Yang | -3 word(s) | 3386 | 2024-01-23 01:38:24 | | |
Video Upload Options
The rapid development of photovoltaic technology has driven the search for novel materials that can improve the cost-effectiveness and efficiency of solar cells. Organic semiconductors offer unique optical tunability and transparency, allowing customization for the absorption of specific optical spectra like near-infrared radiation. Through the molecular engineering of electron donors and acceptors, these materials can be optimized for targeted optical selectivity. This adaptability enables the development of efficient energy-harvesting devices tailored for specific spectral regions. Consequently, organic semiconductors present a promising avenue for specialized applications such as semi-transparent organic solar cells.
1. Introduction
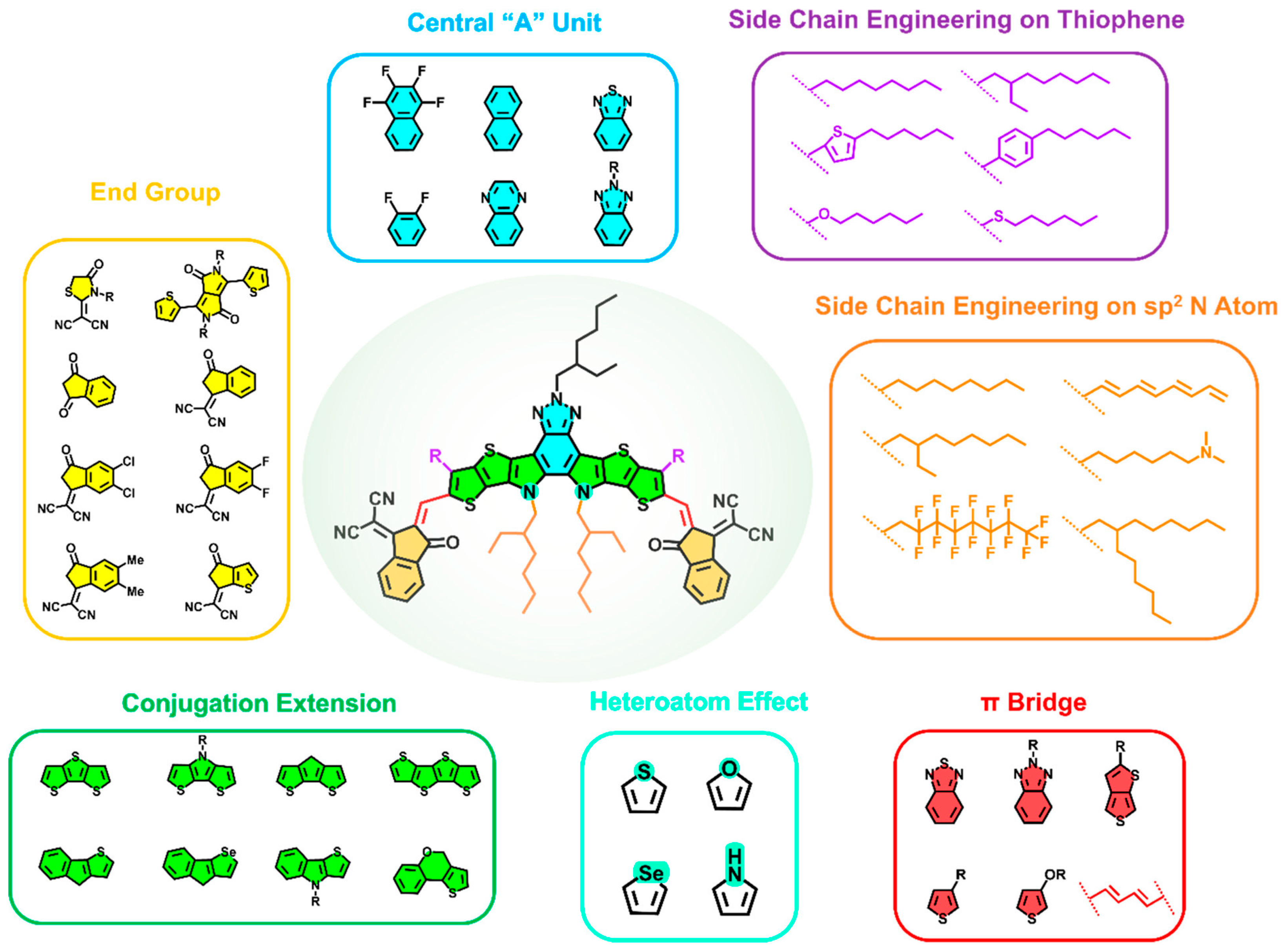
2. Novel Materials for Semi-Transparent OSCs
| Donor | Donor𝑬𝒈𝑶𝒑𝒕. (eV) |
ELUMO/EHOMO (Donor) |
Acceptor | 𝑨𝒄𝒄𝒆𝒑𝒕𝒐𝒓𝑬𝒈𝑶𝒑𝒕. (eV) |
ELUMO/EHOMO (Acceptor) |
VOC (V) |
JSC (mA cm−2) |
FF (%) |
PCE (%) |
AVT (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM6 | 1.81 | 3.50/5.56 | Y6 | 1.33 | 4.10/5.65 | 0.83 | 25.3 | 76.1 | 15.7 | - | [62] |
| PCE10-BDT2F | 1.59 | 3.82/5.42 | Y6 | - | 4.10/5.66 | 0.751 | 20.73 | 69.74 | 10.85 | 41.08 | [45] |
| CS-DP | 1.26 | 3.74/4.96 | PC71BM | - | - | 0.796 | 15.19 | 70.0 | 8.29 | - | [46] |
| PL2 | 1.51 | 3.98/5.49 | Y6:PC61BM | - | - | 0.69 | 24.07 | 59.74 | 9.91 | 40.4 | [50] |
| PM2 | 1.41 | 3.89/5.30 | Y6-BO | 1.30 | 4.38/5.68 | 0.71 | 16.2 | 68.9 | 7.9 | 32.7 | [51] |
| PM6 | 1.84 | 3.70/5.54 | Y6-BO | 1.30 | 4.38/5.68 | 0.81 | 19.1 | 73.5 | 11.4 | 20.9 | [51] |
| PM6 | [62] | - | Y6 | 1.33 | 4.10/5.65 | 0.855 | 25.39 | 72.93 | 15.83 | 50.5 | [49] |
| PBT1-C-2Cl | 1.85 | - | Y6 | 1.33 | - | 0.83 | 15.71 | 67.7 | 9.1 | 40.1 | [48] |
| PBDTTT-E-T | - | - | IEIC | 1.50 | - | 0.90 | 11.7 | 47 | 4.9 | - | [54] |
| PBDTTT-E-T | - | - | IEICO | 1.34 | 3.95/5.32 | 0.82 | 17.7 | 58 | 8.4 | - | [54] |
| PTB7-Th | - | - | IEICO-4F | - | - | 0.718 | 20.59 | 64.0 | 9.48 | 23.7 | [55] |
| PTB7-Th | - | 3.33/5.30 | ACS8 | 1.30 | 4.05/5.54 | 0.74 | 19.9 | 63.8 | 9.4 | 43.2 | [57] |
| PTB7-Th | - | - | A078 | 1.40 | 4.06/5.58 | 0.75 | 21.2 | 73 | 11.3 | 47.8 | [58] |
| PTB7-Th | - | - | SBT-FIC | 1.65 | 4.15/5.81 | 0.71 | 18.7 | 65 | 8.2 | - | [58] |
| PTB7-Th | - | 3.64//5.24 | BFIC | 1.18 | 4.00/5.26 | 0.66 | 13.82 | 65.4 | 6.15 | 38.79 | [59] |
| PTB7-Th | - | 3.04/5.34 | BZO-4Cl | 1.26 | 3.97/5.71 | 0.708 | 19.73 | 66.69 | 9.33 | 43.08 | [60] |
| PM6 | - | 3.64/5.45 | IOEH-N2F | 1.34 | 3.98/5.44 | 0.846 | 22.63 | 74.41 | 14.25 | - | [61] |
| PM6 | - | 3.64/5.45 | IOEH-4F | 1.38 | 3.99/5.46 | 0.874 | 17.81 | 75.55 | 11.77 | - | [61] |
References
- Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 27 September 2023).
- Scott, D.; Hall, C.M.; Gössling, S. Global Tourism Vulnerability to Climate Change. Ann. Tour. Res. 2019, 77, 49–61.
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic Climate Change Has Slowed Global Agricultural Productivity Growth. Nat. Clim. Change 2021, 11, 306–312.
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in Solar Energy Conversion. Chem. Soc. Rev. 2019, 48, 1862–1864.
- Timilsina, G.R.; Kurdgelashvili, L.; Narbel, P.A. Solar Energy: Markets, Economics and Policies. Renew. Sustain. Energy Rev. 2012, 16, 449–465.
- Yu, H.; Wang, J.; Zhou, Q.; Qin, J.; Wang, Y.; Lu, X.; Cheng, P. Semi-Transparent Organic Photovoltaics. Chem. Soc. Rev. 2023, 52, 4132–4148.
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon Heterojunction Solar Cell with Interdigitated Back Contacts for a Photoconversion Efficiency over 26%. Nat. Energy 2017, 2, 17032.
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar Cell Efficiency Tables (Version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15.
- Stuckelberger, M.; Biron, R.; Wyrsch, N.; Haug, F.J.; Ballif, C. Review: Progress in Solar Cells from Hydrogenated Amorphous Silicon. Renew. Sustain. Energy Rev. 2017, 76, 1497–1523.
- Green, M.A.; Dunlop, E.D.; Siefer, G.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Hao, X. Solar Cell Efficiency Tables (Version 61). Prog. Photovolt. Res. Appl. 2023, 31, 3–16.
- Mallick, R.; Li, X.; Reich, C.; Shan, X.; Zhang, W.; Nagle, T.; Bok, L.; Bicakci, E.; Rosenblatt, N.; Modi, D.; et al. Arsenic-Doped CdSeTe Solar Cells Achieve World Record 22.3% Efficiency. IEEE J. Photovolt. 2023, 13, 510–515.
- Szabó, G.; Park, N.-G.; De Angelis, F.; Kamat, P.V. Are Perovskite Solar Cells Reaching the Efficiency and Voltage Limits? ACS Energy Lett. 2023, 8, 3829–3831.
- Wang, J.; Zheng, Z.; Bi, P.; Chen, Z.; Wang, Y.; Liu, X.; Zhang, S.; Hao, X.; Zhang, M.; Li, Y.; et al. Tandem Organic Solar Cells with 20.6% Efficiency Enabled by Reduced Voltage Losses. Natl. Sci. Rev. 2023, 10, nwad085.
- Mohamed El Amine, B.; Zhou, Y.; Li, H.; Wang, Q.; Xi, J.; Zhao, C. Latest Updates of Single-Junction Organic Solar Cells up to 20% Efficiency. Energies 2023, 16, 3895.
- He, W.; Li, H.; Ma, R.; Yan, X.; Yu, H.; Hu, Y.; Hu, D.; Qin, J.; Cui, N.; Wang, J.; et al. In Situ Self-Assembly of Trichlorobenzoic Acid Enabling Organic Photovoltaics with Approaching 19% Efficiency. Adv. Funct. Mater. 2023. early view.
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-Sensitizing Novel ThienoIndole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124.
- Chrispim, M.C. Resource Recovery from Wastewater Treatment: Challenges, Opportunities and Guidance for Planning and Implementation. Ph.D. Thesis, University of Sao Paulo, Sao Paulo, Brazil, 2021.
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A.G. Environmental Impacts of Solar Energy Systems: A Review. Sci. Total Environ. 2021, 754, 141989.
- Li, Y.; Huang, X.; Sheriff, H.K.M.; Forrest, S.R. Semitransparent Organic Photovoltaics for Building-Integrated Photovoltaic Applications. Nat. Rev. Mater. 2023, 8, 186–201.
- Wang, W.; Brown, M.K. Photosensitized - and -Cycloaddition Reactions of N-Sulfonylimines. Angew. Chem. Int. Ed. 2023, 62, e202305622.
- Kini, G.P.; Jeon, S.J.; Moon, D.K. Latest Progress on Photoabsorbent Materials for Multifunctional Semitransparent Organic Solar Cells. Adv. Funct. Mater. 2021, 31, 2007931.
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-Infrared Materials: The Turning Point of Organic Photovoltaics. Adv. Mater. 2022, 34, 2107330.
- Burgués-Ceballos, I.; Lucera, L.; Tiwana, P.; Ocytko, K.; Tan, L.W.; Kowalski, S.; Snow, J.; Pron, A.; Bürckstümmer, H.; Blouin, N.; et al. Transparent Organic Photovoltaics: A Strategic Niche to Advance Commercialization. Joule 2021, 5, 2261–2272.
- Xu, S.; Wang, W.; Liu, H.; Yu, X.; Qin, F.; Luo, H.; Zhou, Y.; Li, Z. A New DiazabenzoFluoranthene-Based D-A Conjugated Polymer Donor for Efficient Organic Solar Cells. Macromol. Rapid Commun. 2022, 43, e2200276.
- Zhang, Y.; Zhou, P.; Wang, J.; Zhan, X.; Chen, X. Designing a Thiophene-Fused Quinoxaline Unit to Build D–A Copolymers for Non-Fullerene Organic Solar Cells. Dye. Pigment. 2020, 174, 108022.
- Chen, L.; Zeng, M.; Tang, X.; Weng, C.; Tan, S.; Shen, P. Development of A-DA′D-A Small-Molecular Acceptors Based on a 6,12-Dihydro-DiindoloPyrazine Unit for Efficient As-Cast Polymer Solar Cells. J. Phys. Chem. C 2020, 124, 21366–21377.
- Lu, B.; Zhang, Z.; Wang, J.; Cai, G.; Wang, J.; Yuan, X.; Ding, Y.; Wang, Y.; Yao, Y. Nonfullerene Electron Acceptors with Electron-Deficient Units Containing Cyano Groups for Organic Solar Cells. Mater. Chem. Front. 2021, 5, 5549–5572.
- Jia, Z.; Qin, S.; Meng, L.; Ma, Q.; Angunawela, I.; Zhang, J.; Li, X.; He, Y.; Lai, W.; Li, N.; et al. High Performance Tandem Organic Solar Cells via a Strongly Infrared-Absorbing Narrow Bandgap Acceptor. Nat. Commun. 2021, 12, 178.
- Cheng, H.W.; Zhao, Y.; Yang, Y. Toward High-Performance Semitransparent Organic Photovoltaics with Narrow-Bandgap Donors and Non-Fullerene Acceptors. Adv. Energy Mater. 2022, 12, 2102908.
- Wei, Q.; Zhang, Y.; Shan, T.; Zhong, H. A Near-Infrared Polymer Enables over 50% Transmittance in Semi-Transparent Organic Solar Cells. J. Mater. Chem. C Mater. 2022, 10, 5887–5895.
- Babu, N.S. Studies of New 2,7-Carbazole (CB) Based Donor-Acceptor-Donor (D-A-D) Monomers as Possible Electron Donors in Polymer Solar Cells by DFT and TD-DFT Methods. ChemistryOpen 2022, 11, e202100273.
- Park, S.H.; Ahn, J.-S.; Kwon, N.Y.; Diem, C.H.; Harit, A.K.; Woo, H.Y.; Cho, M.J.; Choi, D.H. Effect of Fused Thiophene Bridges on the Efficiency of Non-Fullerene Polymer Solar Cells Made with Conjugated Donor Copolymers Containing Alkyl Thiophene-3-Carboxylate. Macromol. Res. 2021, 29, 435–442.
- Zhu, X.; Zhang, Y.; Xie, B.; Miao, J.; Ma, W.; Liu, J.; Wang, L. All-Fused-Ring Small Molecule Acceptors with near-Infrared Absorption. J. Mater. Chem. C Mater. 2023, 11, 2144–2152.
- Li, X.; Weng, K.; Ryu, H.S.; Guo, J.; Zhang, X.; Xia, T.; Fu, H.; Wei, D.; Min, J.; Zhang, Y.; et al. Non-Fullerene Organic Solar Cells Based on BenzoDifuran-Conjugated Polymer with 14% Efficiency. Adv. Funct. Mater. 2020, 30, 1906809.
- Li, X.; Duan, X.; Liang, Z.; Yan, L.; Yang, Y.; Qiao, J.; Hao, X.; Zhang, C.; Zhang, J.; Li, Y.; et al. BenzoDifuran Based Polymer Donor for High-Efficiency (>16%) and Stable Organic Solar Cells. Adv. Energy Mater. 2022, 12, 2103684.
- Al-Isaee, S.; Iraqi, A.; Lidzey, D. Synthesis and Characterisation of a New Series of 2,6-Linked-Anthracene–Benzothiadiazole Based Polymers for Organic Solar Cells Applications. Tetrahedron 2023, 138, 133416.
- Liao, C.; Gong, Y.; Xu, X.; Yu, L.; Li, R.; Peng, Q. Cost-Efficiency Balanced Polymer Acceptors Based on Lowly Fused DithienopyrroloBenzothiadiazole for 16.04% Efficiency All-Polymer Solar Cells. Chem. Eng. J. 2022, 435, 134862.
- Krassas, M.; Polyzoidis, C.; Tzourmpakis, P.; Kosmidis, D.M.; Viskadouros, G.; Kornilios, N.; Charalambidis, G.; Nikolaou, V.; Coutsolelos, A.G.; Petridis, K.; et al. Benzothiadiazole Based Cascade Material to Boost the Performance of Inverted Ternary Organic Solar Cells. Energies 2020, 13, 450.
- Zuo, K.; Dai, T.; Guo, Q.; Wang, Z.; Zhong, Y.; Mengzhen, D.; Wang, H.; Tang, A.; Zhou, E. PTB7-Th-Based Organic Photovoltaic Cells with a High VOCof over 1.0 v via Fluorination and Side Chain Engineering of Benzotriazole-Containing Nonfullerene Acceptors. ACS Appl. Mater. Interfaces 2022, 14, 18764–18772.
- Chen, Y.; Chen, F. Fluorination Effects on Bithiophene Unit in Benzodithiophene-4,8-Dione Based D-A Type Alternating Copolymers for Highly Efficient Polymer Solar Cells. RSC Adv. 2022, 12, 36038–36045.
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259.
- Tetreault, A.R.; Dang, M.T.; Bender, T.P. PTB7 and PTB7-Th as Universal Polymers to Evaluate Materials Development Aspects of Organic Solar Cells Including Interfacial Layers, New Fullerenes, and Non-Fullerene Electron Acceptors. Synth. Met. 2022, 287, 117088.
- Zhong, H.; Ye, L.; Chen, J.Y.; Jo, S.B.; Chueh, C.C.; Carpenter, J.H.; Ade, H.; Jen, A.K.Y. A Regioregular Conjugated Polymer for High Performance Thick-Film Organic Solar Cells without Processing Additive. J. Mater. Chem. A Mater. 2017, 5, 10517–10525.
- Huang, X.; Zhang, L.; Cheng, Y.; Oh, J.; Li, C.; Huang, B.; Zhao, L.; Deng, J.; Zhang, Y.; Liu, Z.; et al. Novel Narrow Bandgap Terpolymer Donors Enables Record Performance for Semitransparent Organic Solar Cells Based on All-Narrow Bandgap Semiconductors. Adv. Funct. Mater. 2022, 32, 2108634.
- Chen, S.; Yan, L.; Xiao, L.; Gao, K.; Tang, W.; Wang, C.; Zhu, C.; Wang, X.; Liu, F.; Peng, X.; et al. A Visible-near-Infrared Absorbing A-Π2-D-Π1-D-Π2-A Type Dimeric-Porphyrin Donor for High-Performance Organic Solar Cells. J. Mater. Chem. A Mater. 2017, 5, 25460–25468.
- Lee, J.; Cha, H.; Yao, H.; Hou, J.; Suh, Y.H.; Jeong, S.; Lee, K.; Durrant, J.R. Toward Visibly Transparent Organic Photovoltaic Cells Based on a Near-Infrared Harvesting Bulk Heterojunction Blend. ACS Appl. Mater. Interfaces 2020, 12, 32764–32770.
- Xie, Y.; Cai, Y.; Zhu, L.; Xia, R.; Ye, L.; Feng, X.; Yip, H.L.; Liu, F.; Lu, G.; Tan, S.; et al. Fibril Network Strategy Enables High-Performance Semitransparent Organic Solar Cells. Adv. Funct. Mater. 2020, 30, 2002181.
- Hu, Z.; Wang, Z.; An, Q.; Zhang, F. Semitransparent Polymer Solar Cells with 12.37% Efficiency and 18.6% Average Visible Transmittance. Sci. Bull. 2020, 65, 131–137.
- Yoon, J.W.; Bae, H.; Yang, J.; Ha, J.W.; Lee, C.; Lee, J.; Yoon, S.C.; Choi, H.; Ko, S.J. Semitransparent Organic Solar Cells with Light Utilization Efficiency of 4% Using Fused-Cyclopentadithiophene Based near-Infrared Polymer Donor. Chem. Eng. J. 2023, 452, 139423.
- Jiang, T.; Zhang, G.; Xia, R.; Huang, J.; Li, X.; Wang, M.; Yip, H.L.; Cao, Y. Semitransparent Organic Solar Cells Based on All-Low-Bandgap Donor and Acceptor Materials and Their Performance Potential. Mater. Today Energy 2021, 21, 100807.
- Wang, J.; Xue, P.; Jiang, Y.; Huo, Y.; Zhan, X. The Principles, Design and Applications of Fused-Ring Electron Acceptors. Nat. Rev. Chem. 2022, 6, 614–634.
- Yang, Y. The Original Design Principles of the Y-Series Nonfullerene Acceptors, from Y1 to Y6. ACS Nano 2021, 15, 18679–18682.
- Yao, H.; Chen, Y.; Qin, Y.; Yu, R.; Cui, Y.; Yang, B.; Li, S.; Zhang, K.; Hou, J. Design and Synthesis of a Low Bandgap Small Molecule Acceptor for Efficient Polymer Solar Cells. Adv. Mater. 2016, 28, 8283–8287.
- Hu, Z.; Wang, Z.; Zhang, F. Semitransparent Polymer Solar Cells with 9.06% Efficiency and 27.1% Average Visible Transmittance Obtained by Employing a Smart Strategy. J. Mater. Chem. A Mater. 2019, 7, 7025–7032.
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra-Narrow Band Gap. Angew. Chem.-Int. Ed. 2017, 56, 3045–3049.
- Chen, J.; Li, G.; Zhu, Q.; Guo, X.; Fan, Q.; Ma, W.; Zhang, M. Highly Efficient Near-Infrared and Semitransparent Polymer Solar Cells Based on an Ultra-Narrow Bandgap Nonfullerene Acceptor. J. Mater. Chem. A Mater. 2019, 7, 3745–3751.
- Li, Y.; Guo, X.; Peng, Z.; Qu, B.; Yan, H.; Ade, H.; Zhang, M.; Forrest, S.R. Color-Neutral, Semitransparent Organic Photovoltaics for Power Window Applications. Proc. Natl. Acad. Sci. USA 2020, 117, 21147–21154.
- Zhang, Y.; Wei, Q.; He, Z.; Wang, Y.; Shan, T.; Fu, Y.; Guo, X.; Zhong, H. Efficient Optoelectronic Devices Enabled by Near-Infrared Organic Semiconductors with a Photoresponse beyond 1050 Nm. ACS Appl. Mater. Interfaces 2022, 14, 31066–31074.
- Xu, X.; Wei, Q.; Zhou, Z.; He, H.; Tian, J.; Yip, H.L.; Fu, Y.; Lu, X.; Zhou, Y.; Li, Y.; et al. Efficient Semitransparent Organic Solar Cells with CRI over 90% Enabled by an Ultralow-Bandgap A-DA’D-A Small Molecule Acceptor. Adv. Funct. Mater. 2023. early view.
- Zhu, J.; Hao, R.; Wang, J.; Sun, D.; Song, X.; Zhu, M.; Zhang, B.; Liu, Y.; Tan, H.; Zhu, W. Boosting Efficiency and Stability for Organic Solar Cells by Subtle Management Electron-Withdrawing Terminal of Electron Acceptors Based on Asymmetric Dual-Donor Centra. Dye. Pigment. 2023, 210, 111003.
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.L.; Lau, T.K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151.




