Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | GAZI A. K. M. RAFIQUL BARI | -- | 1310 | 2024-01-22 14:38:22 | | | |
| 2 | Jessie Wu | Meta information modification | 1310 | 2024-01-23 01:48:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bari, G.A.K.M.R.; Jeong, J. Gel Classifications. Encyclopedia. Available online: https://encyclopedia.pub/entry/54202 (accessed on 06 February 2026).
Bari GAKMR, Jeong J. Gel Classifications. Encyclopedia. Available at: https://encyclopedia.pub/entry/54202. Accessed February 06, 2026.
Bari, Gazi A. K. M. Rafiqul, Jae-Ho Jeong. "Gel Classifications" Encyclopedia, https://encyclopedia.pub/entry/54202 (accessed February 06, 2026).
Bari, G.A.K.M.R., & Jeong, J. (2024, January 22). Gel Classifications. In Encyclopedia. https://encyclopedia.pub/entry/54202
Bari, Gazi A. K. M. Rafiqul and Jae-Ho Jeong. "Gel Classifications." Encyclopedia. Web. 22 January, 2024.
Copy Citation
Gel materials are non-fluid colloidal or polymer networks saturated with liquid, exhibiting characteristics of solid materials as three-dimensional networks form within the liquid phase. Nanostructured gels provide a highly porous architecture with abundant defects, facilitating efficient mass transfer and offering opportunities for composition tunability in the generation of catalytic responsive materials. The ease of synthesis and functionalization of gel materials enables the production of hierarchical porous structures with high surface areas capable of hosting various catalysts, thereby enhancing mass transport during electrocatalysis processes.
gel
electrocatalyst
energy conversion
CO2RR
OER
ORR
1. Introduction
The pressing global need for sustainable and efficient clean energy is paramount in countering the extensive use of fossil fuels and mitigating the adverse impacts of climate risks [1][2]. An electrocatalytic energy conversion system in clean energy processes holds the potential to transform environmental pollutants, such as CO2, into valuable and environmentally safe energy compounds. The success of this energy conversion process is crucial to meeting the demands of practical applications, considering factors such as cost-effectiveness, stability, and operational flexibility [3]. Metal–air batteries, fuel cells, and water splitting represent pivotal electrochemical energy conversion systems, encompassing processes such as the CO2 reduction reaction (CO2RR), oxygen evolution reaction (OER), and oxygen reduction reaction (ORR). Practical application of these processes is hindered by sluggish kinetics, efficiency constraints, and selectivity limitations [4][5][6]. While noble metals exhibit efficient catalytic activity, their scarcity and high costs raise significant concerns regarding real-world applications [7]. Recent advancements in gel materials have addressed these challenges by tuning electronic properties, enhancing catalytic active sites, and modulating morphology [8][9]. These improvements contribute to the optimization of electrochemical processes in energy conversion systems, enabling a reduction or dilution of expensive noble metal utilization. Gel-based electrocatalysts thus serve as a guided approach towards enhancing the efficiency and feasibility of these electrochemical processes [10][11][12].
In terms of physicochemical properties, gels present a significant number of active sites for reactant or intermediate species, allowing for adsorption, desorption, activation, and conversion or transformation [8]. The formation of these newly developed gel materials involves physical or chemical interactions between the building blocks themselves or between the building blocks and crosslinking agents. Common forces utilized in the gel formation process include Coulomb forces, π–π interactions, hydrogen bonds, hydrophobic interactions, and covalent bonds [11][12].
Polymeric gels offer a three-dimensional porous network structure, where the hydrophilic component facilitates water absorption within the structure. Simultaneously, the organic component resists dissolution in water due to crosslinking among the polymeric chains. Gels are categorized based on various factors, including source/origin, polymeric composition, structure/configuration, response to stimuli, durability, network electrical charge, and the presence of crosslinking, air, or water components in the structure. This discussion focuses on two main categories of gels: hydrogels and aerogels. The subsequent exploration delves into their applications and fundamental properties.
2. Hydrogels
Gelation chemistry serves as a fundamental framework for modulating a diverse array of physicochemical properties within hydrogels. These properties encompass ionic and electronic conductivities, structural flexibility, stretchability, mechanical strength, swelling characteristics, and responsiveness to external stimuli, including pH, temperature, light, pressure, magnetic fields, and electrical fields [13][14]. Consequently, this versatility broadens the spectrum of applications in the fields of energy conversion and storage, extending to supercapacitors, batteries, fuel cells, electrocatalysts, wearable electronic devices, solar desalination, and water purification [15]. Hydrogels are designed with variable functionalities tailored for specific applications, such as self-healing conductive hydrogels, double-network conductive hydrogels, stimuli-responsive conductive hydrogels, and gamma radiation-induced conductive hydrogels. Self-healing hydrogels find utility in flexible devices or wearable applications, whether covalently bonded or non-covalently bonded (Figure 1). Covalently bonded hydrogels incorporate imine, hydrazine, borate, and disulfide bonds, while non-covalently bonded hydrogels involve coordination bonds of metals, hydrogen bonding, and host–guest π–π stacking bonds. Self-healing hydrogels exhibit dual characteristics through physical and chemical crosslinking, encompassing hydrogen and ionic bonding [16][17][18].
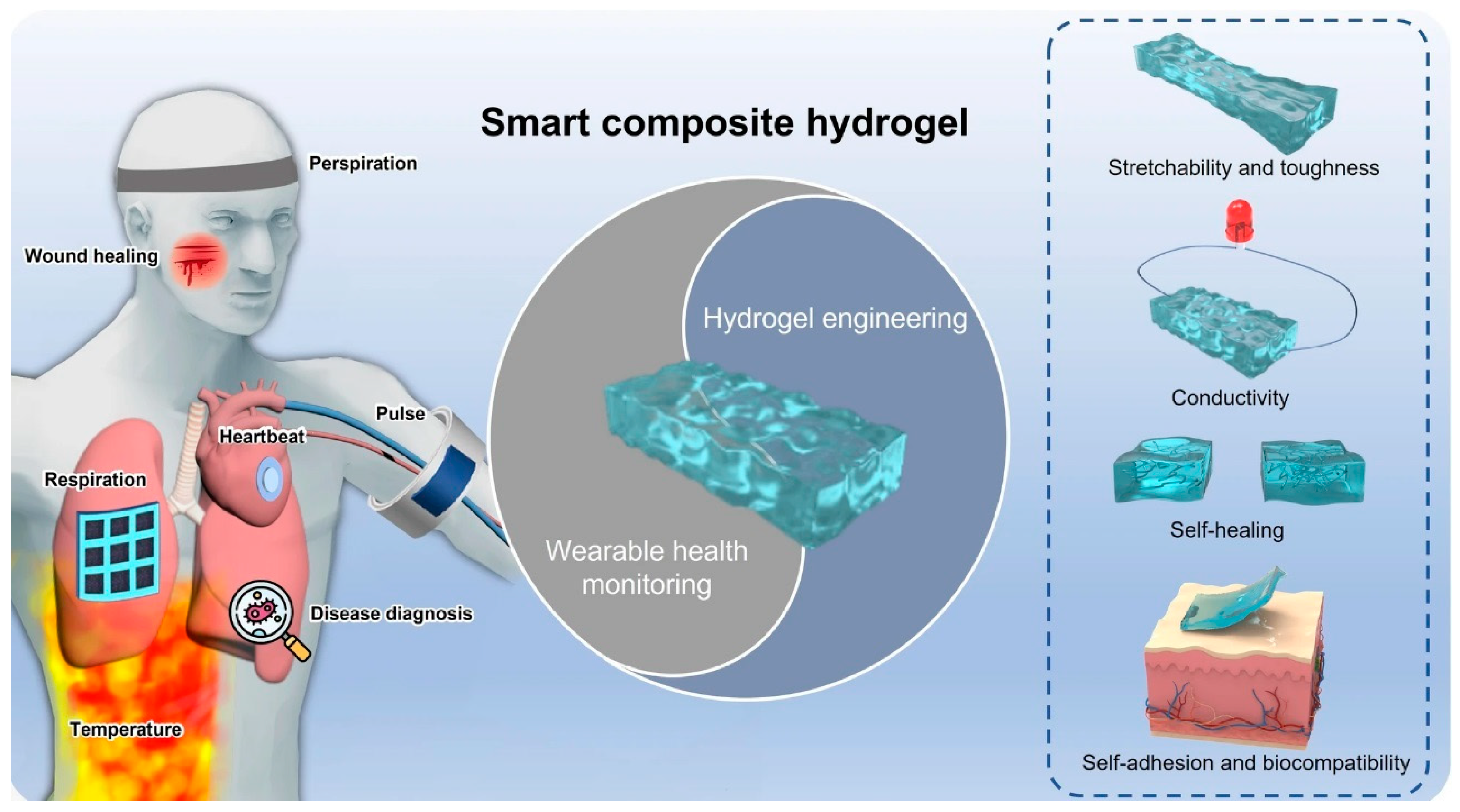
Figure 1. Schematic smart and advanced composite hydrogels for wearable disease monitoring systems. (Adapted with permission from Ref. [19], copyright © 2023 Li et al.).
Organic–inorganic hydrogels, featuring metal ions and counter ions, facilitate electron flow and delocalized electrons, enhancing conductivity across the hydrogel networks. Double-network hydrogels are engineered to incorporate rigid and potentially brittle segments alongside flexible and stretchable components, both interpenetrating each other. This technology finds application in wearable smart clothing and devices, where the delocalized double network imparts conductivity to the polymer [20][21]. Stimuli-responsive hydrogels react to external stimuli such as temperature, electric fields, pH, pressure, and near-infrared light, rendering them versatile materials for diverse applications. Their combination of conductive and responsive properties propels technological advancements, particularly in areas like sensors, biomedicine, and tissue engineering [22][23].
3. Aerogels
Aerogel is a porous structure with micro-/meso-/macro-pores or nanofibrils, where the dispersed phase is gas. The classification or definition of aerogel is not based on materials or synthesis processes (Figure 2). An aerogel is a structural scaffold soaked with air [24][25]. Aerogel is sometimes referred to as “frozen smoke”, “solid smoke”, “solid air”, or “blue smoke” due to its translucent nature and the way light scatters within the material. Electrocatalytic and electrical properties of aerogels are significant in the field of energy storage, energy conversion, supercapacitors, lightweight optics, and wearable devices. Drying aerogels is a challenging step for scaling them up to an industrial scale [25]. The electrocatalytic activity required for the conversion of their different phases is enhanced due to their 3D structure, which provides a higher surface area, more catalytic active sites, and improved mass and electron transport properties.

Figure 2. Classification of an aerogel based on preparation, composition, physical state, and structural porosity.
In the sol–gel process, the formation of a stable alkoxide or solvated metal from precursors occurs in the solution phase (Figure 3) [26]. Alcohol or oxide bridge networks form the gel through polycondensation or polyesterification, leading to an increase in the viscosity of the solution. The gel undergoes aging until it transforms into a solid mass, resulting in the contraction of the gel network and solvent removal. The aging process is a crucial factor for preventing cracks in the gel. The subsequent drying process determines the desired shape of the gel, resulting in either a highly porous aerogel or a denser xerogel. The solvent evaporation method is employed to remove the solvent and form a wet gel, subsequently producing the xerogel [26][27].
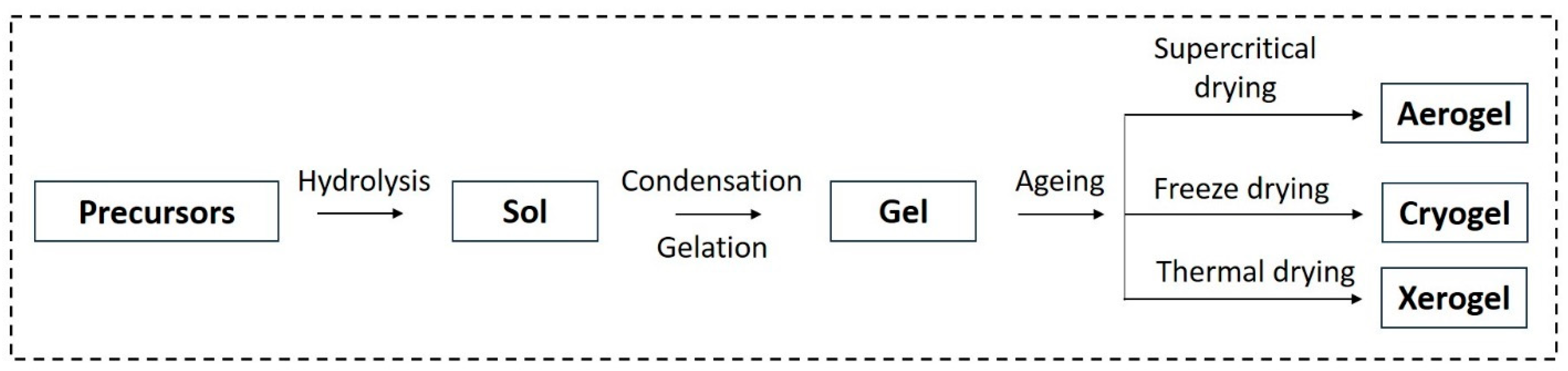
Figure 3. Schematic of aerogel, cryogel, and xerogel production.
In the case of aerogel drying through a supercritical solvent removal process, a porous network with unhindered shrinkage and low density is formed, maintaining porosity (15–50%) and a high surface area (150–1000 m2 g−1) within the micro-to-meso-pore range (1–10 nm). The removal of the fluid from the gel tunes the pore morphology, with strong capillary forces generating the xerogel, weak capillary forces creating ambigel, and zero capillary forces producing the aerogel [26].
In the sol–gel method, parameters such as the pH, solvents, and temperature play critical roles. pH control influences the hydrolysis step, leading to the generation of nanoparticles and a gel network. Solvents dissolve the nanoparticles and facilitate their joining together [28]. Temperature governs the formation of gel networks, with a slower formation resulting in a uniform structure and excellent mechanical properties. Acid-based catalysts enhance or accelerate the chemical reaction process, and their tuning can shorten the gel formation process from weeks to days or even weeks to minutes [29].
The supercritical drying process is employed to extract liquid in a controlled manner, ensuring not to cross the liquid–gas boundary during the liquid-to-gas phase transition. In the supercritical phase, high temperature and pressure are applied, where the transition from liquid to gas does not cross any phase boundary but passes through the supercritical region. Carbon dioxide (31.1 °C at 73.9 bar) and nitrous oxide are suitable fluids for supercritical drying, exhibiting similar physical behavior, with the latter demonstrating strong oxidizer behavior in the supercritical state. In a supercritical drying process, alcohol first washes away water, and then alcohol is removed via the liquefied CO2. Finally, the liquefied CO2 is heated beyond the critical point, resulting in a pressure release, allowing the escape and yielding of the dried products [26][30].
References
- Lee, T.-G.; Kang, H.-J.; Bari, G.A.R.; Park, J.-W.; Seo, H.-W.; An, B.-H.; Hwang, H.J.; Jun, Y.-S. Macroscopic graphitic carbon nitride monolith for efficient hydrogen production by photocatalytic reforming of glucose under sunlight. Chemosphere 2021, 283, 131174.
- Kang, H.-J.; Lee, T.-G.; Bari, G.A.K.M.R.; Seo, H.-W.; Park, J.-W.; Hwang, H.J.; An, B.-H.; Suzuki, N.; Fujishima, A.; Kim, J.-H.; et al. Sulfuric acid treated G-CN as a precursor to generate high-efficient G-CN for hydrogen evolution from water under visible light irradiation. Catalysts 2021, 11, 37.
- Zhou, B.; Liu, L.; Yang, Z.; Li, X.; Wen, Z.; Chen, L. Porous Organic Polymer Gel Derived Electrocatalysts for Efficient Oxygen Reduction. ChemElectroChem 2019, 6, 485–492.
- Pei, Z.; Tan, H.; Gu, J.; Lu, L.; Zeng, X.; Zhang, T.; Wang, C.; Ding, L.; Cullen, P.J.; Chen, Z.; et al. A polymeric hydrogel electrocatalyst for direct water oxidation. Nat. Commun. 2023, 14, 818.
- Gu, C.; Li, J.; Liu, J.-P.; Wang, H.; Peng, Y.; Liu, C.-S. Conferring supramolecular guanosine gel nanofiber with ZIF-67 for high-performance oxygen reduction catalysis in rechargeable zinc–air batteries. Appl. Catal. B Environ. 2021, 286, 119888.
- Feng, X.; Xiao, Y.; Huang, H.; Wang, Q.; Wu, J.; Ke, Z.; Tong, Y.; Zhang, J. Phytic Acid-Based FeCo Bimetallic Metal-Organic Gels for Electrocatalytic Oxygen Evolution Reaction. Chem. Asian J. 2021, 16, 3213–3220.
- Jena, B.K.; Raj, C.R. Electrocatalytic applications of nanosized pt particles self-assembled on sol-gel-derived three-dimensional silicate network. J. Phys. Chem. C 2008, 112, 3496–3502.
- Yang, H.; Hu, H.; Xia, C.; You, F.; Yao, J.; Jiang, X.; Xia, B.Y. Progress on nanostructured gel catalysts for oxygen electrocatalysis. Nano Res. 2022, 15, 10343–10356.
- Lu, J.; Hao, W.; Wu, X.; Shen, X.; Cui, S.; Shi, W. Electronic Modulation of the 3D Architectured Ni/Fe Oxyhydroxide Anchored N-Doped Carbon Aerogel with Much Improved OER Activity. Gels 2023, 9, 190.
- Wang, H.; Chen, B.; Liu, D. Metal–Organic Frameworks and Metal–Organic Gels for Oxygen Electrocatalysis: Structural and Compositional Considerations. Adv. Mater. 2021, 33, 2008023.
- Xiang, Q.; Liu, Y.; Zou, X.; Hu, B.; Qiang, Y.; Yu, D.; Yin, W.; Chen, C. Hydrothermal Synthesis of a New Kind of N-Doped Graphene Gel-like Hybrid As an Enhanced ORR Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 10842–10850.
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, e1804881.
- Zhang, H.; Zhang, D.; Wang, Z.; Xi, G.; Mao, R.; Ma, Y.; Wang, D.; Tang, M.; Xu, Z.; Luan, H. Ultrastretchable, Self-Healing Conductive Hydrogel-Based Triboelectric Nanogenerators for Human-Computer Interaction. ACS Appl. Mater. Interfaces 2023, 15, 5128–5138.
- Wu, S.; Wang, B.; Chen, D.; Liu, X.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Ge, S.; Liu, W. Highly sensitive and self-healing conductive hydrogels fabricated from cationic cellulose nanofiber-dispersed liquid metal for strain sensors. Sci. China Mater. 2023, 66, 1923–1933.
- Wang, X.; Weng, L.; Zhang, X.; Guan, L.; Li, X. Constructing conductive and mechanical strength self-healing hydrogel for flexible sensor. J. Sci. Adv. Mater. Devices 2023, 8, 100563.
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583.
- Green, R.A.; Hassarati, R.T.; Goding, J.A.; Baek, S.; Lovell, N.H.; Martens, P.J.; Poole-Warren, L.A. Conductive Hydrogels: Mechanically Robust Hybrids for Use as Biomaterials. Macromol. Biosci. 2012, 12, 494–501.
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905.
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 105.
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W.; et al. Polyacrylamide/Chitosan-Based Conductive Double Network Hydrogels with Outstanding Electrical and Mechanical Performance at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953.
- Song, B.; Ren, Z.; Gu, H. Totally dynamically cross-linked dual-network conductive hydrogel with superb and rapid self-healing ability for motion detection sensors. Mater. Today Commun. 2023, 35, 105919.
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54.
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123.
- Rai, N.; Chauhan, I. Multifunctional Aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Sol-Gel Sci. Technol. 2023, 105, 324–336.
- Thapliyal, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 127049.
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of silica aerogel by supercritical drying method. Procedia Eng. 2013, 57, 200–206.
- Obrey, K.A.; Wilson, K.V.; Loy, D.A. Enhancing mechanical properties of silica aerogels. J. Non-Cryst. Solids 2011, 357, 3435–3441.
- Lee, S.; Cha, Y.C.; Hwang, H.J.; Moon, J.-W.; Han, I.S. The effect of pH on the physicochemical properties of silica aerogels prepared by an ambient pressure drying method. Mater. Lett. 2007, 61, 3130–3133.
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87.
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306.
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
752
Revisions:
2 times
(View History)
Update Date:
23 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




