
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Avneesh Kumar | -- | 3337 | 2024-01-22 08:36:12 | | | |
| 2 | Lindsay Dong | Meta information modification | 3337 | 2024-01-23 01:27:49 | | |
Video Upload Options
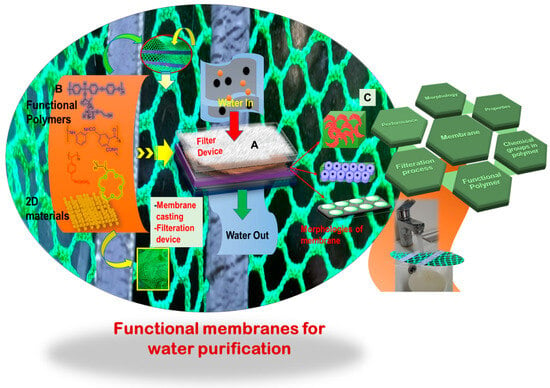
Conventional polymers, endowed with specific functionalities, are extensively utilized for filtering and extracting a diverse set of chemicals, notably metals, from solutions. The main structure of a polymer is an integral part for designing an efficient separating system. However, its chemical functionality further contributes to the selectivity, fabrication process, and resulting product morphology. One example would be a membrane that can be employed to selectively remove a targeted metal ion or chemical from a solution, leaving behind the useful components of the solution. Such membranes or products are highly sought after for purifying polluted water contaminated with toxic and heavy metals. An efficient water-purifying membrane must fulfill several requirements, including a specific morphology attained by the material with a specific chemical functionality and facile fabrication for integration into a purifying module Therefore, the selection of an appropriate polymer and its functionalization become crucial and determining steps.
1. Introduction
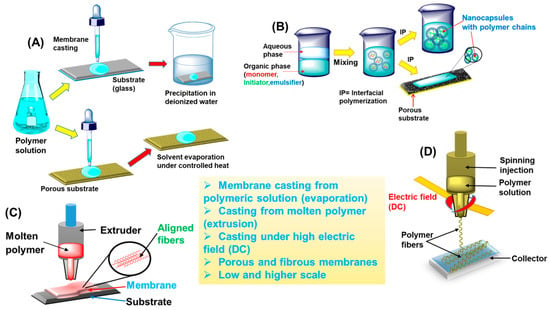
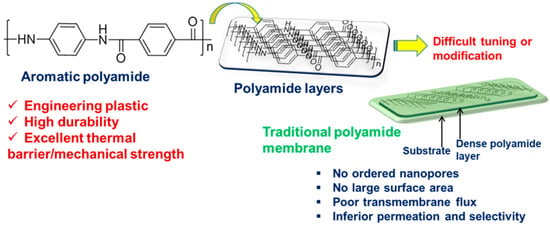
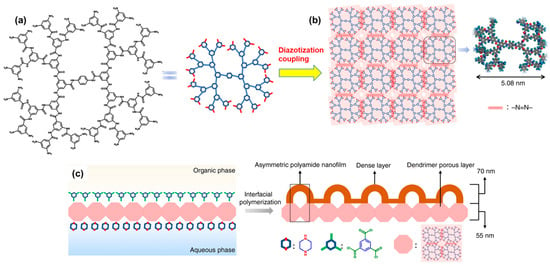
2. Membranes Based on PA and Its Derivatives
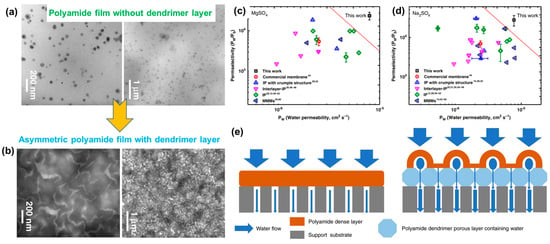
3. Membrane Based on Polyvinylidene Fluoride (PVDF) and Its Derivatives
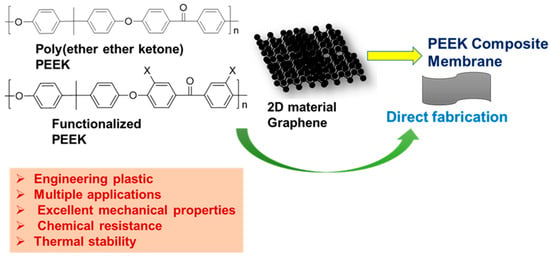
4. Membrane Based on Poly (PEEK) and Its Derivatives
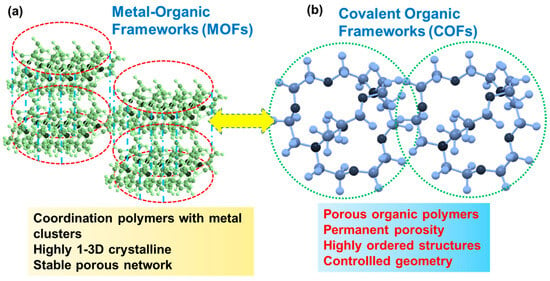
5. Membranes Based on Porous Organic Polymers and Covalent or Metal Organic Frameworks (COFs/MOFs)
6. Conclusions
References
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667.
- Flörke, M.; Schneider, C.; McDonald, R.I. Water competition between cities and agriculture driven by climate change and urban growth. Nat. Sustain. 2018, 1, 51–58.
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288.
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, K. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965.
- Zinabu, E.; Kelderman, P.; van der Kwast, J.; Irvine, K. Impacts and Policy Implications of Metals Effluent Discharge into Rivers within Industrial Zones: A Sub-Saharan Perspective from Ethiopia. Environ. Manag. 2018, 61, 700–715.
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222.
- Zhitcovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629.
- Skidmore, M.E.; Sims, K.M.; Gibbs, H.K. Agricultural intensification and childhood cancer in Brazil. PNAS 2013, 120, e2306003120.
- Wang, M.; Abad, D.; Kickhoefer, V.A.; Rome, L.H.; Mahendra, S. Vault Nanoparticles Packaged with Enzymes as an Efficient Pollutant Biodegradation Technology. ACS Nano 2015, 9, 10931–10940.
- Dong, C.; Wang, Z.; Yang, C.; Li, X. Dual-functional single-atomic Mo/Fe clusters–decorated C3N5 via three electron-pathway in oxygen reduction reaction for tandemly removing contaminants from water. PNAS 2023, 120, e2305883120.
- Wu, J.; Cao, M.; Tong, D.; Finkelstein, Z.; Hoek, E.M.V. A critical review of point-of-use drinking water treatment in the United States. NPJ Clean Water 2021, 4, 40.
- Lewis, S.R.; Datta, S.; Gui, M.; Bhattacharya, D. Reactive nanostructured membranes for water purification. PNAS 2011, 108, 8577–8582.
- Liu, Z.; Ma, Z.; Qian, B.; Chan, A.Y.H.; Wang, X.; Liu, Y.; Xin, J.H. A Facile and Scalable Method of Fabrication of Large-Area Ultrathin Graphene Oxide Nanofiltration Membrane. ACS Nano 2021, 15, 15294–15305.
- Sarkar, A.K.; Bediako, J.K.; Choi, J.W.; Yun, Y.S. Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification. NPG Asia Mater. 2019, 11, 4.
- Abbo, H.S.; Gupta, K.C.; Khaligh, N.G.; Titinchi, S.J.J. Carbon Nanomaterials for Wastewater Treatment. ChemBioEng 2021, 8, 463–489.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662.
- Andersson, J.; Castello, G.F.D.; Bilotto, P.; Hook, F.; Valtiner, M.; Dahlin, A. Control of Polymer Brush Morphology, Rheology, and Protein Repulsion by Hydrogen Bond Complexation. Langmuir 2021, 37, 4943–4952.
- Hurst, P.J.; Graham, A.A.; Patterson, J.P. Gaining Structural Control by Modification of Polymerization Rate in Ring-Opening Polymerization-Induced Crystallization-Driven Self-Assembly. ACS Polym. Au 2022, 2, 501–509.
- Huo, N.; Ye, S.; Ouderkirk, A.J.; Tenhaeff, W.E. Porous Polymer Films with Tunable Pore Size and Morphology by Vapor Deposition. ACS Appl. Polym. Mater. 2022, 4, 7300–7310.
- Nitti, A.; Carfora, R.; Assanelli, G.; Notari, M.; Pasini, D. Single-Chain Polymer Nanoparticles for Addressing Morphologies and Functions at the Nanoscale: A Review. ACS Appl. Nano Mater. 2022, 5, 13985–13997.
- Umermura, A.; Diring, S.; Furukawa, S.; Uehara, H.; Tsuruoka, T.; Kitagawa, S. Morphology Design of Porous Coordination Polymer Crystals by Coordination Modulation. J. Am. Chem. Soc. 2011, 133, 15506–15513.
- Malile, B.; Chen, J.I.L. Morphology-Based Plasmonic Nanoparticle Sensors: Controlling Etching Kinetics with Target-Responsive Permeability Gate. J. Am. Chem. Soc. 2013, 135, 16042–16045.
- Li, C.P.; Zhou, H.; Chen, J.; Wang, J.J.; Du, M.; Zhou, W. A Highly Efficient Coordination Polymer for Selective Trapping and Sensing of Perrhenate/Pertechnetate. ACS Appl. Mater. Interfaces 2020, 12, 15246–15254.
- Zhu, Y.; Gui, L.; Wang, R.; Wang, Y.; Fang, W.; Elimelech, M.; Lin, S.; Jin, J. Regulation of molecular transport in polymer membranes with voltage-controlled pore size at the angstrom scale. Nat. Comm. 2023, 14, 2373.
- Abe, M.; Kametani, Y.; Uemura, T. Fabrication of Double-Stranded Vinyl Polymers Mediated by Coordination Nanochannels. J. Am. Chem. Soc. 2023, 145, 2448–2454.
- Mardle, P.; Chen, B.; Holdcroft, S. Opportunities of Ionomer Development for Anion-Exchange Membrane Water Electrolysis. ACS Energy Lett. 2023, 8, 3330–3342.
- Getachew, B.A.; Kim, S.R.; Kim, J.H. Self-Healing Hydrogel Pore-Filled Water Filtration Membranes. Environ. Sci. Technol. 2017, 51, 905–913.
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Highly Permeable Polymer Membranes Containing Directed Channels for Water Purification. ACS Macro Lett. 2012, 1, 723–726.
- Sun, Q.; Aguila, B.; Song, Y.; Ma, S. Tailored Porous Organic Polymers for Task-Specific Water Purification. Acc. Chem. Res. 2020, 53, 812–821.
- Luo, H.; Chang, K.; Bahati, K.; Geise, G.M. Engineering Selective Desalination Membranes via Molecular Control of Polymer Functional Groups. Environ. Sci. Technol. Lett. 2019, 6, 462–466.
- Benkhaled, B.T.; Montheli, T.; Lapinte, V.; Monge, S. Hydrosoluble phosphonic acid functionalized poly(2-ethyl-2-oxazoline) chelating polymers for the sorption of metallic cations. J. Polym. Sci. 2020, 58, 2875–2886.
- Ahn, H.; Kim, Y.M.; Yang, H.S.; Yeo, S.Y.; Yu, W.R.; Lee, B.S. Moisturized Polyacrylonitrile Copolymer for Stronger Precursor Fibers. ACS Appl. Polym. Mater. 2021, 3, 6285–6293.
- Yu, S.; Guerrero, J.M.; Tai, Y.; Yang, S.; Choi, Y.Y.; Nam, J.; Myung, N.V. Maximizing Polyacrylonitrile Nanofiber Piezoelectric Properties through the Optimization of Electrospinning and Post-thermal Treatment Processes. ACS Appl. Polym. Mater. 2022, 4, 635–644.
- Facure, M.H.M.; Mercante, L.A.; Correa, D.S. Polyacrylonitrile/Reduced Graphene Oxide Free-Standing Nanofibrous Membranes for Detecting Endocrine Disruptors. ACS Appl. Nano Mater. 2022, 5, 6376–6384.
- Falireas, P.G.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Straightforward Synthesis of Well-Defined Poly(vinylidene fluoride) and Its Block Copolymers by Cobalt-Mediated Radical Polymerization. Macromolecules 2019, 52, 1266–1276.
- Meringolo, C.; Mastropietro, T.F.; Poerio, T.; Fontananova, E.; De Filpo, G.; Curcio, E.; Di Profio, G. Tailoring PVDF Membranes Surface Topography and Hydrophobicity by a Sustainable Two-Steps Phase Separation Process. ACS Sustain. Chem. Eng. 2018, 6, 10069–10077.
- Sadare, O.O.; Daramola, M.O. Blended Polysulfone/Polyethersulfone (PSF/PES) Membrane with Enhanced Antifouling Property for Separation of Succinate from Organic Acids from Fermentation Broth. ACS Sustain. Chem. Eng. 2021, 9, 13068–13083.
- Bernstein, R.; Anton, E.; Ulbricht, M. UV-Photo Graft Functionalization of Polyethersulfone Membrane with Strong Polyelectrolyte Hydrogel and Its Application for Nanofiltration. ACS Appl. Mater. Interfaces 2012, 4, 3438–3446.
- Ben-Haida, A.; Colquhoun, H.M.; Hodge, P.; Williams, D.J. Synthesis of a Catechol-Based Poly(ether ether ketone) (“o-PEEK”) by Classical Step-Growth Polymerization and by Entropically Driven Ring-Opening Polymerization of Macrocyclic Oligomers. Macromolecules 2006, 39, 6467–6472.
- Anderson, L.J.; Yuan, X.; Fahs, G.B.; Moore, R.B. Blocky Ionomers via Sulfonation of Poly(ether ether ketone) in the Semicrystalline Gel State. Macromolecules 2018, 51, 6226–6237.
- Mulhearn, W.D.; Stafford, C.M. Highly Permeable Reverse Osmosis Membranes via Molecular Layer-by-Layer Deposition of Trimesoyl Chloride and 3,5-Diaminobenzoic Acid. ACS Appl. Polym. Mater. 2021, 3, 116–121.
- Zhou, W.; Zhang, X.; Gong, X.; Ding, M.; Yu, J.; Zhang, S.; Ding, B. Environmentally Friendly Polyamide Nanofiber Membranes with Interconnective Amphiphobic Channels for Seawater Desalination. ACS Appl. Mater. Interfaces 2022, 14, 35287–35296.
- Phillips, S.J.; Ginder, N.C.; Lear, B.J. Rapid Photothermal Synthesis of Polyurethane from Blocked Isocyanates. Macromolecules 2022, 55, 7232–7239.
- Alberti, S.; Dodero, A.; Sartori, E.; Vicini, S.; Ferrati, M.; Castelleno, M. Composite Water-Borne Polyurethane Nanofibrous Electrospun Membranes with Photocatalytic Properties. ACS Appl. Polym. Mater. 2021, 3, 6157–6166.
- Sato, K.; Kajita, T.; Noro, A. Synthesis of a Cross-Linked Polymer Electrolyte Membrane with an Ultra-High Density of Sulfonic Acid Groups. ACS Appl. Polym. Mater. 2023, 5, 3480–3488.
- Zhang, Y.; Almodovar-Arbelo, N.E.; Weidman, J.L.; S-Corti, D.; Boudoris, B.W.; Phillip, W.A. Fit-for-purpose block polymer membranes molecularly engineered for water treatment. NPJ Clean Water 2018, 1, 2.
- Lu, T.D.; Wang, Q.; Gu, S.S.; Sun, S.P. Beyond Symmetry: Exploring Asymmetric Electrospun Nanofiber Membranes for Liquid Separation. Adv. Funct. Mater. 2023. early on-line view 2310218.
- Bachler, S.; Ort, M.; Kramer, S.D.; Dittrich, P.S. Permeation Studies across Symmetric and Asymmetric Membranes in Microdroplet Arrays. Anal. Chem. 2021, 93, 5137–5144.
- White, N.; Misovich, M.; Yaroschuk, A.; Bruening, M.L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628.
- Wang, K.Y.; Ong, R.C.; Chung, T.S. Double-Skinned Forward Osmosis Membranes for Reducing Internal Concentration Polarization within the Porous Sublayer. Ind. Eng. Chem. Res. 2010, 49, 4824–4831.
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28.
- Zhu, Z.; Liu, Z.; Tan, G.; Qi, J.; Zhou, Y.; Li, J. Interlayered Interface of a Thin Film Composite Janus Membrane for Sieving Volatile Substances in Membrane Distillation. Environ. Sci. Technol. 2023, 57, 7612–7623.
- Koros, W.J.; Zhang, C. Materials for Next-Generation Molecularly Selective Synthetic Membranes. Nat. Mater. 2017, 16, 289–297.
- Seo, M.; Moll, D.; Silvis, C.; Roy, A.; Querelle, S.; Hilmayer, M.A. Interfacial Polymerization of Reactive Block Polymers for the Preparation of Composite Ultrafiltration Membranes. Ind. Eng. Chem. Res. 2014, 53, 18575–18579.
- Nielen, W.M.; Willot, J.D.; de Vos, W.M. Aqueous Phase Separation of Responsive Copolymers for Sustainable and Mechanically Stable Membranes. ACS Appl. Polym. Mater. 2020, 2, 1702–1710.
- Clarizia, G.; Tasselli, F.; Simari, C.; Nicotera, I.; Bernardo, P. Solution Casting Blending: An Effective Way for Tailoring Gas Transport and Mechanical Properties of Poly(vinyl butyral) and Pebax2533. J. Phys. Chem. C 2019, 123, 11264–11272.
- Raje, A.; Koll, J.; Schneider, E.S.; Gerogopanos, P. A novel organic solvent-free method for manufacturing polyethersulfone hollow fiber membranes using melt extrusion. J. Membr. Sci. 2023, 683, 121837.
- Tekin, F.S.; Zeynep, P.; Emecen, C. Controlling Cellulose Membrane Performance via Solvent Choice during Precursor Membrane Formation. ACS Appl. Polym. Mater. 2023, 5, 2185–2194.
- Lavielle, N.; Hebraud, A.; Schlatter, G.; Meyer, L.T.; Rossi, R.M.; Popa, A.M. Simultaneous Electrospinning and Electrospraying: A Straightforward Approach for Fabricating Hierarchically Structured Composite Membranes. ACS Appl. Mater. Interfaces 2013, 5, 10090–10097.
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green Chem. 2022, 24, 2347–2375.
- Magat, E.E.; Faris, B.F.; Reith, J.E.; Salisbury, L.F. Acid-catalyzed Reactions of Nitriles. I. The Reaction of Nitriles with Formaldehyde. J. Am. Chem. Soc. 1951, 73, 1028–1031.
- Yuan, B.; Zhao, S.; Hu, P.; Cui, J.; Niu, Q.J. Asymmetric polyamide nanofilms with highly ordered nanovoids for water purification. Nat. Commun. 2020, 11, 6102.
- Zapsas, G.; Patil, Y.; Gnanou, Y.; Ameduri, B.; Hadjichristidis, N. Poly(vinylidene fluoride)-based complex macromolecular architectures: From synthesis to properties and applications. Prog. Polym. Sci. 2020, 104, 101231.
- Pantuso, E.; Ahmed, E.; Fontananova, E.; Brunetti, A.; Tahir, I.; Karothu, D.P.; Alnaji, N.A.; Dushaq, G.; Rasras, M.; Naumov, P.; et al. Smart dynamic hybrid membranes with self-cleaning capability. Nat. Commun. 2023, 14, 5751.
- Ahmed, E.; Karothu, D.P.; Warren, M.; Naumov, P. Shape-memory effects in molecular crystals. Nat. Commun. 2019, 10, 3723.
- Wei, L.; Wang, G.; Cai, M. Synthesis and properties of novel poly(ether ketone ketone)s containing 1,4-naphthylene units by electrophilic solution copolycondensation. Polym. Int. 2021, 70, 1048–1056.
- Senra, M.R.; Vieira Marques, M.F.; Monteiro, S.N. Poly (Ether-Ether-Ketone) for Biomedical Applications: From Enhancing Bioactivity to Reinforced-Bioactive Composites—An Overview. Polymers 2023, 15, 373.
- Markova, D.; Kumar, A.; Kalpper, M.; Mullen, K. Phosphonic acid-containing homo-, AB and BAB block copolymers via ATRP designed for fuel cell applications. Polymer 2009, 50, 3411–3421.
- Kumar, A. Self Assemblies of Poly (ether ether ketone) Block Copolymers for Biomedical Applications. ChemistrySelect 2021, 6, 9060–9068.
- Aristizábal, S.L.; Chisca, S.; Pulido, B.A.; Nunes, S.P. Preparation of PEEK Membranes with Excellent Stability Using Common Organic Solvents. Ind. Eng. Chem. Res. 2020, 59, 5218–5226.
- Zhang, S.; Chen, C.; Su, Z.; Qin, X.; Jiang, M.; Liu, P. High efficiency and flexible construction of hydrophilic polymer brushes on polyether ether ketone hollow-fiber membrane surface for improving permeability separation, and anti-fouling performances. Chem. Eng. J. 2023, 454, 140176.
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Wang, H.; Zhao, S.; Liu, Y.; Yao, R.; Wang, X.; Cao, Y.; Ma, D.; Zou, M.; Cao, A.; Feng, X.; et al. Membrane adsorbers with ultrahigh metal-organic framework loading for high flux separations. Nat. Commun. 2019, 10, 4204.
- Shefer, I.; Peer-Haim, O.; Leifman, O.; Epsztein, R. Enthalpic and entropic selectivity of water and small ions in polyamide membranes. Environ. Sci. Technol. 2021, 55, 14863–14875.
- Wang, H.; Zhai, Y.; Li, Y.; Cao, Y.; Shi, B.; Li, R.; Zhu, Z.; Jiang, H.; Guo, Z.; Wang, M.; et al. Covalent organic framework membranes for efficient separation of monovalent cations. Nat. Commun. 2022, 13, 7123.




