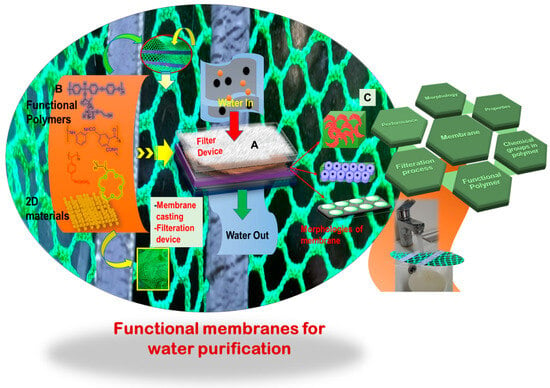
Conventional polymers, endowed with specific functionalities, are extensively utilized for filtering and extracting a diverse set of chemicals, notably metals, from solutions. The main structure of a polymer is an integral part for designing an efficient separating system. However, its chemical functionality further contributes to the selectivity, fabrication process, and resulting product morphology. One example would be a membrane that can be employed to selectively remove a targeted metal ion or chemical from a solution, leaving behind the useful components of the solution. Such membranes or products are highly sought after for purifying polluted water contaminated with toxic and heavy metals. An efficient water-purifying membrane must fulfill several requirements, including a specific morphology attained by the material with a specific chemical functionality and facile fabrication for integration into a purifying module Therefore, the selection of an appropriate polymer and its functionalization become crucial and determining steps.
1. Introduction
Human activities and interventions in ecosystems have led to numerous global challenges [
1,
2]. Among these, the availability of freshwater for drinking stands out as a significant concern. Scarcity of potable water in many regions worldwide presents a formidable challenge for sustaining life on this planet [
3]. Large quantities of chemicals from industrial plants and households are dumped into rivers and other natural water reservoirs [
4]. These chemicals or pollutants mainly comprise heavy metal ions, dyes, and other organic contaminants. They accumulate in water bodies, posing risks to living organisms and causing not only water pollution but also potentially life-threatening illnesses, such as cancer [
5,
6,
7,
8]. Consequently, there is an urgent need for efficient methods to remove or decompose these pollutants, prompting extensive research in this area. Most of these techniques involve incorporating functional materials, such as polymers or hybrid composites, within the performing device [
9,
10,
11,
12]. While bulk materials have also been utilized for this purpose, there has been a growing emphasis on focusing on nanoscale functional materials. This is because nanomaterials can provide several benefits ranging from control over the morphology to selectivity and fabrication [
13,
14,
15,
16]. Among these nanomaterials, polymers are of particular interest as they offer multiple features (as mentioned above) suitable for similar applications. In polymers, the introduction of a specific chemical functional group (polar or nonpolar) before or post synthesis can help determine the final morphology and selectivity for a particular pollutant and its removal during the water treatment process [
17,
18,
19,
20,
21,
22,
23,
24]. The chemical and physical properties of a polymer, characterized by a well-designed backbone and desired functional groups, play a considerable role in the fabrication of water purification devices or units [
25,
26,
27]. Functional polymers employed in water treatment processes are modified with polar or ionic groups, such as hydroxyl, carboxylic, amine, phosphonic, and sulfonic groups, among others, to remove a specific pollutant (for example, a metal) [
28,
29,
30,
31]. Therefore, when designing functional polymers, the removal pathway in the water treatment process must be considered. To further understand the holistic approach applied to functional polymers concerning their morphology, selectivity, and role in the water treatment processes, it is essential to summarize and highlight all the recent research efforts aimed at developing efficient functional polymers dedicated to pollutant removal.
A well-controlled and directed morphology further dictates the formation of nanopores within the membrane. The size and shape of these nanopores are crucial for the pathway of water flux.
Figure 2 provides a detailed schematic overview showcasing a few polymers, the membrane casting in a device, and the resultant membrane morphology. Common polymers, including polyacrylonitrile (PAN), polyvinylidene fluoride (PVDF), polyethersulfone (PES), polysulfone (PSF), poly(ether ether ketone) (PEEK), poly(amide) (PA), polyurethane (PU), and poly(styrene), along with their derivatives, such as copolymers, have been extensively utilized for membrane filtration [
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48]. Crucial considerations when casting or processing membranes in a device involves evaluating the chemical structure, morphology, mechanical, and thermal properties of these polymers [
43,
44,
46,
47]. These parameters significantly impact the membrane’s performance in the device. Membranes produced from these synthetic polymers are categorized into the following groups: (1) isotropic or symmetric and (2) anisotropic or asymmetric. This categorization depends on factors like nonporosity, as well as the presence of charged or uncharged polymeric chains (as fabricated through solvent-based or other techniques) [
49,
50].
Figure 2. (A) A water filter device composed of different components, such as a separating layer, substrate, and a polymeric membrane cast from a relevant functional polymer; (B) chemical structures of a few polymers, including their two-dimensional (2D) materials counterparts, highlighting active functional groups crucial for selectivity and filtration, as well as a specific nanostructure suitable for an effective membrane and its processability; and (C) various morphologies of polymeric membranes showcasing diverse features, including nanopores, three-dimensional (3D) interconnected channels and homogenous distribution of uniform nanopores, and structure–properties correlation.
In isotropic or symmetric membranes, the porosity and pore size regulate the filtration process, that is, the microfiltration (MF) of molecules or particles with varying sizes or dimensions. Alternatively, nonporous yet dense isotropic membranes have undergone testing for their performance. These membranes allow water to pass through while retaining other “species” or pollutants depending on their solubility and diffusivity. This phenomenon is observed in gas separation, pervaporation, and reverse osmosis (RO) processes. In isotropically charged membranes, such as those used in electrodialysis, the separation process occurs via ionic interactions (regardless of porosity) between the polymeric network’s ionic groups in the membrane and the positively/negatively charged molecules of the impurities [
51]. Conversely, anisotropic (asymmetric) membranes exhibit structural or chemical heterogeneity in their networks and are classified into two subgroups: (i) Loeb and Sourirajan membranes and (ii) Thin-film composite (TFC) membranes [
52,
53,
54,
55]. Loeb and Sourirajan membranes are fabricated using polymer-forming layers with indistinguishable pore sizes and porosities. In contrast, TFC membranes are prepared employing two different polymers, creating a dense surface layer responsible for separation and a thick, porous layer for providing mechanical support [
52,
53,
54,
55]. Several techniques are employed for fabricating polymer-based membranes, serving both research and industrial purposes. Notable methods include interfacial polymerization (IP), sputtering, solution casting, extruding, melt pressing, phase inversion, and electrospinning, all commonly applied for producing polymeric membranes [
56,
57,
58,
59,
60,
61,
62].
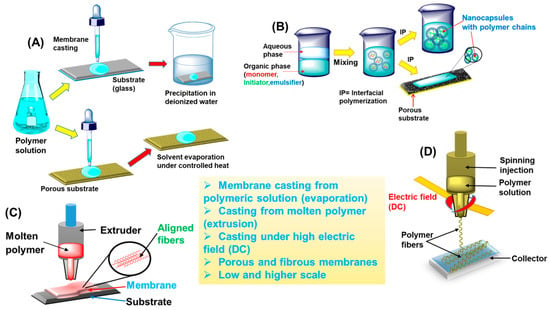
Figure 3 illustrates a graphical depiction of some of these fabrication techniques.
Figure 3. Various techniques for fabricating membranes from polymeric precursors: (
A) casting a membrane from a polymeric solution on a substrate and evaporating the solvent either via precipitation in a nonsolvent (DI-water) or under controlled heat; (
B) membrane formation via IP of a monomer at the interface of organic–aqueous phase, resulting in nanocapsules with aligned polymer chains on a porous substrate; (
C) solvent-free extrusion of a molten polymer, generating aligned fibers in a membrane on a substrate; and (
D) formation of polymeric fibers and a membrane via electrospinning, achieved by injecting a viscous polymeric solution through needles under electrodynamics or high DC voltage.
2. Membranes Based on PA and Its Derivatives
In 1930, at DuPont Wallace, Carothers synthesized a PA, commonly known as nylon [
82]. As engineering plastics, PAs are usually synthesized via step-growth polymerization and exhibit high durability and excellent thermal, barrier, and mechanical strengths owing to their rigid backbone. They are used in textiles, the automotive industry, carpets, kitchen utensils, and sportswear.
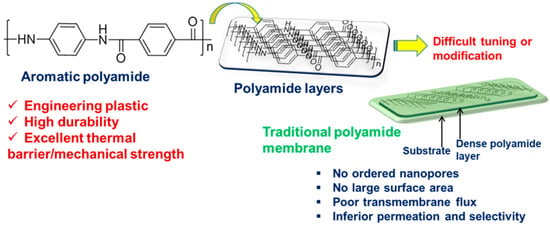
Figure 4 illustrates the typical structure of an aromatic PA, emphasizing its high-performance attributes and the presence of dense layers in a film or membrane.
Figure 4. Chemical structure of a representative aromatic PA, categorized as a high-performance or engineering plastic due to its high durability and excellent thermal and mechanical properties. The layers of PA chains in a film are dense and inert to further modifications, necessitating advanced and optimized methodologies for the same.
Synthetic aromatic PAs are preferred over their aliphatic counterparts because the latter exhibits increased moisture susceptibility, resulting in mechanical weakness and dimensional deformities. Notably, aromatic PAs such as polyphthalamides (PPAs) are processable in the molten state and have accordingly attracted considerable commercial attention. Aromatic PAs have found significant utility exploited as membrane materials owing to their superior hydrolytic and permselective properties. Despite PAs being the gold standard in both academic and industrial efforts for membrane preparation, achieving precise tuning of the PA layer remains a challenging task (Figure 4).
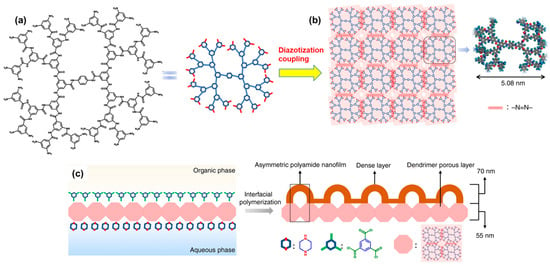
Recently, a remarkable approach for fabricating asymmetric PA films with well-ordered nanopores and dense layers was reported. This method involves the successive utilization of a PA-based dendrimer and IP of two different monomers to create an efficient double-layered membrane for NF [
86].
Figure 5 illustrates a schematic representation of this innovative approach. In the study, PA dendrimers of different generations were employed to create the lower porous layer of a membrane. Since PA dendrimers are hyperbranched and possess a nearly spherical structure, they display peculiar features such as well-defined functional periphery and uniform intramolecular voids or cavities. For this purpose, PA dendrimers with 32-amine terminal groups at the periphery were synthesized and subsequently treated in a salt solution (pH = 1) on the surface of a PSF support. This treatment facilitated their coupling with each other via a diazotization-coupling reaction involving a sodium nitrite solution, culminating in a covalent assembly (
Figure 5).
Figure 5. Strategy for creating a double-layered membrane comprising the following: (
a) hyper-branched–amine-terminated dendrimer which, upon further coupling via diazotization, forms (
b) a porous layer on a support, followed by (
c) IP of two different monomers, namely PIP and TMC, conducted on the porous layer. This process aims to produce a dense and thin asymmetric successive layer of PA with an optimized transport pathway and ordered nanovoids. Reprinted with permission from Nature Comm. [
86], Copyright 2020 Nature Portfolio. The article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
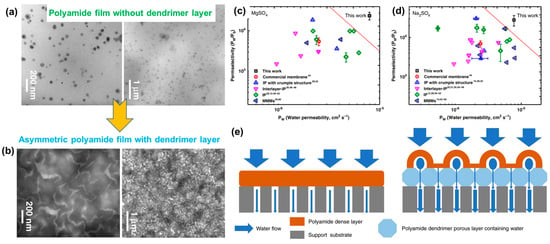
Morphological investigations of these double-layered membranes using transmission electron microscopy (TEM) clearly delineated two different layers corresponding to the covalent assembly of dendrimers and asymmetric PAs produced after IP. Figure 6 displays the TEM micrographs showcasing the single-layer PA nanofilms, revealing PA aggregations throughout. However, following the IP process, the structural features of the membrane transformed significantly, characterized by the appearance of uniform nanovoids. At high resolution, an array of interconnected, ordered nanovoids (width of 70–120 nm) was also observed. Further analysis with atomic force microscopy (AFM) and X-ray techniques confirmed the formation of an asymmetric PA membrane, comprising a porous layer of spherical dendrimers and a dense PA layer. Such features are targeted to reduce the transmembrane resistance of water and increase the permeation flux. With regard to the separation performance of these membranes, studies have demonstrated a 3.7- to 4.3-fold higher water flux for various salt solutions (2000 ppm) compared to traditional PA membranes. To further examine the superiority of these double-layered membranes, their permeability and selectivity were tested and compared with the data estimated for different types of PA membranes. These included commercial PA membranes, crumpled PA membranes (IP with crumpled structures), PA membranes prepared using interlayers, traditional IP membranes, and mixed-matrix PA membranes (Figure 6).
Figure 6. Morphological features of an asymmetric PA membrane: (
a) TEM micrographs without the dendrimer; (
b) with the covalent assemblies of dendrimer; (
c,
d) permeation and selectivity of an asymmetric membrane produced via IP on dendrimer and other available membranes reported in the literature, including commercial ones; and (
e) schematic representation illustrating the transportation of water (salt water) across the membrane without and with a dendrimer porous layer, as fabricated via diazotization prior to IP. Reprinted with permission from Nature Comm. [
86], Copyright 2020 Nature Portfolio. The article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
3. Membrane Based on Polyvinylidene Fluoride (PVDF) and Its Derivatives
Following the extensive utilization of PAs, PVDF (–(CH
2CF
2)
n–) has emerged as another favored polymer for fabricating membranes due to its impressive mechanical strength, chemical resistance, and thermal stability [
94]. Its solubility in various common solvents and easy processability allow the manufacture of flat sheets, hollow fibers, or tubular membranes suitable for MF or UF.
Further progress towards the development of PVDF membranes has explored more innovative and unique approaches, including the utilization of thermosalient (TS) crystal monomers such as 1,2,4,5-tetrabromobenzene (TBB). This compound is recognized as an energy-efficient–dynamic crystalline material due to its phase transition occurring above room temperature [
97,
98]. These dynamic functional crystals can activate the membrane via mechanical or heat responses, thus increasing the mass transfer. To fabricate these smart, responsive membranes, a layer of PVDF was integrated with a polyvinyl alcohol (PVA) hydrogel. The fabrication process involves incorporating dynamic crystals of TBB into an aqueous solution containing PVA and glutaraldehyde (GA), resulting in a suspension. Treatment of this suspension with hydrochloric acid or HCl leads to its casting on the porous surface of the PVDF film. Subsequent polymerization between TBB and GA was performed to obtain a smart PVDF–PVA membrane with dispersed TBB crystals. Experiments conducted to evaluate the transmembrane flux of these membranes showcase improved and superior performance compared with the undoped PVDF–PVA membrane. Even after the fifth operational cycle, the average flux of TBB-doped membranes remains consistently higher, surpassing 160%. Notably, the phase transition of TBB crystals likely impacts the transport properties upon heating and cooling. Applications of these membranes include distillation and antifouling. Additionally, with regard to hybrid PVDF membranes, another example involves the fabrication of polyaniline (PANI) layers within a PVDF matrix to optimize the hydrophilicity, porosity, antifouling properties, solvent content, and water flux.
In the study, a phase-inversion method is employed to fabricate PVDF membranes with various PANI concentrations. The addition of PANI (PANI (1–4 wt.%) increases the hydrophilicity of the resulting membrane with reduced pore size, as evidenced by the contact angle and SEM measurements. The operational experimental results demonstrate an enhancement in water flux from 28 to 47 L m−2 h, signifying increased hydrophilicity upon addition of PANI to PVDF. Furthermore, the performance tests exhibit high rejection of dye molecules (allura red and methyl orange).
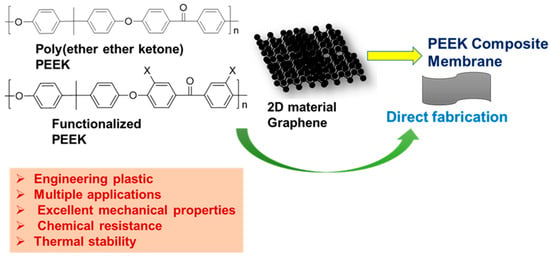
4. Membrane Based on Poly (PEEK) and Its Derivatives
PEEK, a high-performance polymer, exhibits high performance, excellent mechanical properties, chemical resistance, thermal stability, and self-lubrication properties (
Figure 12) [
107,
108,
109]. Owing to its processability in various solvents, it has also been used in multiple sectors, spanning aerospace, chemical industry, biomedicine, and membrane technology [
108,
109,
110,
111]. By introducing carbon fibers, carbon nanotubes, and various nanoparticles into its matrix, the properties of PEEK can be further tailored, with the resulting composites finding applications in membrane production (
Figure 12).
Figure 12. Typical PEEK polymer with its functionalized and composite derivatives that are used for membrane fabrication.
This entry is adapted from the peer-reviewed paper 10.3390/polym16020271