Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shengjie Wang | -- | 2353 | 2024-01-20 06:40:44 | | | |
| 2 | Fanny Huang | -13 word(s) | 2340 | 2024-01-22 07:44:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Su, Z.; Li, W.; Lei, Z.; Hu, L.; Guo, L.; Wang, S. Regulation of Angiogenesis by Non-Coding RNAs in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/54140 (accessed on 07 February 2026).
Su Z, Li W, Lei Z, Hu L, Guo L, Wang S. Regulation of Angiogenesis by Non-Coding RNAs in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/54140. Accessed February 07, 2026.
Su, Zhiyue, Wenshu Li, Zhe Lei, Lin Hu, Lingchuan Guo, Shengjie Wang. "Regulation of Angiogenesis by Non-Coding RNAs in Cancer" Encyclopedia, https://encyclopedia.pub/entry/54140 (accessed February 07, 2026).
Su, Z., Li, W., Lei, Z., Hu, L., Guo, L., & Wang, S. (2024, January 20). Regulation of Angiogenesis by Non-Coding RNAs in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/54140
Su, Zhiyue, et al. "Regulation of Angiogenesis by Non-Coding RNAs in Cancer." Encyclopedia. Web. 20 January, 2024.
Copy Citation
Non-coding RNAs, including microRNAs, long non-coding RNAs, and circular RNAs, have been identified as crucial regulators of various biological processes through epigenetic regulation, transcriptional regulation, and post-transcriptional regulation. Growing evidence suggests that dysregulation and activation of non-coding RNAs are closely associated with tumor angiogenesis, a process essential for tumor growth and metastasis and a major contributor to cancer-related mortality. Therefore, understanding the molecular mechanisms underlying tumor angiogenesis is of utmost importance. Numerous studies have documented the involvement of different types of non-coding RNAs in the regulation of angiogenesis.
miRNA
lncRNA
circRNA
tumor angiogenesis
1. Introduction
Non-coding RNA (ncRNA) refers to a class of RNA molecules that are transcribed from the genome but do not have the ability to code for proteins [1][2]. They can be classified into two main types: housekeeping ncRNA and regulatory ncRNA [3]. Regulatory ncRNAs can be further categorized as microRNA (miRNA), small interfering RNA (siRNA), piwi-interacting RNA (piRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) [3]. In recent years, there has been extensive research on regulatory ncRNAs, especially in the field of cancer research. These regulatory ncRNAs play important biological functions through epigenetic regulation, transcriptional regulation, and post-transcriptional regulation.
Tumor angiogenesis refers to the process of new blood vessel formation during the development of malignant tumors, which provides nutrients and oxygen to tumor cells [4][5]. Moreover, tumor angiogenesis offers a pathway for tumor metastasis, which is the leading cause of death in cancer patients [6][7]. Sustaining angiogenesis is a significant hallmark of cancer [8]. When vascular support is lacking, tumors may become necrotic or even apoptotic [9]. Therefore, targeting angiogenesis is a promising strategy for cancer treatment [10].
2. The Characteristics of Tumor Angiogenesis
Angiogenesis is a sequential, multi-step process that includes the destruction of the extracellular matrix, the budding and elongation of endothelial cells, the migration and proliferation of endothelial cells, and the formation and maturation of tubes [11]. A variety of cell types, including tumor cells, endothelial cells, immune cells, and fibroblasts are involved in tumor angiogenesis, which correlates with the complexity of the tumor microenvironment (TME) [12]. There is a diverse group of mediators secreted from these cells including growth factors, matrix-degrading enzymes, cytokines, bioactive lipids, and a variety of small molecules in TEM [13]. Among these mediators, vascular endothelial growth (VEGF) factors are thought to play a crucial role in regulating tumor angiogenesis. It can activate intracellular signaling pathways by binding to the corresponding receptors on endothelial cell membranes, ultimately forming blood vessels [14]. Hypoxia is a major feature of the tumor microenvironment, leading to the activation of the hypoxia-inducible factor-1 (HIF-1) transcription factor in tumor cells, which promotes the expression of VEGF [15][16]. HIF-1 complex activity can also be influenced by inflammation and cellular stress among other factors [17]. Fibroblasts accumulate in the early stages of tumor tissue formation and participate in regulating angiogenesis by secreting plasminogen activators (PAs) [18]. Immune cells, such as tumor-associated macrophages, secrete various growth factors and chemical mediators like VEGF, fibroblast growth factor-2, and angiogenesis-modulating enzymes. These substances directly or indirectly affect the process of angiogenesis [19]. In addition, several signaling pathways, such as transforming growth factor-beta (TGF-β) and signal transducer and activator of transcription (STAT), are involved in regulating the expression of these mediators [20][21]. The above features contribute to the complex nature of tumor angiogenesis. Additionally, these newly formed blood vessels often exhibit irregular, incomplete, and highly permeable characteristics, which promote cancer cell growth and metastasis [5][22]. Taken together, tumor angiogenesis is a complex process characterized by multi-step composition, the involvement of multiple cell types, and multi-factorial regulation.
3. miRNA and Tumor Angiogenesis
miRNAs are a type of small RNA molecules that exist naturally within organisms and are approximately 20–24 nucleotides long [23]. They are present in various organisms and play a significant role in regulating gene expression [24]. They achieve this regulation mainly by targeting specific recognition sites in the 3′ untranslated region (UTR), leading to mRNA degradation [25] (Figure 1). Dysregulation of miRNAs can lead to the development and progression of several diseases. In particular, aberrant miRNA expression has been linked to tumor angiogenesis [26][27]. Understanding the roles and behaviors of miRNAs offers valuable insights into the mechanisms of angiogenesis and potential targets for therapeutic interventions.
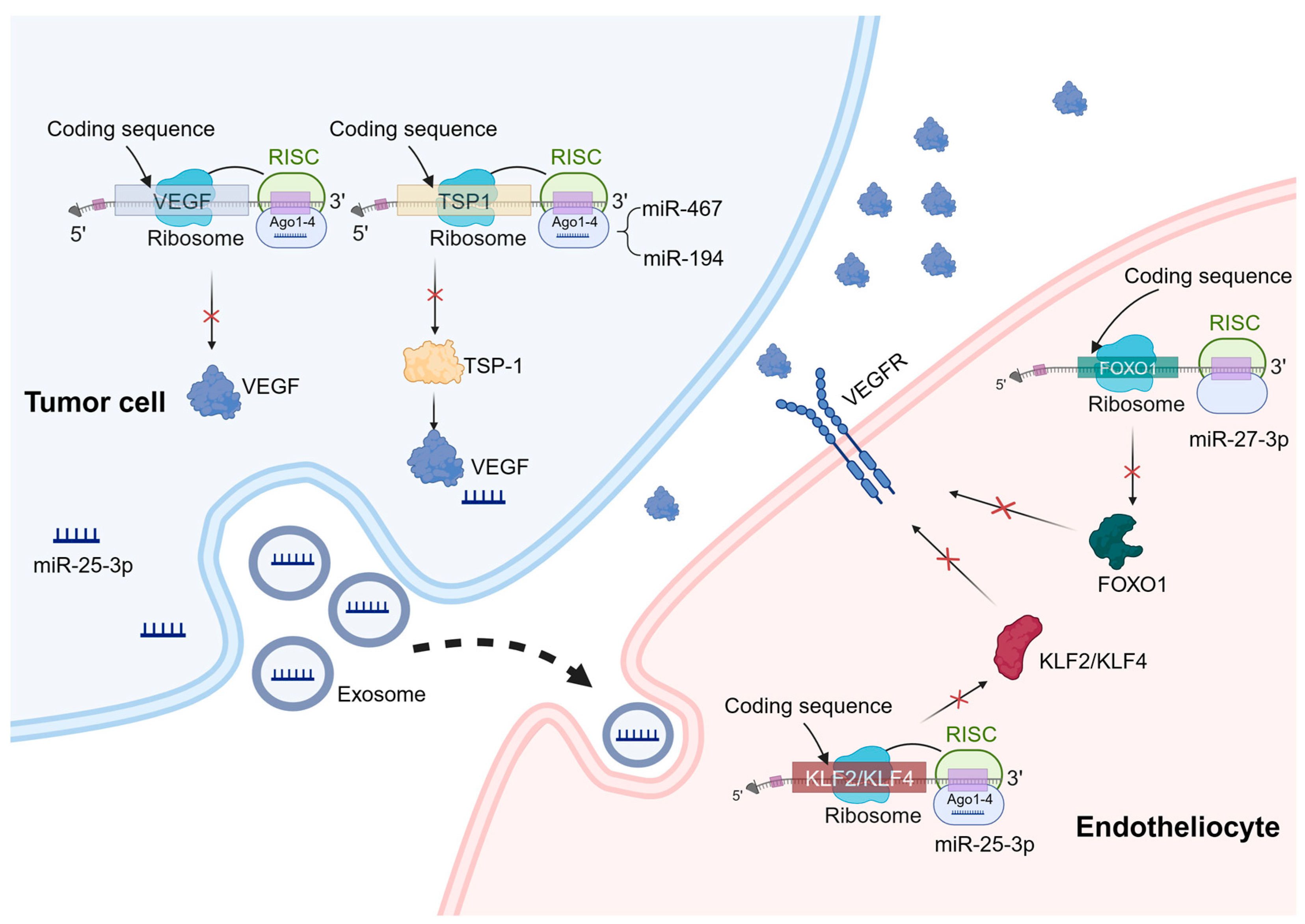
Figure 1. Mechanisms of miRNA regulation of tumor angiogenesis. miRNAs can play a role in tumor cells and vascular endothelial cells to regulate tumor angiogenesis. In the process of tumor angiogenesis, miRNAs primarily function by binding to the 3′-UTR of specific mRNAs, leading to mRNA degradation or translation inhibition. These target genes mainly include pro-angiogenic or anti-angiogenic factors, as well as genes involved in angiogenesis signaling pathways. A similar mechanism also exists in vascular endothelial cells. For example, miR-27b-3p targets the 3′-UTR region of FOXO1 mRNA and downregulates its expression, thereby regulating the expression of VEGFR. In addition, exosomal miRNAs have a significant effect on angiogenesis. For example, miR-25-3p targets and silences KLF2 and KLF4, ultimately regulating the expression of genes associated with VEGFR2 and ZO-1 in endothelial cells.
miRNAs have a promoting effect on tumor angiogenesis. Since miRNAs are repressive for the regulation of target genes, angiogenesis inhibitors such as platelet response protein 1 (TSP-1) become prime targets when miRNAs promote tumor angiogenesis [28]. It has been demonstrated that miR-467 targets TSP-1, leading to increased inflammation resolution and angiogenesis [29]. Moreover, overexpression of miR-194 has been observed in advanced colorectal cancer, which binds to the 3′-UTR region of the TSP1 gene [30]. On the other hand, when miRNAs directly regulate angiogenic factors, they inhibit tumor angiogenesis. One crucial group of angiogenic factors is the VEGF family, and overactivation of these factors can result in abnormal angiogenesis [31]. For example, studies have demonstrated that miR-126 downregulates VEGF-A expression, inducing apoptosis and impeding tumor angiogenesis across various cancer types including breast cancer, lung cancer, oral cancer, and esophageal cancer [32][33][34][35].
miRNAs also play important roles in vascular endothelial cells to regulate tumor angiogenesis. Specifically, miR-27b can suppress the activation of inflammatory pathways, consequently inhibiting intrinsic apoptosis. Mechanistically, miR-27b-3p targets the 3′-UTR region of FOXO1 mRNA, thereby downregulating its expression and subsequently attenuating the activation of the AKT/FOXO1 pathway [36].
In addition to miRNAs present within endothelial cells, extracellular vesicle miRNAs (exosomal miRNAs) have a notable impact on angiogenesis. For instance, recent studies have revealed that exosomal transfer of miR-25-3p from colorectal cancer (CRC) cells to endothelial cells promotes CRC metastasis. Mechanistically, this exosomal miRNA targets and silences KLF2 and KLF4, ultimately regulating gene expression associated with VEGFR2 and ZO-1 within endothelial cells. Consequently, this process promotes vascular permeability and neovascularization [37]. Importantly, extracellular vesicle miRNAs can also exert their influence on tumor angiogenesis by modulating immune cells. Research conducted by Zhao et al. demonstrates that exosomal miR-934 derived from CRC cells induces M2 macrophage polarization through downregulation of PTEN expression and activation of the PI3K/AKT signaling pathway. As a result, M2 macrophages produce various growth factors and cytokines like CXCL13 and CXCR5 that play crucial roles in regulating tumor growth, facilitating migration, and promoting angiogenesis [38]. In vivo, exosomes derived from stem cells of human deciduous exfoliated teeth significantly reduce the micro-vascular formation of tumors generated from xenografted oral squamous cell carcinoma cells via the transfer of miR-100-5p and miR-1246 [39].
4. lncRNA and Tumor Angiogenesis
lncRNAs, a group of RNA molecules longer than 200 nucleotides with a structure similar to mRNA, have been shown to possess more versatile mechanisms for regulating gene expression compared to miRNAs [40][41][42]. In recent years, lncRNAs have been implicated in various biological processes, including tumor proliferation, migration, invasion, and angiogenesis [43]. An overview of the molecular mechanisms by which lncRNAs function as miRNA sponges, protein scaffolds, and coding peptides in the context of tumor angiogenesis is presented (Figure 2).
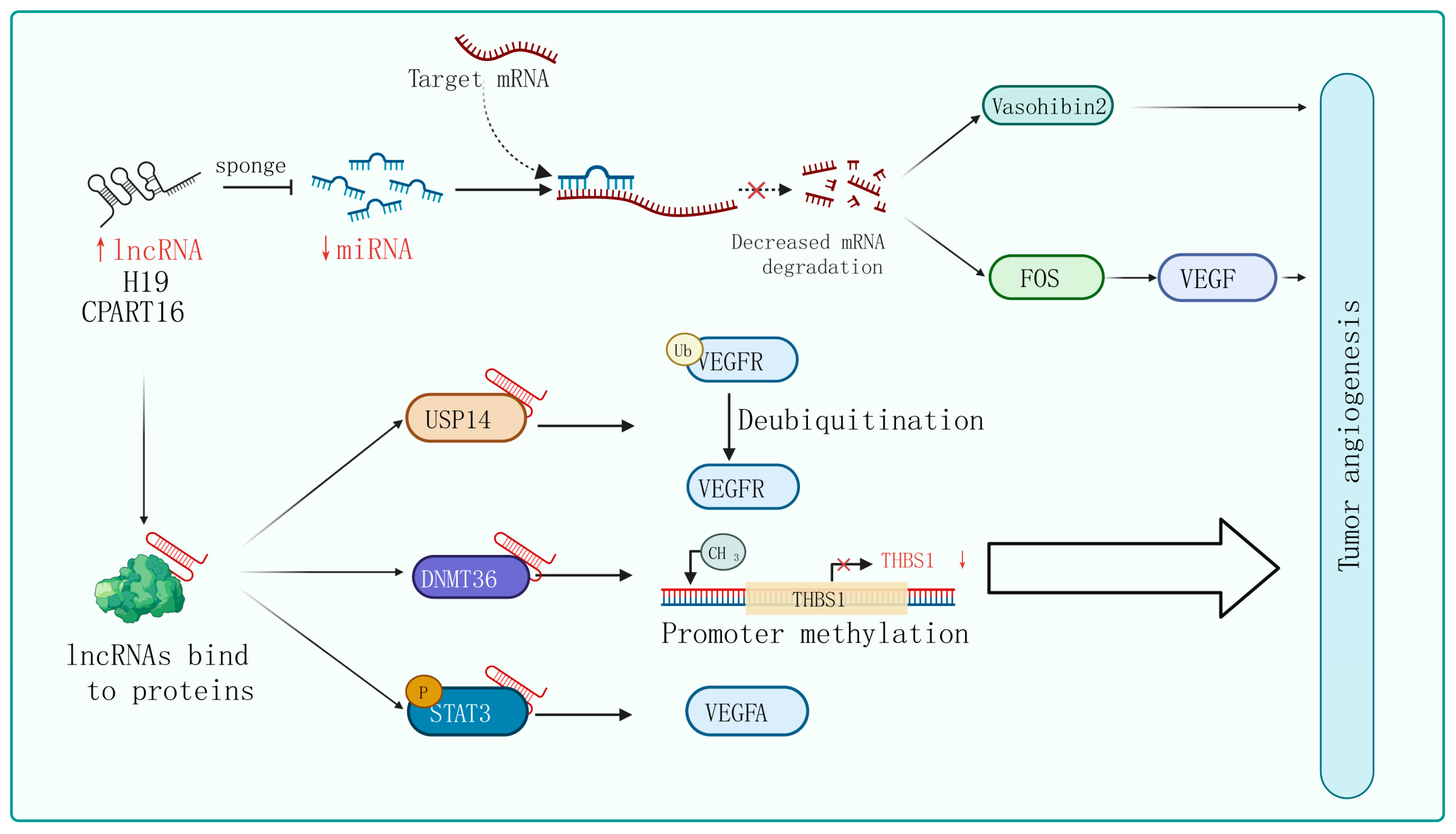
Figure 2. Mechanisms of lncRNA regulation of tumor angiogenesis. In certain tumor cells and specific tissues, some lncRNAs carry specific sequences that can adsorb miRNA, acting in a similar way to sponges in order to bind with miRNA, thereby preventing miRNA from binding to its target mRNA. On the other hand, lncRNA can interact with certain proteins, affecting their post-translational modifications, protein stability, transcription, and translation activities, ultimately affecting tumor angiogenesis through the regulation of downstream target genes.
The phenomenon of lncRNAs acting as sponges that absorb miRNAs to promote target gene expression is universal in tumor angiogenesis. For instance, lncRNA H19 is upregulated in glioblastoma and plays an important role in driving angiogenesis by sequestering miR-29a and increasing the expression of vasohibin 2, an angiogenesis factor [44]. Similarly, Zhang et al. identified an lncRNA called CRART16 that is significantly overexpressed in gastric cancer tissue. They found that CRART16 acts as a sponge for miR-122-5p and upregulates the expression of the oncogene FOS. The overexpression of FOS leads to an increase in VEGF levels, promoting cancer cell growth and angiogenesis [45]. In addition, using the xenograft animal model, lncRNA ZNRD1-AS1 has been proven to inhibit the development of lung cancer by attenuating tumor angiogenesis [46].
The interactions between lncRNAs and various types of proteins confer a diversity of modalities for regulating tumor angiogenesis. Firstly, they can influence post-translational modifications of proteins. For instance, lncRNA PCAT6 binds to USP14 (a deubiquitinase) to induce the deubiquitination of VEGFR2, thereby increasing VEGFR2 expression levels and promoting angiogenesis in triple-negative breast cancer (TNBC) [47]. Secondly, lncRNAs can regulate gene transcription and translation. For example, in breast cancer cells, lncRNA RAB11B-AS1 promotes VEGFA and ANGPTL4 gene transcription by recruiting RNA Pol II to their promoter, enhancing tumor angiogenesis [48]. Additionally, lncRNA BZRAP1-AS1 indirectly enhances the methylation of the THBS1 promoter by increasing the stability of the DNMT3b protein, which inhibits the transcription of the anti-angiogenic gene THBS1 and promotes the angiogenesis process in tumors [49]. Furthermore, lncRNA HITT weakens the binding between YB-1 proteins and the 5′-UTR of HIF1α mRNA, impairing HIF1α translation. Finally, lncRNA can regulate signaling pathway activity. lncPVT1 interacts with phosphorylated STAT3 directly in the cell nucleus and activates the STAT3 signaling pathway, leading to increased expression of VEGFA [50]. Similarly, in non-small-cell lung cancer, researchers have found that lncRNA EPIC1 promotes tumor angiogenesis by activating the Ang2/Tie2 axis [51].
Although lncRNAs do not have typical protein-coding ability, some specific lncRNAs are capable of encoding small peptides due to their open reading frames (ORFs). Researchers found that LINC00908 can encode a 60-amino acid peptide in the TNBC. Interestingly, the peptide can directly interact with STAT3 and reduce the phosphorylation level of STAT3, thereby decreasing the expression of VEGF [52].
5. cirRNA and Tumor Angiogenesis
circRNAs are a unique form of non-coding RNA that possesses a closed circular structure, providing it with exceptional stability. Consequently, circRNAs are abundantly found in various cells and tissues and play significant roles in diverse biological processes, including tumor angiogenesis. Previous studies have elucidated several mechanisms by which circRNAs function: (1) They act as sponges, sequestering miRNAs and thereby inhibiting their regulatory effects on target genes. (2) They interact with proteins to modulate their localization, post-translational modifications, and stability. (3) Notably, circRNAs themselves can serve as potential sources for encoding small functional peptides that exhibit specific biological activities (Figure 3).
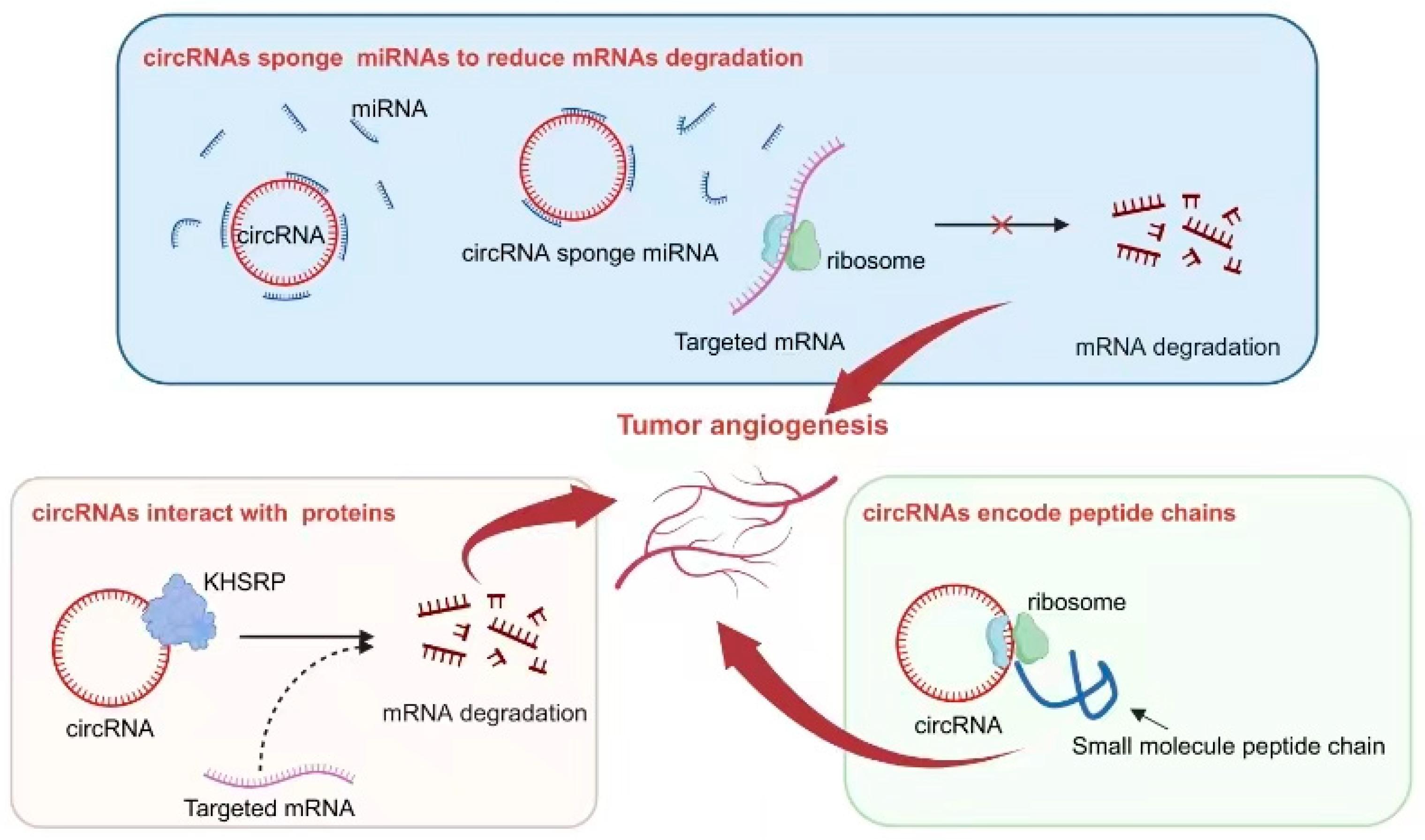
Figure 3. Mechanisms of circRNA regulation of tumor angiogenesis. Some circRNAs function as sponges, absorbing miRNAs, inhibiting the binding of miRNAs to target genes, reducing the degradation of target mRNA, and thereby affecting tumor angiogenesis. Certain circRNAs have binding sites with the protein KHSRP, influencing the degradation of target mRNA and impacting tumor angiogenesis. The small peptides encoded by circRNAs play a role in influencing tumor angiogenesis.
As a crucial regulator of tumor angiogenesis, VEGF serves as an excellent indicator for investigating the role of circRNAs in regulating this process. The abnormal activation of circRNAs often directly or indirectly influences the expression of VEGF, thereby exerting an impact on tumor angiogenesis. For instance, circ-29 enhances gastric cancer invasion and angiogenesis by sequestering miR-29a and consequently boosting the VEGF signaling pathway. The underlying mechanism involves the interaction between circ-29 and miR-29a, which reduces the latter’s ability to target the 3′-UTR region of VEGF mRNA and ultimately increases VEGF expression. Elevated levels of circ-29 contribute to the enhancement of invasive capabilities in gastric cancer cells and the promotion of neovascularization within the tumor microenvironment [53].
Interactions between circRNAs and various proteins play a crucial role in the process of tumor angiogenesis. One example is circLMP2A, which forms a complex with KHSRP, an RNA-splicing regulatory protein. This interaction inhibits the stability of VHL mRNA and relieves its inhibitory effect on HIF1α-induced VEGFA expression [54]. Another notable interaction involves circSHKBP1 and HSP90. In this case, their interaction inhibits the degradation of HSP90 by STUB1, the E3 ubiquitin ligase, resulting in enhanced VEGF expression in tumor tissue. As a result, progression and angiogenesis are enhanced in gastric cancer [55].
Interestingly, some specific circRNAs have been found to possess the capability of encoding small peptides. This ability is attributed to the presence of an ORF within their sequence. In the context of tumor angiogenesis, this phenomenon has been extensively studied and reported. One such example is circ-0000437, which has been identified as encoding a small peptide comprising 47 amino acids. Through research efforts, it has been discovered that this particular peptide plays a significant role in inhibiting the interaction between ARNT and TACC3 proteins. Consequently, this inhibition leads to a reduction in VEGF expression, ultimately resulting in the suppression of tumor angiogenesis [56].
In addition to its effects on tumor cells, circRNA can regulate other cells in the tumor microenvironment through the secretion of exosomes by tumor cells. Exosomes are nanoscale lipid-enclosed structures with a diameter of 30–100 nm that are released by cells and contain proteins, nucleic acids, and other substances. This regulation contributes to the promotion of tumor angiogenesis. For instance, circHIPK3, which is highly expressed in breast cancer, can be carried by exosomes and enter human endothelial cells after being released into the extracellular space. circHIPK3 relieves the inhibitory effect of miR-124-3p on MTDH gene expression, ultimately promoting angiogenesis [57]. Furthermore, other cells from the tumor microenvironment can impact angiogenesis. Studies have demonstrated that M2 macrophages promote angiogenesis in cutaneous squamous cell carcinoma (cSCC). One potential mechanism involves the interaction between circ_TNFRSF21 and miR-3619-5p, leading to the increased expression of the ROCK2 gene and subsequent promotion of angiogenesis [58].
References
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29.
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62.
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027.
- Voravud, N.; Charuruk, N. Tumor angiogenesis. J. Med. Assoc. Thai. 1999, 82, 394–404.
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis—Characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212.
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695.
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48.
- Suresh, R.; Diaz, R.J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 2021, 14, 101051.
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435.
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529.
- Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? Int. J. Mol. Sci. 2021, 22, 929.
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322.
- Kim, H.J.; Ji, Y.R.; Lee, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharm. Res. 2022, 45, 401–416.
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264.
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15.
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480.
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066.
- Nyberg, P.; Salo, T.; Kalluri, R. Tumor microenvironment and angiogenesis. Front. Biosci. 2008, 13, 6537–6553.
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell Mol. Med. 2009, 13, 2822–2833.
- Pardali, E.; ten Dijke, P. Transforming growth factor-beta signaling and tumor angiogenesis. Front. Biosci. 2009, 14, 4848–4861.
- Haura, E.B.; Turkson, J.; Jove, R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2005, 2, 315–324.
- Maishi, N.; Hida, K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017, 108, 1921–1926.
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610.
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159.
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207.
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647.
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007.
- Cao, Y. Antiangiogenic cancer therapy. Semin Cancer Biol. 2004, 14, 139–145.
- Gajeton, J.; Krukovets, I.; Muppala, S.; Verbovetskiy, D.; Zhang, J.; Stenina-Adognravi, O. Hyperglycemia-Induced miR-467 Drives Tumor Inflammation and Growth in Breast Cancer. Cancers 2021, 13, 1346.
- Sundaram, P.; Hultine, S.; Smith, L.M.; Dews, M.; Fox, J.L.; Biyashev, D.; Schelter, J.M.; Huang, Q.; Cleary, M.A.; Volpert, O.V.; et al. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011, 71, 7490–7501.
- Perez-Gutierrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834.
- Alhasan, L. MiR-126 Modulates Angiogenesis in Breast Cancer by Targeting VEGF-A-mRNA. Asian Pac. J. Cancer Prev. 2019, 20, 193–197.
- Liu, B.; Peng, X.C.; Zheng, X.L.; Wang, J.; Qin, Y.W. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer 2009, 66, 169–175.
- Sasahira, T.; Kurihara, M.; Bhawal, U.K.; Ueda, N.; Shimomoto, T.; Yamamoto, K.; Kirita, T.; Kuniyasu, H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br. J. Cancer 2012, 107, 700–706.
- Kong, R.; Ma, Y.; Feng, J.; Li, S.; Zhang, W.; Jiang, J.; Zhang, J.; Qiao, Z.; Yang, X.; Zhou, B. The crucial role of miR-126 on suppressing progression of esophageal cancer by targeting VEGF-A. Cell. Mol. Biol. Lett. 2016, 21, 3.
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells. Redox Biol. 2023, 62, 102681.
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395.
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156.
- Liu, P.; Zhang, Q.; Mi, J.; Wang, S.; Xu, Q.; Zhuang, D.; Chen, W.; Liu, C.; Zhang, L.; Guo, J.; et al. Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of miR-100-5p and miR-1246. Stem Cell Res. Ther. 2022, 13, 89.
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166.
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307.
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46.
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015.
- Jia, P.; Cai, H.; Liu, X.; Chen, J.; Ma, J.; Wang, P.; Liu, Y.; Zheng, J.; Xue, Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016, 381, 359–369.
- Zhang, J.; Pang, X.; Lei, L.; Zhang, J.; Zhang, X.; Chen, Z.; Zhu, J.; Jiang, Y.; Chen, G.; Wu, Y.; et al. LncRNA CRART16/miR-122-5p/FOS axis promotes angiogenesis of gastric cancer by upregulating VEGFD expression. Aging 2022, 14, 4137–4157.
- Wang, J.; Tan, L.; Yu, X.; Cao, X.; Jia, B.; Chen, R.; Li, J. lncRNA ZNRD1-AS1 promotes malignant lung cell proliferation, migration, and angiogenesis via the miR-942/TNS1 axis and is positively regulated by the m(6)A reader YTHDC2. Mol. Cancer 2022, 21, 229.
- Dong, F.; Ruan, S.; Wang, J.; Xia, Y.; Le, K.; Xiao, X.; Hu, T.; Wang, Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020, 11, 728.
- Niu, Y.; Bao, L.; Chen, Y.; Wang, C.; Luo, M.; Zhang, B.; Zhou, M.; Wang, J.E.; Fang, Y.V.; Kumar, A.; et al. HIF2-Induced Long Noncoding RNA RAB11B-AS1 Promotes Hypoxia-Mediated Angiogenesis and Breast Cancer Metastasis. Cancer Res. 2020, 80, 964–975.
- Wang, W.; Chen, G.; Wang, B.; Yuan, Z.; Liu, G.; Niu, B.; Chen, Y.; Zhou, S.; He, J.; Xue, H. Long non-coding RNA BZRAP1-AS1 silencing suppresses tumor angiogenesis in hepatocellular carcinoma by mediating THBS1 methylation. J. Transl. Med. 2019, 17, 421.
- Zhao, J.; Du, P.; Cui, P.; Qin, Y.; Hu, C.; Wu, J.; Zhou, Z.; Zhang, W.; Qin, L.; Huang, G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 2018, 37, 4094–4109.
- Hou, Y.; Jia, H.; Cao, Y.; Zhang, S.; Zhang, X.; Wei, P.; Xie, J.; Dong, W.; Wang, B. LncRNA EPIC1 promotes tumor angiogenesis via activating the Ang2/Tie2 axis in non-small cell lung cancer. Life Sci. 2021, 267, 118933.
- Wang, Y.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Guo, B.; Zhang, S.; Wu, R.; Zhang, Z.; et al. LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J. Exp. Med. 2020, 217, e20190950.
- Li, S.; Li, J.; Zhang, H.; Zhang, Y.; Wang, X.; Yang, H.; Zhou, Z.; Hao, X.; Ying, G.; Ba, Y. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells. Biochem. Biophys. Res. Commun. 2021, 560, 37–44.
- Du, Y.; Zhang, J.Y.; Gong, L.P.; Feng, Z.Y.; Wang, D.; Pan, Y.H.; Sun, L.P.; Wen, J.Y.; Chen, G.F.; Liang, J.; et al. Hypoxia-induced ebv-circLMP2A promotes angiogenesis in EBV-associated gastric carcinoma through the KHSRP/VHL/HIF1alpha/VEGFA pathway. Cancer Lett. 2022, 526, 259–272.
- Xie, M.; Yu, T.; Jing, X.; Ma, L.; Fan, Y.; Yang, F.; Ma, P.; Jiang, H.; Wu, X.; Shu, Y.; et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer 2020, 19, 112.
- Li, F.; Cai, Y.; Deng, S.; Yang, L.; Liu, N.; Chang, X.; Jing, L.; Zhou, Y.; Li, H. A peptide CORO1C-47aa encoded by the circular noncoding RNA circ-0000437 functions as a negative regulator in endometrium tumor angiogenesis. J. Biol. Chem. 2021, 297, 101182.
- Shi, P.; Liu, Y.; Yang, H.; Hu, B. Breast cancer derived exosomes promoted angiogenesis of endothelial cells in microenvironment via circHIPK3/miR-124-3p/MTDH axis. Cell. Signal. 2022, 95, 110338.
- Ma, J.; Huang, L.; Gao, Y.B.; Li, M.X.; Chen, L.L.; Yang, L. M2 macrophage facilitated angiogenesis in cutaneous squamous cell carcinoma via circ_TNFRSF21/miR-3619-5p/ROCK axis. Kaohsiung J. Med. Sci. 2022, 38, 761–771.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
547
Revisions:
2 times
(View History)
Update Date:
22 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




