The main functions of bone tissue include: structural support; protection of internal organs and soft tissues from damage; locomotion; mineral storage; production of blood cells; endocrine regulation.
1. Introduction
The human skeletal system is a marvel of biological engineering. From the explanation of the main functions of bones and their composition to the precise mechanisms of bone formation and remodeling, scholars seek to unravel and shed light on the pivotal roles that bones play beyond mere structural support with a focus on research and insights from the fields of physiology, anatomy, and histology of bone tissue.
Bones are not just lifeless scaffolds; they are living, dynamic organs that play crucial roles in support, protection, movement, and even blood cell production. Bone distinguishes itself from other connective tissues through its rigidity and firmness. These distinctive qualities arise from the incorporation of inorganic salts into a matrix composed of collagen fibers, non-collagenous proteins, and so on. Furthermore, bone possesses the remarkable ability to self-repair, adapting its shape, mass, and other properties in response to mechanical demands.
2. Bone Physiology and Structure
2.1. Main Functions of Bone Tissue
The main functions of bone tissue include:
The basic mechanical property of bone tissue is its strength, which provides both support and protection and ensures a framework for the body
[1]. Skeletal muscles attach to bones via tendons, and when a skeletal muscle contracts, it produces a pulling force that is transmitted through the tendon to the bone. This interaction between the muscle and the bone is what enables the movement of the body
[2].
Bone tissue also plays an important role as a mineral store, especially for calcium and phosphorus. These minerals are involved in various metabolic processes within our body and can be released into the bloodstream when needed. In situations where the calcium level in the blood is low, specialized cells in the bone, known as osteoclasts, break down bone tissue, releasing the minerals into the bloodstream.
Bones may appear inert and rock-solid, but in reality, they are porous inside and house the bone marrow, which is responsible for the production of blood cells. The process of producing mature blood cells from precursor cells is called hematopoiesis or hematopoiesis. Bone marrow contains stem cells such as pluripotent and hematopoietic stem cells, which can proliferate and differentiate to give rise to different types of blood cells, including red blood cells (erythrocytes), white blood cells (leucocytes), and platelets (thrombocytes)
[3].
The function of an endocrine organ is to control remote functions by releasing either a peptide or a steroid hormone. Bone cells can function as endocrine cells and regulators of many metabolic processes by secreting specific hormones and signaling molecules
[4].
2.2. Microstructure of Bone Tissue
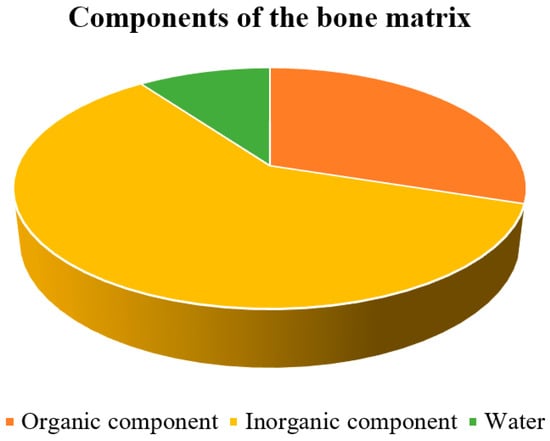
Bone is a mineralized tissue consisting of about 60% inorganic components, mainly hydroxyapatite, along with 10% water and 30% organic components (
Figure 1), primarily proteins
[5]. Because of its combination of inorganic and organic elements, it is one of the most rigid structures in the body. The organic element of the bone matrix is primarily collagen, a fibrous protein that gives bone flexibility and tensile strength
[6]. Collagen makes up a large portion of the organic matrix, accounting for approximately 85 to 90% of the organic matrix of bone
[7].
Figure 1. Distribution of bone matrix components.
2.2.1. Organic Component of the Bone Matrix
Collagen fibers are found in all types of connective tissue and are made up of the protein collagen. These fibers are very tensile (an elongation of 10 to 20% may result in a fiber break) and flexible and can form either sparse networks of thin collagen fibrils or dense bundles depending on the function and placement
[8].
Among the different types of collagens, type I collagen is the predominant form found in the bone matrix. It forms the characteristic triple helix structure, provides structural support, and contributes to the mechanical properties of bone, such as its flexibility and resistance to tensile forces. Type III and type V collagen occur in trace amounts, where type III collagen provides additional flexibility to the bone tissue, and type V collagen helps regulate the structure and organization of collagen fibers in the bone matrix. FACIT (Fibril-Associated Collagens with Interrupted Triple Helices) collages are also present in the bone matrix and have a very similar function to type V collagen
[7].
Non-collagenous proteins make up approximately 10 to 15% of the total protein content in bone. These proteins play important roles in various biological processes, including mineralization, bone remodeling, cell signaling, and regulation of bone cell activity
[7].
2.2.2. Inorganic Component of the Bone Matrix
The inorganic component of bone primarily consists of minerals, with calcium and phosphate being the most important minerals. These minerals form hydroxyapatite crystals (Ca
10(PO
4)
6(OH)
2), which are embedded in the collagen matrix. Calcium and phosphate provide hardness and much of the rigidity, providing structural strength, and they contribute to approximately 60–70% of the dry weight of bone
[5]. Hydroxyapatite, as a vital component of bone composition, plays an important role not only in maintaining bone structure but also in facilitating bone regeneration, especially in these two key processes: osteoinduction (refers to the mechanism responsible for the generation of new bone tissue and the conversion of immature cells into preosteoblasts, crucial cellular units involved in the development of new bone) and osteoconduction (refers to the ability of bone-forming cells to migrate across a bone structure and replace it with new bone tissue)
[9][10].
2.2.3. Cells of Bone
In the bone tissue, scholars distinguish four types of bone cells:
-
Osteoclasts;
-
Osteoblasts;
-
Osteocytes;
-
Lining cells.
The first three bone cell types represent different stages in the maturation process of a single cell type. Together with their progenitor cells, they form the osteoblastic cell lineage (a subgroup of the lineage that includes connective tissue cells), while osteoclasts belong to the hematopoietic cell family together with all types of blood cells
[11].
Osteoclasts are large cells that have multiple nuclei, which are essential for their high metabolic activity and efficient resorption (breakdown) of bone tissue. The cytoplasm of the osteoclasts contains several well-developed organelles such as mitochondria, endoplasmic reticulum, Golgi apparatus, transport secretory vesicles, and microtubule arrays. One prominent feature of the osteoclast cytoplasm is the presence of a specialized structure called the ruffled border, which is a highly folded cell membrane that forms numerous microvilli-like projections forcing the bone surface.
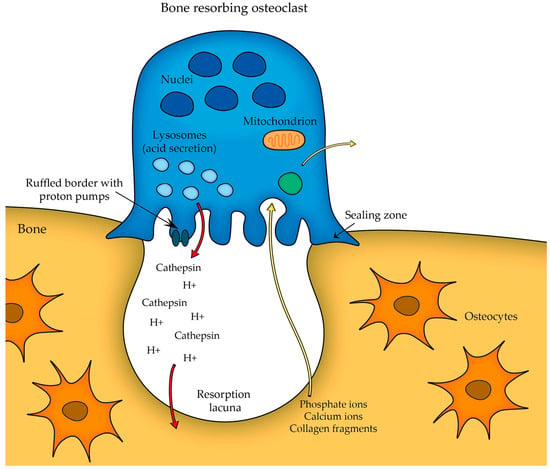
Osteoclasts play a pivotal role in bone remodeling. A fully mature osteoclast must maintain a strong attachment to the bone to effectively resorb it—thus, the initial step in osteoclast differentiation involves the attachment of osteoclast precursor to the bone surface. This leads to the formation of a structure known as the sealing zone and the development of the ruffled border membrane rich in proton pumps, which actively transport protons into the resorption lacuna (Figure 2).
Figure 2. Osteoclast sealing zone formation and proton transport.
The attachment mechanism of osteoclasts to the bone surface allows these cells to concentrate their resorptive activity in a specific area. Integrins (cell adhesion molecules expressed by osteoclasts) are responsible for binding to specific extracellular matrix proteins in bone, such as collagen (the integrin α2β1) and other bone matrix proteins (integrin αVβ3 binds to osteopontin, bone sialoprotein, etc.).
While the osteoclast attaches to the bone surface, the actual bone resorption takes place exactly at the ruffled border (as seen in
Figure 2), which serves as the site where the osteoclast secretes acid (protons H
+) that acidifies the local environment and other lysosomal enzymes (e.g., cathepsin K, which is responsible for degrading the organic components of the bone matrix) to dissolve the mineralized bone matrix. The acidic conditions help to dissolve the hydroxyapatite crystals present in the bone, releasing calcium and phosphate ions into the resorption cavity. Thus, the combination of acidic conditions and lysosomal enzymes effectively degrades the bone matrix
[11].
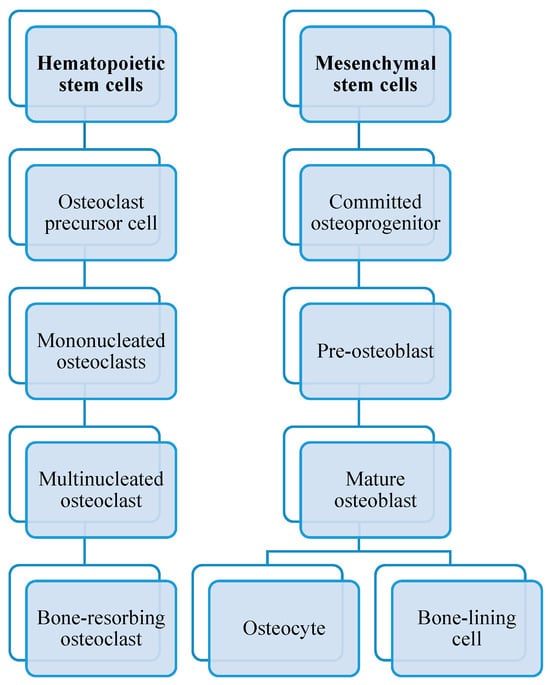
As you can see in Figure 3, osteoclasts originate from hematopoietic stem cells. They belong to the monocyte/macrophage family. Initially, hematopoietic stem cells develop into macrophage colony-forming units CFU-M when exposed to the M-CSF precursor.
Figure 3. Differences in osteoblastogenesis and osteoclastogenesis.
The role of bone morphogenetic proteins (BPMs) is to stimulate or induce mesenchymal stem cells to differentiate into osteoblasts. These proteins play essential roles in various biological processes (particularly in tissue regeneration) and belong to the transforming growth factor-beta (TGF-β) superfamily of proteins. Hence, osteoblasts arise from multipotent mesenchymal precursors, and when BPMs bind to their specific receptors, these signals activate specific genes that promote the expression of transcription factors, such as Runt-related transcription factors 2, RUNX2, and Osterix, which are crucial for osteoblast formation.
Osteoblasts, in contrast to osteoclasts, are bone-forming cells responsible for synthesizing and storing new bone tissue, as well as regulating osteoclasts. They play a key role in bone formation and remodeling throughout life. Bone remodeling is a continuous and dynamic process in which old or damaged bone tissue is removed by osteoclasts or the bone-resorbing cells (bone resorption is considered to be much faster than bone formation) and replaced by new bone tissue formed by osteoblasts.
Osteoblasts are mononuclear cells. The cytoplasm contains various cellular organelles such as the enlarged Golgi apparatus (involved in the processing and packaging of bone matrix proteins, such as collagen and osteocalcin, which bind to the hydroxyapatite crystals present in bone, and a number of glycoproteins, before they are secreted into the extracellular space), prominent rough endoplasmic reticulum involved in protein synthesis, many secretory vesicles, etc.
[3][11][12][13].
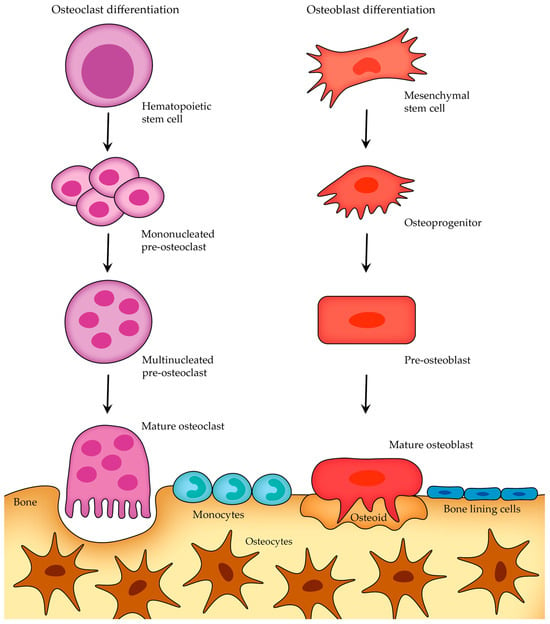
Although osteoblasts and osteoclasts have distinct functions and arise from different developmental origins, both cell types undergo a similar short life cycle involving stages of activation, activity, and eventual removal (once they have completed their task, they undergo apoptosis—programmed cell death) (Figure 4)
Figure 4. Osteoblast and osteoclast differentiation and life cycle before apoptosis.
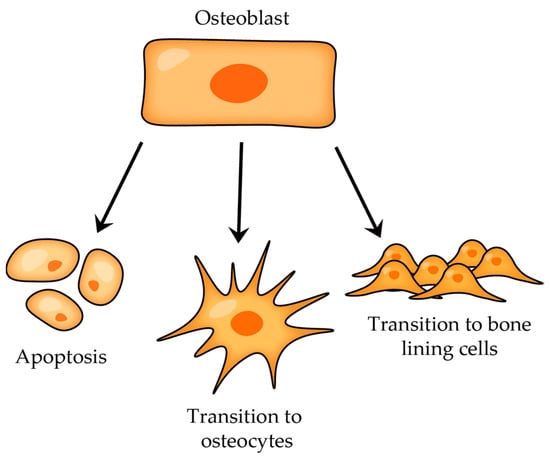
After the bone-forming phase, osteoblasts can have three different fates (
Figure 5): some osteoblasts become osteocytes, which are embedded in the mineralized bone matrix, other osteoblasts can transform into bone-lining cells that cover the bone surface, and a certain subgroup of osteoblasts undergo programmed cell death (apoptosis)
[14].
Figure 5. Osteoblast transitions after bone-forming phase.
Osteocytes are the most abundant cells in mature adult bone tissue, comprising about 90% to 95% of the cellular component. They originate from osteoblasts (once osteoblasts complete their bone-forming role, some of them become embedded within a lacuna as osteocytes; those that do not experience this transition either become bone-lining cells or undergo apoptosis; Figure 5) and have a distinct morphology characterized by dendritic processes that extend from the cell body, connecting with other bone cells (either osteocytes themselves or with cells on the bone surface) or blood vessels.
The differentiation of osteoblasts is governed by various transcription factors and signaling proteins, which encompass Runx2 (Runt-related transcription factor 2); Osterix (also known as SP7 or Osx); and the Wnt signaling pathway, or Sclerostin. The presence of Runx2 is vital for the determination of the osteoblast lineage and bone development, as evidenced by the absence of both endochondral and membranous bones in Runx2-deficient mice. Osterix is probably downstream of Runx2 (as evidenced by the absence of detectable Osx transcripts in mice that lack functional Runx2 genes) and is expressed in all osteoblasts. It is important for the differentiation of pre-osteoblasts into mature osteoblasts.
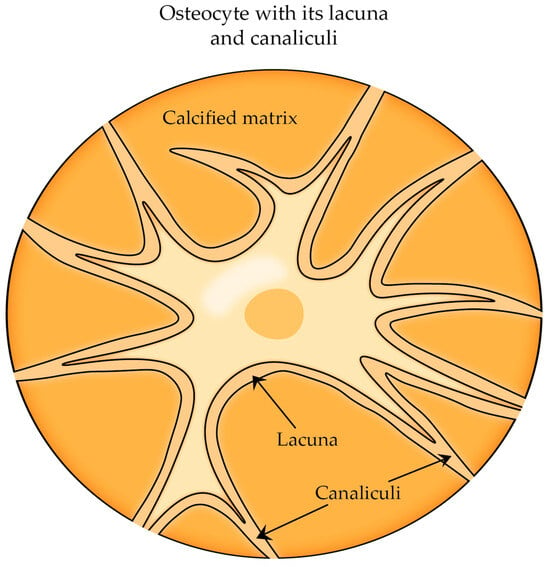
At the end of the osteoblast-to-osteocyte differentiation process, fully developed osteocytes are enclosed within a network of microscopic bony cavities referred to as lacunae (where the cellular bodies are situated) and canaliculi (where the arborization of cytoplasmic processes is contained) and are surrounded by bone fluid responsible for oxygen and nutrient supply (
Figure 6)
[15][16].
Figure 6. The cell body of osteocyte located in lacuna, and the cytoplasmic processes are situated in canaliculi.
Even though osteocytes are relatively abundant within bone tissue, a definitive function for them has not yet been clearly defined. It is believed that osteocytes can function as mechanosensors and form a sensory network responsible for sensing mechanical loads and tissue damage. Furthermore, osteocytes can facilitate communication between deep sites within the bone and the external environment and might serve as agents for repairing the bone matrix located deep within the bone, away from the remodeling activities of osteoblasts and osteoclasts. They are found to be involved in the control of mineral metabolism and in releasing calcium stores within the skeleton, a process particularly notable during lactation. During the second half of the last century, various observations hinted at the possibility of osteocytes engaging in bone resorption and thus enlarging their lacunae.
Osteocytic osteolysis: Even though the concept of osteocytic osteolysis lost favor by the late 1970s as it became evident that osteoclasts were the primary cells responsible for bone resorption, in 1962, a study conducted by Baud utilized electron transmission microscopy to observe and demonstrate that osteocytic lacunae, the small spaces in which osteocytes are housed within bone, exhibited irregular borders that bore resemblance to the characteristics associated with osteolytic activity
[17][18].
Mechanosensation: In general, osteoclasts and osteoblasts are believed to control functional adaption, but the mechanism through which these cells receive instructions to do so remains unclear. These cells act on the bone tissue’s surface, while mechanical loads result in strains across the tissue or even deeper. Hence, osteoblasts and osteoclasts must be informed about the local tissue requirements, and the detection of an abnormal strain is most effectively carried out by cells dispersed within the bone tissue—osteocytes. It can therefore say that osteocytes play a significant role in the anabolic response triggered by mechanical stimuli. They have the capacity to perceive physical changes stemming from mechanical strain on the bone and translate these changes into signals that can prompt either bone resorption or bone formation. This process is commonly referred to as “mechanotransduction”
[19][20][21].
Osteocytes as regulators of bone remodeling: Osteocytes are involved in bone formation regulation, notably through their influence on the Wnt/β-catenin complex, a critical signaling pathway that governs bone formation in osteoblasts. This complex stimulates osteoblastogenesis through the activation of β-catenin. This occurs when Wnt binds to its receptor complex, comprising Frizzled and LRP5/6 (this process was described in more detail in the previous subsection concerning the communication between osteoclasts and osteoblasts). Osteocytes are able to inhibit bone formation by releasing sclerostin and Dkk-1 (Dickkopf protein-1), both of which bind to LRP5/6, acting as inhibitors of the Wnt/β-catenin signaling pathway.
Sclerostin, which is produced by osteocytes and encoded by the SOST gene, acts as a negative regulator of bone formation by acting as an inhibitor of the canonical Wnt signaling pathway. Intermittent PTH treatment can reduce SOST expression and increase bone formation. The use of a sclerostin antibody, Scl-Ab, leads to enhanced bone formation either by attracting new osteoblasts to dormant surfaces or by directly activating quiescent bone-lining cells. Hence, the use of a sclerostin antibody, Scl-Ab, has emerged as a promising approach for osteoporosis treatment
[22].
Bone micro-damage repair: Osteocyte apoptosis is believed to serve as a trigger mechanism for local bone remodeling and micro-crack repairing by attracting osteoclasts toward the sites of bone damage. When micro-damage forms, alternations in bone fluid composition and reduced oxygen levels occur. Osteocytes are able to detect these changes, and as a result, they undergo apoptosis, and neighboring osteocytes release signals (e.g., TNF-α, IL-6, IL-11) that attract osteoclasts to remove the damaged bone
[16].
2.2.4. Communication between Bone Cells
It is important to note that osteoblasts and osteoclasts communicate with each other, and the dynamic coupling between bone resorption and bone formation is necessary for regulating and coordinating their activities and maintaining bone homeostasis, which refers to the balanced and stable state of bone tissue. Three primary mechanisms and some examples of signaling molecules and factors through which they communicate are
[23]:
Ephrin-B2/ephB4: Ephrin-B2 is a membrane-bound ligand on osteoclasts, and ephB4 is its corresponding receptor on osteoblasts and osteocytes. When the ligand ephrin-B2 binds to the ephB4 receptor, this interaction affects bone resorption and regulates osteoblast function and bone formation. The forward signaling, observed in vitro, starts with the expression of ephB4 and promotes the expression of genes related to osteogenic differentiation, whereas reverse signaling, starting with Ephrin-B2 expression, initiates negative feedback and inhibits osteoclast differentiation
[12][23][24][25].
Semaphorin 3A: semaphorins are a family of membrane-associated proteins that have been shown to influence bone remodeling and osteoblast–osteoclast communication. Semaphoring 3A is considered to be a diffusible axonal chemorepellent (in layman’s terms, chemorepulsion is also called negative chemotaxis, which means that when axons encounter the molecule, it signals them to change their direction) that plays a significant role in nervous system development.
FAS ligand (FASL)-FAS: estrogen has been shown to promote osteoclast apoptosis, and one of the mechanisms through which estrogen induces osteoclast apoptosis is by induction of FASL (Factor associated suicide ligand, also known as CD95 or APO-1). First of all, estrogen binds to estrogen receptors on osteoblasts, and this binding induces the osteoblasts to produce and release FASL. The FASL released by osteoblasts then interacts with its receptor on the surface of pre-osteoclasts, FAS, and this binding leads to the activation of apoptosis in these osteoclast precursors.
Osteoblast-Secreted Factors
Macrophage colony stimulating factor (M-CSF), also known as colony-stimulating factor 1 (CSF-1): M-CSF is a cytokine that plays a crucial role in the differentiation, survival, and proliferation of monocytes, macrophages, osteoclasts, and their precursor cells. This growth factor is secreted from osteoblasts and binds to its receptor, c-FMS, on the surface of osteoclast precursor cells. This binding contributes to forming multinucleated mature osteoclasts and maintaining the functional lifespan of osteoclasts.
Receptor Activator of NF-κB (Nuclear Factor-Kappa B) Ligand (RANKL): RANKL is a membrane-bound cytokine and a member of the tumor necrosis factor (TNF) superfamily. It is primarily produced by osteoblasts. RANKL is not only an osteoclast differentiation factor but also serves as an osteoprotegerin ligand OPGL. When bone remodeling is required, osteoblasts express RANKL on their cell surface. RANKL subsequently binds to its receptor, RANK (Receptor Activator of Nuclear Factor Kappa-B), present on osteoclast precursor cells. This binding (along with co-stimulatory signaling that leads to Ca
2+ oscillation, which is essential for the production of a transcription factor NFATc1) induces differentiation of osteoclast precursors into mature osteoclasts and is necessary for the proper functioning of mature osteoclasts in bone resorption. If RANKL is knocked out in mice, it leads to a phenotype characterized by osteopetrosis
[12][23][26].
Osteoprotegerin OPG (osteoclastogenesis inhibitory factor): osteoprotegerin is a member of the tumor necrosis factor receptor superfamily (TNFR). It is named like that to reflect its role in protecting bone against bone loss. OPG is produced by various cell types, including osteoblasts and cells in the liver, spleen, or heart. This glycoprotein lacks transmembrane and cell-association signals, suggesting that it likely acts in the extracellular environment.
Leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4, also known as GRP48): LGR4 is a member of the G-protein-coupled receptor family, and it was found to be expressed in osteoblasts, among others. LGR4 plays a dual role in regulating both osteoclastogenesis and osteoblastogenesis, thereby impacting bone homeostasis. It competes with RANK for binding to RANKL, which reduces the availability of RANKL to interact with its receptor on osteoclast precursor cells. This competition leads to the inhibition of osteoclast differentiation and maturation.
The Wnt family: a group of signaling molecules that are involved in various developmental processes, including embryogenesis, as it regulates cell fate determination, postnatal development, and so on. Wnt proteins also play a significant role in regulating bone homeostasis. They can bind to two receptor complexes on the cell surface, leading to the activation of two main pathways: the β-catenin-dependent canonical pathway and the β-catenin-independent noncanonical pathway. The canonical pathway is distinguished by its reliance on the stabilization of β-catenin, whereas the non-canonical pathway operates independently of β-catenin.
-
β-catenin-dependent canonical pathway (Figure 8)—in this pathway, Wnt ligands bind to a receptor complex consisting of Frizzled and LRP5/6, which leads to the initiation of a signaling cascade where glycogen synthase kinase 3 stabilizes β-catenin → as a result, β-catenin is no longer targeted for degradation and accumulates in the cytoplasm, whereupon it translocates from there into the nucleus, leading to the activation of gene transcription program and initiation of the expression of target genes involved in various cellular processes, such as osteoblastogenesis, osteocyte formation, and bone tissue development.
-
β-catenin-independent non-canonical pathway—Wnt5a, expressed in osteoblast-lineage cells, is a main ligand for non-canonical Wnt signaling that binds to its receptor complex of Frizzled and ROR2, present on the surface of osteoclasts, and it has been shown to activate both the Wnt-Ca
2+ and Wnt-JNK pathways; Wnt5a was found to enhance RANKL-induced osteoclastogenesis due the upregulation of RANK expression in osteoclasts and activation of the Jun-N-terminal kinase MAPK pathway (JNK)
[12][27][28].
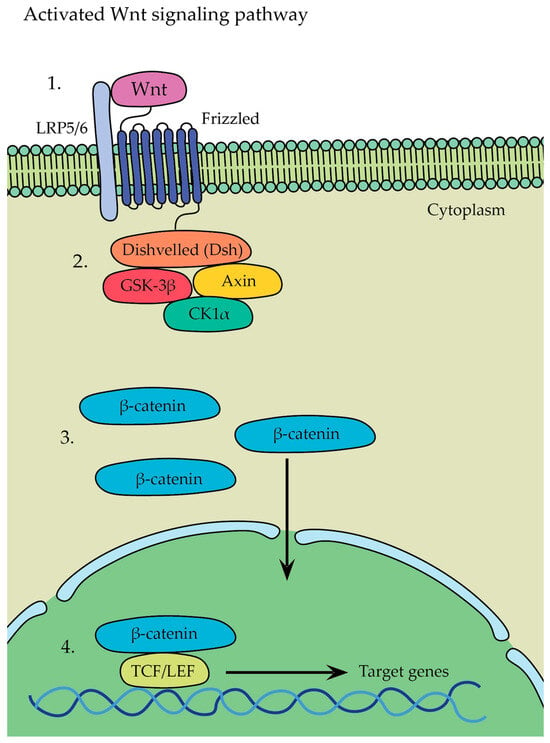
The Wnt ligand binds to Frizzled and LRP5/6 receptors located on the surface of osteoblasts (
Figure 7(1)). Frizzled interacts with Disheveled (Dsh), which inhibits GSK-3β activity, preventing the phosphorylation and degradation of β-catenin (
Figure 7(2)). The inhibition of GSK-3β by Dsh disrupts the GSK-3β/Axin/CK1α destruction complex and recruits it to the membrane, leading to the accumulation of β-catenin in the cytoplasm of the osteoblast. This accumulation allows β-catenin to translocate into the nucleus (
Figure 7(3)). Inside the nucleus, β-catenin forms a complex with various enhanceosome proteins, such as the LEF/TCF family of DNA-bound transcription proteins, initiating the transcription of specific target genes involved in osteoblast differentiation and bone formation (
Figure 7(4))
[29].
Figure 7. Activated Wnt signaling pathway: (1) the Wnt ligand binds to Frizzled and LRP5/6 receptors; (2) Frizzled interacts with Disheveled (Dsh); (3) β-catenin translocates into the nucleus; (4) β-catenin forms a complex with various enhanceosome proteins.
- 1.
-
Osteoclast-Secreted Factors
Atp6v0d2 (also known as d2 isoform of vacuolar ATPase, or v-ATPase, V0 domain): Atp6v0d2 is expressed in osteoclasts, and its role is to regulate the function and assembly of the v-ATPase complex in osteoclasts because proper v-ATPase activity is essential for osteoclast function and bone resorption. When Atp6v0d2 is deactivated in mice, there is a significant increase in bone mass. This is attributed to impaired osteoclasts and enhanced bone formation.
Semaphorin 4D: Sema4D is an axon guidance molecule belonging to the semaphoring family of cell-signaling molecules. Sema4D is involved in many cellular processes; apart from suppressing osteoblast differentiation, it plays a pivotal role in immune regulation and cancer bone metastases. It is expressed on osteoclasts and binds to its receptor Plexin-B1 on the surface of osteoblasts. After that, Plexin-B1 forms a receptor complex with the tyrosine kinase receptor ErbB2 (erythroblastic leukemia viral oncogene homolog 2) in osteoblasts. The binding of Sema4D to Plexin-B1 induces the phosphorylation of ErbB2, which activates its kinase function in osteoblasts. The Sema4D-Plexin-B1-ErbB2 complex induces activation of GTPase RhoA.
Sphingosine 1-phosphate (S1P): S1P is a lipid molecule formed by the phosphorylation of sphingosine by sphingosine kinase (SPHK) enzymes. When S1P binds to its receptor located on the surface of osteoblasts, the expression of RANKL is upregulated, resulting in the promotion of osteoclast differentiation
Collagen Triple Helix Repeat Containing 1 (CTHRC1): CTHRC1 is a protein that is predominantly expressed by osteoclasts during their bone-resorbing activity. Research has shown that the expression of the CTHRC1 gene can be influenced by exposure to hydroxyapatite and calcium. Furthermore, CTHRC1 has been shown to stimulate osteoblast differentiation, promoting bone formation. To understand the role of osteoclast-derived CTHRC1 in bone remodeling, the mice were bred to gain a conditional CTHRC1 knockout allele.
Complement component C (C3): osteoclast-derived C3 plays a crucial role in bone homeostasis, serving as a vital link between osteoclasts and osteoblasts. It has a significant effect on bone cell growth, differentiation, and development. Its bioactive fragment, C3a, enhances osteoclast formation. On the other hand, its receptor, C3aR, is expressed during the differentiation of human mesenchymal stem cells (MSCs) into mature osteoblasts, suggesting that C3a may play a role in both osteoblast differentiation (promoting osteoblastogenesis) and bone resorption. C3 deficiency has been associated with certain autoimmune conditions, such as systemic lupus erythematosus
[23][30].
- 2.
-
Examples of other factors influencing either osteoblast or osteoclast behavior
Sclerostin: The SOST gene, responsible for producing sclerostin, is predominantly expressed in osteocytes. When sclerostin interacts with the LRP5/6 and Frizzled co-receptors on the surface of osteoblasts, the Wnt/β-catenin signaling pathway is inhibited. This inhibition leads to a decrease in osteoblast differentiation, proliferation, and activity, ultimately resulting in reduced bone formation by osteoblasts.
MicroRNAs: microRNAs are a class of short, single-stranded, non-coding RNAs that have significant implications in various diseases, including cardio-vascular diseases, cancer, and osteoporosis. On the other hand, research has highlighted the potential of microRNAs as valuable tools for disease diagnosis or biomarkers.
Parathyroid hormone (PTH): this is an endocrine hormone secreted by the parathyroid glands. Its primary role is to maintain calcium metabolism in the body and help to regulate bone remodeling. When PTH is injected daily as a treatment for osteoporosis, it has a positive effect on bone health by stimulating bone formation. However, it is important to note that continuous infusion of PTH can have detrimental effects on bone health—extended exposure to high levels of PTH can lead to severe bone loss. Thus, since PTH has dual effects on bone, as it stimulates both bone formation and bone resorption, the resulting net effect of PTH on bone mass, either anabolic or catabolic, depends on the duration and periodicity of its exposure. Continuous treatment results in catabolic effects on the skeleton, while intermittent PTH administration results in osteoanabolic effects.
Transforming Growth Factor β1 (TGF-β1): in bone metabolism, TGF-β1 is a key player, influencing both osteoblasts and osteoclasts. It helps to regulate bone remodeling and maintain a delicate balance between the processes of bone resorption and bone formation throughout life.
2.2.5. Osteon Structure (Haversian System)
There are two types of bone tissue: solid cortical bone, which provides mechanical strength and most of the support for the body, and spongy trabecular bone, which is less dense than cortical bone and has a honeycomb-like structure. Osteons are the fundamental structural and functional units of cortical bone. They can be categorized into two main types
[31]:
-
Primary osteons—they are situated near primary bone, and they usually contain fewer circular lamellae compared to the secondary osteons,
-
Secondary osteons—also known as Haversian systems, they are the primary functional units of cortical bone that develop from primary osteons during bone remodeling. Each secondary osteon has concentric lamellae arranged around the central canal that houses blood vessels, nerves, and connective tissue, and these systems are distinguished from each other by clear cement lines that define secondary osteon boundaries.
Because of the ongoing bone-remodeling process, osteons are constantly being renewed. This leads to the presence of osteons at various levels of calcification within the adult compact bone. In general, osteons in the early stages of development are less mineralized and appear more transparent to X-rays compared to fully mineralized osteons that have reached their final stage of formation
[32]. When compared to trabecular bone, osteons carry out better physiological functions and dynamic adaptability. Within an osteon’s structure, both osteocytes and the lacunocanalicular network LCN play pivotal roles in sensing and transducing mechanical stress, rendering the bone highly responsive to external stimuli.
As already mentioned in the chapter called Cells of bone, osteocytes are the most common cell type in bones and represent fully matured bone cells that are embedded within the mineralized bone matrix. Osteocytes are distinctive in their stellate-shaped morphology, with numerous dendrites extending from their cell body. These dendritic projections enable extensive cellular connections and communication within the bone tissue.
When it comes to the formation of dendritic processes, E11/gp38 may induce and regulate the formation of these projections. This protein is selectively associated with osteocytes (not with osteoblasts) and appears in the developing dendritic processes of the osteocytes.
The lacunocanalicular network represents the intricate network formed by the spaces containing osteocyte cell bodies (lacunae) and their dendrites (canaliculi). It serves as a conduit for nutrient transportation and waste removal. Also, the fluid-filled spaces within the lacunocanalicular network enable osteocytes to detect mechanical cues, translating them into cellular signals that initiate cellular responses. This response involves the release of signaling molecules that influence bone remodeling, mineral homeostasis, etc.
[31][33].
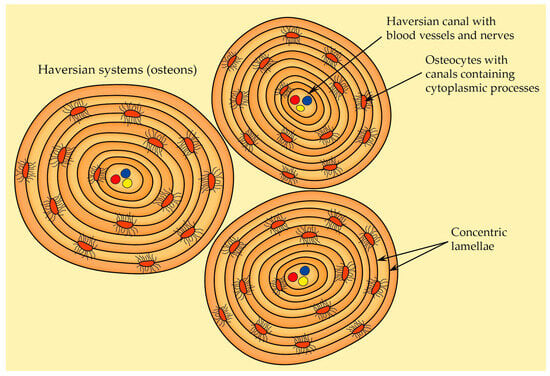
The most prominent and recognizable characteristic of osteons is the arrangement of concentric lamellae (
Figure 8). A mature secondary osteon generally consists of four to eight layers of these lamellae. They can be described as circular layers of bone tissue, and when observed under polarized light microscopy, the lamellae within osteons exhibit a distinct alternating pattern in appearance. Both lamellar bone and the osteonic bone (the type of bone found within osteons) reveal an alternation between isotropic and anisotropic lamellae. This phenomenon is a result of the intricate organization, thickness, and alignment of collagen fibers within bone tissue. The gaps between the Haversian systems are occupied by interstitial lamellae
[31][32].
Figure 8. The structure of Haversian system (osteon). Each osteon contains a single central Haversian canal encircled by concentric lamellae.
2.2.6. Bone Blood Supply
The long bone is normally supplied by four types of arteries: nutrient arteries, periosteal arteries, metaphyseal arteries, and epiphyseal arteries. One or two primary nutrient arteries penetrate the shaft through openings called nutrient foramina, which lead to nutrient canals. Inside these canals, the nutrient arteries do not divide, but within the medullary cavity, they divide into ascending and descending branches. These branches subsequently divide into smaller vessels, which can eventually reach the endosteal surface. Close to the epiphyses, these vessels can anastomose with terminal branches from metaphyseal (originating from nearby systemic vessels) and epiphyseal arteries (originating from networks of blood vessels that are formed around the bone surfaces adjacent to the joints)
[1].
Haversian canals are cylindrical spaces that are enclosed by the innermost layer of osteon lamellae and run parallel to the primary axis of an osteon (this inner surface of the innermost lamella is covered by flat cells surrounded by densely packed collagen fibers). These canals house nerve fibers and blood vessels, facilitating the nourishment of osteocytes. Typically, one to two blood vessels can be observed within a Haversian canal. Additionally, it serves as the initial location for the remodeling of osteons
[31].
2.3. Overview of Bone Types and Their Organization
Woven bone lacks the distinct layers seen in lamellar bone and is notable for its random arrangement of type I collagen fibers. This type of bone is the initial form that appears during embryonic development and serves as a framework—subsequently, layers of lamellar bone are added onto this framework. Thus, woven bone is typically temporary, and in adults, it is replaced by the more organized lamellar bone, with a few exceptions, such as near the sutures of the skull and within certain tendon attachments
[8][34]. There are two different groups of osteoblast cells contributing to the creation of woven and lamellar bone. The first, mesenchymal osteoblasts, surround themselves with collagen fibers arranged randomly, forming woven bone. The second group, surface osteoblasts, arrange themselves in a linear pattern on the surface of woven bone, producing parallel-fibered lamellar bone. This whole process can be divided into the following four stages
[34]:
-
Stage I—the initial differentiation of pre-osteoblasts from their stem (mesenchymal) cells.
-
Stage II—mesenchymal osteoblasts encircle themselves with matrix fibers oriented in a random manner.
-
Stage III—the woven matrix serves as a scaffold where surface osteoblasts start synthesizing bone with a parallel-fibered lamellar structure.
-
Stage IV—gradual reduction in woven bone and progression from woven to lamellar bone.
Lamellar bone, also known as secondary or mature bone, earns its name because it replaces the woven or primary bone, which is gradually resorbed after birth through bone remodeling (however, under certain pathological circumstances, e.g., bone tumors, woven bone can reappear).
2.4. Overview of Macroscopic Properties of Bone
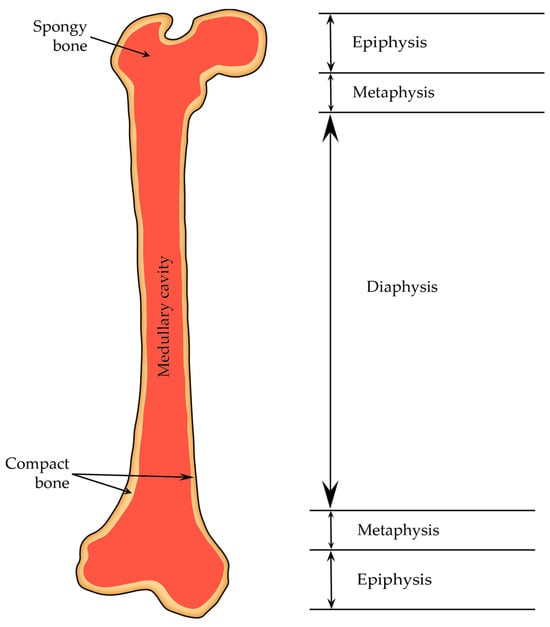
2.4.1. Diaphysis and Epiphyses
A typical adult long bone is divided into the following parts: a central cylindrical core known as the diaphysis and two wider ends called the epiphyses (they are wider than the diaphysis because they encompass joints covered by articular cartilage, and sustaining consistent loads require more substantial areas of this cartilage than bone alone). The diaphysis is linked to each epiphysis through conical regions referred to as the metaphysis (
Figure 9)
[11].
Figure 9. Structure of a long bone. The distal and proximal ends (epiphyses) are separated from the shaft (diaphysis) by metaphyses.
2.4.2. Periosteum and Endosteum
The periosteum is composed of two layers: an outer fibrous membrane (characterized by irregular and dense connective tissue that consists of fibroblasts, collagen and elastin fibers, and a network of nerves and microvessels) and an inner cellular layer, or cambium layer, that directly contacts the bone surface (composed of osteoprogenitor cells; hence, the inner cellular layer is also referred to as the osteogenetic layer. Within the inner layer, osteoblasts are present in young bones, and in the case of adult bones, osteoblasts might not be consistently present, but they become active whenever needed) [35][36]. The inner cambium layer reaches its maximum thickness in the fetus and gradually becomes thinner with age. In adulthood, the density of blood vessels and cells decreases; thus, it becomes indistinguishable from the fibrous layer above it [37].
The periosteum is absent in regions where tendons or ligaments attach to bone, on bone ends covered by articular cartilage, on the surface of sesamoid bones, etc. Additionally, because of its rich blood supply, the periosteum contains a significant number of endothelial pericytes (cells that are in direct contact with capillary endothelial cells and can transform into various cell types, including osteoblasts). These cells could potentially act as an additional reservoir of osteoprogenitor cells
[35][37].
The endosteum is a membrane that covers the inner surfaces of the bone. It lines the Haversian canal as well as all the internal spaces within the bone. The endosteum is composed of a layer of flattened osteoprogenitor cells and type III collagen fibers, also known as reticular fibers. Notably, the endosteum is thinner compared to the periosteum
[36].
2.4.3. Bone Marrow
Bone marrow is a vital organ for the process of hematopoiesis (which is the production of various types of blood cells). This continuous production of blood cells is essential for various physiological processes, including oxygen transport, immune response, and blood clotting. The bone marrow also provides a specialized and unique environment that supports the formation and development of blood cells. This microenvironment, often referred to as the hematopoietic niche, consists of various cellular and molecular components that interact to regulate the balance between stem cell self-renewal and differentiation
[38].
Bone marrow is found in the cavities of bones. In newborns, all bone marrow is red and actively involved in blood cell production (red bone marrow represents myeloid tissue and is rich in hematopoietic cells). However, as a child grows, much of the red bone marrow gradually transitions to yellow marrow (it contains more fat cells—adipocytes— and fewer blood-forming cells; thus, it is considered less active in terms of blood cell production and primarily serves as a storage site for adipose tissue).
2.5. Bone Development and Growth
Skeletal development and bone ossification (osteogenesis) start with the aggregation of mesenchymal cells into condensed structures. These condensations serve as the initial foundation for bone formation
[11]. Bone ossification specifically starts around the sixth to seventh weeks of embryonic growth and persists until approximately the age of twenty-five, although it can slightly differ from person to person
[39].
There are two types of bone ossification: intramembranous and endochondral. Both processes initiate from a precursor mesenchymal tissue, but their transformations into bone are different. In intramembranous ossification, mesenchymal tissue is directly converted into bone, giving rise to flat bones of the skull and jaws, as well as the scapula or clavicle. On the other hand, endochondral ossification starts with mesenchymal tissue turning into an intermediate cartilage stage, which is eventually substituted by bone. This process contributes to the axial skeleton’s remaining parts and the formation of long bones
[39].
2.6. Bone Remodeling
Bone remodeling is a dynamic mechanism where bones change their shape due to physiological cues or mechanical pressures. This leads to a gradual adaptation of the skeleton to the various forces it encounters. Bones tend to increase in width with aging due to the addition of new bone on the outer surface (periosteal apposition) and the removal of old bone from the inner surface (endosteal resorption). Wolf’s law describes the concept that the structure of long bones adapts to handle the specific stresses they experience
[7].
Bone remodeling starts before birth and continues throughout an individual’s life. It encompasses two fundamental processes: bone formation by osteoblasts and bone resorption by osteoclasts. These activities of bone formation and resorption are not always tightly coupled (except for the remodeling process itself). Even though bone formation and resorption can occur independently on different surfaces, they are not entirely independent—both processes occur concurrently throughout the skeleton and are coordinated to shape bones effectively
[7][40].
Bone remodeling can be categorized as either targeted or stochastic. In targeted remodeling, a specific local signal prompts osteoclasts to initiate remodeling at a given site (this could be due to microdamage or osteocyte apoptosis, which can sometimes be related to each other when microdamage can lead to osteocyte apoptosis; other factors, such as high mineralization, might also play a role). Stochastic remodeling, on the other hand, is characterized by random osteoclast activity that does not rely on specific signals. It is believed to contribute more to calcium regulation
[40][41].