Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amira Abdelrasoul | -- | 3301 | 2024-01-19 04:51:13 | | | |
| 2 | Rita Xu | Meta information modification | 3301 | 2024-01-19 06:33:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ehsani, M.; Westphalen, H.; Doan, H.; Lohi, A.; Abdelrasoul, A. Faba Bean Protein Purification Using Membrane Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/54077 (accessed on 07 February 2026).
Ehsani M, Westphalen H, Doan H, Lohi A, Abdelrasoul A. Faba Bean Protein Purification Using Membrane Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/54077. Accessed February 07, 2026.
Ehsani, Masoume, Heloisa Westphalen, Huu Doan, Ali Lohi, Amira Abdelrasoul. "Faba Bean Protein Purification Using Membrane Technology" Encyclopedia, https://encyclopedia.pub/entry/54077 (accessed February 07, 2026).
Ehsani, M., Westphalen, H., Doan, H., Lohi, A., & Abdelrasoul, A. (2024, January 19). Faba Bean Protein Purification Using Membrane Technology. In Encyclopedia. https://encyclopedia.pub/entry/54077
Ehsani, Masoume, et al. "Faba Bean Protein Purification Using Membrane Technology." Encyclopedia. Web. 19 January, 2024.
Copy Citation
Plant-based proteins are gaining popularity because of their appeal to vegetarians and vegans, alignment with scientific and regulatory recommendations, and the environmental impact associated with livestock production. Several techniques are employed for the separation, isolation, and purification of plant-based proteins including membrane-based separation, diafiltration, centrifugation, chromatography, electrophoresis, micellar precipitation, and isoelectric precipitation.
membrane technology
faba bean
isolate
protein purification
1. Introduction
It has been proven that proteins play a vital role in the growth and development of the body and are essential for a healthy lifestyle. Increasing awareness about the importance of high-quality proteins in the diet has led researchers and nutritionists to seek environmentally friendly and sustainable protein sources. Proteins are found in both animals and plants. However, there are a number of issues associated with animal protein, including cost, supply, direct environmental impact, biodiversity loss, and even human health issues. In addition, there has been a growth in the population of vegetarians, vegans, and people who have difficulty relying on animal proteins [1][2][3][4][5]. In terms of environmental impact, a significant amount of greenhouse gas emissions (GHG) is attributable to the modern food system, which constitutes 21 to 37% of total greenhouse gas emissions. According to GHG life cycle assessments, livestock production accounts for 18% of anthropogenic greenhouse gas emissions [6]. In this regard, a wide range of plant-based proteins are increasingly being utilized in human diets as economical and versatile substitutes for animal proteins. Alternative dairy and meat products made from plant-derived proteins can meet the same nutritional needs at considerably good prices while preserving forests and reducing greenhouse gas emissions [7]. In addition to their anti-diabetic properties, plant proteins are low in calories and fat, and have a high level of antioxidant activity, essential amino acids, minerals, and vitamins [8].
Industrial-scale production and commercialization of plant-derived proteins have become commonplace, such that they are widely used in edible products including food supplements, edible coatings, food stabilizers, bioactive peptides (BAPs), and hydrogels [1][7]. Legumes, soy proteins, lentils, and cereals are the most common plant-based protein sources. Proteins can be isolated and purified using a wide range of approaches, determined by their physicochemical properties and the biological characteristics of their sources.
A growing market for plant-based proteins has led to many studies on legumes, including faba beans. Faba beans are the third most popular legume after soy and peas and like other legumes, they provide a high amount of lysine-rich protein. In addition to its essential nutrients, the faba bean is one of the most affordable protein sources in developing countries [9][10][11]. Although faba beans are becoming increasingly popular as a source of protein, some of their low functional properties and antinutritional compounds such as pyrimidine glycosides (vicine and convicine), condensed tannins, and protease inhibitors limit their use. Thus, various processing methods would be required to remove or degrade antinutritional compounds from faba beans in order to improve their features and ensure their safety for consumption [12][13][14][15].
Protein purification takes place to achieve high purity standards, concentration enrichment, inhibition of undesired catalysis, meet product specifications, improve protein stability, and minimize protein denaturation. There are a number of methods that can be utilized to purify proteins, including membrane-based separation, diafiltration, centrifugation, chromatography, electrophoresis, micellar precipitation, and isoelectric precipitation [1][16][17][18][19]. Although these techniques have been used for protein purification for years, they still have some limitations, such as: scale-up challenges, high solvent consumption, chemical/biological disposal, low purity, and potential loss of proteins due to precipitation or elution [20][21][22].
Membrane technology has been proven to be one of the most sustainable and cost-effective approaches for protein purification/recovery [23]. The use of membrane-based processes has gained growing attention in recent years owing to their ability to separate and purify proteins based on their size and charge. It has been found that pressure-driven processes such as microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF) are the most promising techniques among the membrane-based separation processes [24][25][26][27][28][29]. Membrane filtration possesses beneficial features such as its ability to work at mild operational conditions (low temperature and pressure) without phase changes, bioactivity preservation, molecular separation, high separation efficiency, low footprint and chemical consumption, high protein recovery yield, and easy scale-up, which make it suitable for a broad range of applications [30][31][32].
2. Faba Bean Protein Isolate
Faba bean isolate refers to a protein extracted from faba beans, which is used as an ingredient in various food products. The goal of using plant-based protein isolates in food products is to create more sustainable and environmentally friendly food choices by reducing reliance on animal protein sources [7][33]. Faba bean isolate consists of approximately 80–95% crude protein [34] and has two main protein fractions: globulin and albumin. The globulin fraction is composed of vicilin and legumin, and the faba bean protein isolate consists of this fraction [35]. Multari et al. reports that the production of protein isolates and concentrates can significantly improve the nutritive value of legumes [36] and the protein structure plays a very important role in its functional properties. Various chemical, enzymatic, and physical treatments can be used to modify protein structures and tailor protein isolates to specific applications [35].
Because the composition of faba beans includes antinutritional components, the production of the protein isolate is a promising approach to produce high-quality functional nutritional foods and supplements free of favism-inducing components [36][37]. However, the manufacturing process, that is, the method and conditions of isolation, is a determinant factor on the composition and functional properties of the protein isolate, such as solubility, foam expansion, gelation capacity, emulsifying capacity, and others [36].
According to GEA, protein isolate manufacturing consists of three main processes: extraction, purification, and drying, as shown in Figure 1. For the first step, the alkaline condition promotes the dissolution of protein fractions in the aqueous extract, yielding a vegetable isolate with a protein content of 80% [38]. However, various techniques can be used besides alkaline extraction, and different extraction techniques can yield different properties. Afterwards, by using an appropriate technique such as decantation or isoelectric precipitation, the aqueous extract can be separated from other solids. The proteins that are dissolved in water can be precipitated and separated. Further dilution, pH adjustments, thermal treatment, and finally drying are necessary to obtain the protein isolate powder [38].
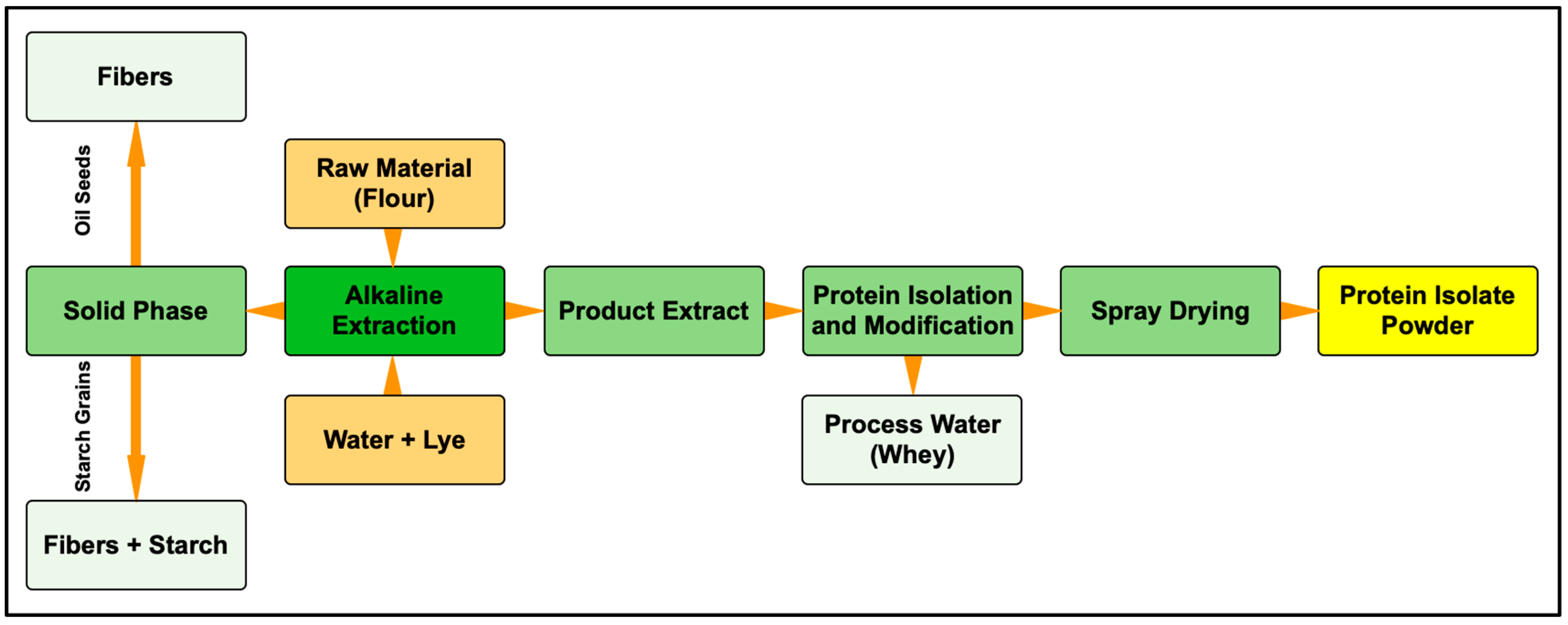
Figure 1. Example of protein isolate process.
Figure 2 shows the protein isolate process based on alkaline extraction used by Vioque et al. [37]. According to the authors [37], the process yielded 92% protein isolate with a high oil absorption capacity; favism-inducing components were almost completely eliminated. Furthermore, by-products presented a great potential use in the food industry.

Figure 2. Production process for Vicia faba protein isolates.
Martínez-Velasco et al. investigated the effect of high-intensity ultrasound treatment of faba bean proteins. Physicochemical and surface properties were analysed, as well as foaming ability, stability, morphology, bubble size, and rheology foams. Lower interfacial tension, zeta potential and viscosity, and higher solubility were observed. Furthermore, the structure and relative digestibility of the faba bean protein isolate were studied. According to the response surface methodology, an optimized faba bean protein isolate was obtained under amplitude of 72.7% for 17.3 min [39].
3. Membrane Technology for Purification of Plant-Based Protein Isolate
Following the enrichment procedure, proteins need to be purified. There has been an increasing implementation of membrane technologies in the industrial processing of food products/by-products. Membrane separation processes have been adopted as purification/concentration procedures in the production of plant-based proteins and protein nanofibrils due to their selective separation, simple operational conditions, and easy automation [40][41][42][43]. The difference between protein isolates and concentrates is the protein content in each one. Protein isolates should have at least 70% of protein content and protein concentrates at least 90% of protein content, both on a dry basis [44]. Moreover, membrane separation can provide specific benefits for the purification of different plant-based proteins, including preserved protein properties, low recovery cost, and high recovery yields and purity [31][44][45][46][47].
3.1. Advantages of Membrane-Based Separation in Plant-Based Protein Purification
In the realm of plant-based protein purification, membrane-based separation offers a number of distinct advantages over other methods. Membrane-based separation has been proven to be a viable technique for large-scale production in industrial applications [48]. Membrane filtration can be used for the separation/purification of soluble proteins, based on their size, through a pressure-driven process (microfiltration (MF), ultrafiltration (UF) and nanofiltration (NF)) without altering their structure (preserving their functional and nutritional properties) [32][49][50][51]. Ultrafiltration (mean pore size of 1–100 nm) is the most effective process to purify/isolate proteins and other macromolecules, while microfiltration is ideal for separating fine particles sized 0.1–10.0 μm [24]. Nanofiltration (mean pore size of 0.2–2 nm) can also be employed in the separation/purification of the smaller proteins and some types of peptides/amino acids. Wang et al. [52] employed UF and NF processes for the purification of glutathione after extraction. According to their findings, the combination of UF and NF processes showed promising results for the concentration/purification of glutathione from yeast extracts. UF was used first to concentrate glutathione in the permeate stream (larger particles were separated). Subsequently, the NF process was applied to the glutathione-rich solution obtained from the UF permeate to purify glutathione.
The efficacy of protein hydrolysates can be enhanced through the use of UF. The application of appropriate UF membranes would produce highly purified, food-grade proteins with a desired molecular size [53]. The functional properties of faba bean protein isolates were found to be inadequate for use in food applications owing to their low solubility. In this regard, Eckert et al. [54] aimed to enhance the solubility and functional properties of faba bean proteins through the application of the UF technique following enzymatic hydrolysis. UF membranes with two different molecular weight cut-offs of 10 and 5 kDa have been used for the fractionation of faba bean hydrolysates. According to their results, the use of ultrafiltration resulted in enhanced foaming and oil holding capacity, as well as significantly improved emulsifying capacity. Therefore, it was deduced that ultrafiltration following enzymatic hydrolysis is a viable approach for markedly enhancing the solubility and functional characteristics of faba bean proteins. The protein composition of faba beans reveals that a significant portion, ranging from 69 to 78% of the storage proteins, is comprised of salt-soluble globulins, which are primarily located in the membrane-bound protein bodies. In order to produce clean-label proteins that are low in salt or free of added salt, filtration techniques such as dialysis, ultrafiltration, or diafiltration could be employed over wet processing, which can denature proteins through heat and pH changes [34][55][56]. Membrane-based separation presents a promising alternative to the conventional acid-leaching process for protein separation and isolation. This approach involves the use of a variety of membranes that selectively separate and extract components based on their molecular sizes [56]. As reported by Vose [57], ultrafiltration was utilized to isolate protein from faba beans, and it resulted in a protein yield of 94% (w/w). The protein obtained from this process exhibited comparable foaming and emulsifying properties to those obtained from isoelectric precipitation, which yields 91% (w/w) of proteins. In another study by Jeganathan et al. [58], ultrafiltration and dialysis were employed to isolate faba bean proteins without using alkali/acid and thermal treatments (clean-label proteins). The results demonstrated that protein isolates obtained using a cellulose membrane with a molecular weight cut-off of 6–8 kDa, following water extraction, at 35 °C and a solvent/feed (S/F) ratio of two, had a higher protein yield, recovery rate, and protein content, as compared to the protein concentrates that have been produced through alkali extraction followed by acid precipitation. However, due to the impracticality of performing large-scale dialysis for the extraction, ultrafiltration (Nuetch filter, LJ Star W.T. Maye) was used as a substitute. High-tannin faba bean dehulled flour was subjected to water extraction at a S/F ratio of three, followed by ultrafiltration and spray drying, which yielded a protein fraction of 16.46 ± 0.12% with a purity of 82.80 ± 0.03% and recovery rate of 40.08 ± 0.28%.
3.2. Diverse Applications of Ultrafiltration in Protein Processing
One specific membrane technology that has gained significant prominence in protein processing is ultrafiltration. Its diverse applications extend across various facets of protein purification and processing, offering unique advantages for different protein sources and end products. Aside from in faba bean isolation, ultrafiltration was used in a study on protein enrichment from ryegrass and alfalfa, and it was compared to coagulation/centrifugation. Despite the fact that crude protein yields were almost identical between these methods, ultrafiltration resulted in a higher protein solubility and 14% higher crude protein recovery [59]. In another study, Vishwanathan et al. assessed the ability of MF and UF membranes of various pore sizes/MWCO to eliminate non-protein substances from okara—a by-product of the soymilk production—and soy protein extract [44]. Figure 3 shows the lab-scale procedure utilized in the study. The system consisted of a cross-flow flat sheet membrane operated in batch mode. The results obtained by Vishwanathan et al. indicated that both MF and UF are feasible processes for the purification/concentration of okara and soy protein concentrates. The protein content in okara extract increased by approximately 13% for both processes, reaching about 80%. Soy extract presented better protein content improvement, reaching 85%. All membranes tested had a similar performance, but the larger pore size offered reduced processing time as the result of a higher average flux. Overall, the study indicated that membrane technology can be successfully applied to produce protein concentrates without compromising protein properties and adding value to underutilized products such as okara [44]. In another study, a UF membrane was used in the purification of Lupin proteins following liquid/solid extraction at various pH levels by Albe-Slabi et al. [60]. The protein retention rate and permeate flux were measured using an Akta Flux®6 system coupled with a hollow fiber cartridge with different molecular weight cut-offs (MWCO) (10, 30, 100, and 300 kDa). The UF membrane with MWCO of 10 kDa retained proteins completely with a flux of 0.09 mL/min·cm2. It has also been reported that even 300 kDa MWCO results in 97% protein retention (flux of 0.11 mL/min·cm2). After washing with five diafiltration volumes (DV) using ultrapure water, the rejected proteins were collected and freeze-dried.

Figure 3. Scheme for processing of SPC/OPC using membrane technology.
Soybeans have played an important role in the human diet as a rich source of protein; and its functional forms such as flours, isolates, and concentrates became very popular. Traditionally, the proteins from the soybean were extracted using defatted flour with acid or alcohol, followed by a separation process such as centrifugation or filtration [45]. Kumar et al. adopted a membrane separation process to produce soybean protein concentrates as shown in Figure 4. Using a polyvinylidene difluoride membrane (18 kDa), ultrafiltration (UF) was conducted in batch mode while diafiltration (DF) was operated continuously. According to the study, UF presented higher yields than conventional processes but still presented limitations. The retention of salts in the retentate, high viscosity, and higher solids losses in the permeate at high concentration factors limited the protein level to 60–70%. To overcome these limitations, UF was combined with DF, and optimum results were obtained with the following configuration: UF-DF-UF. A higher protein content was obtained (90%), while sugars were almost completely removed [45].
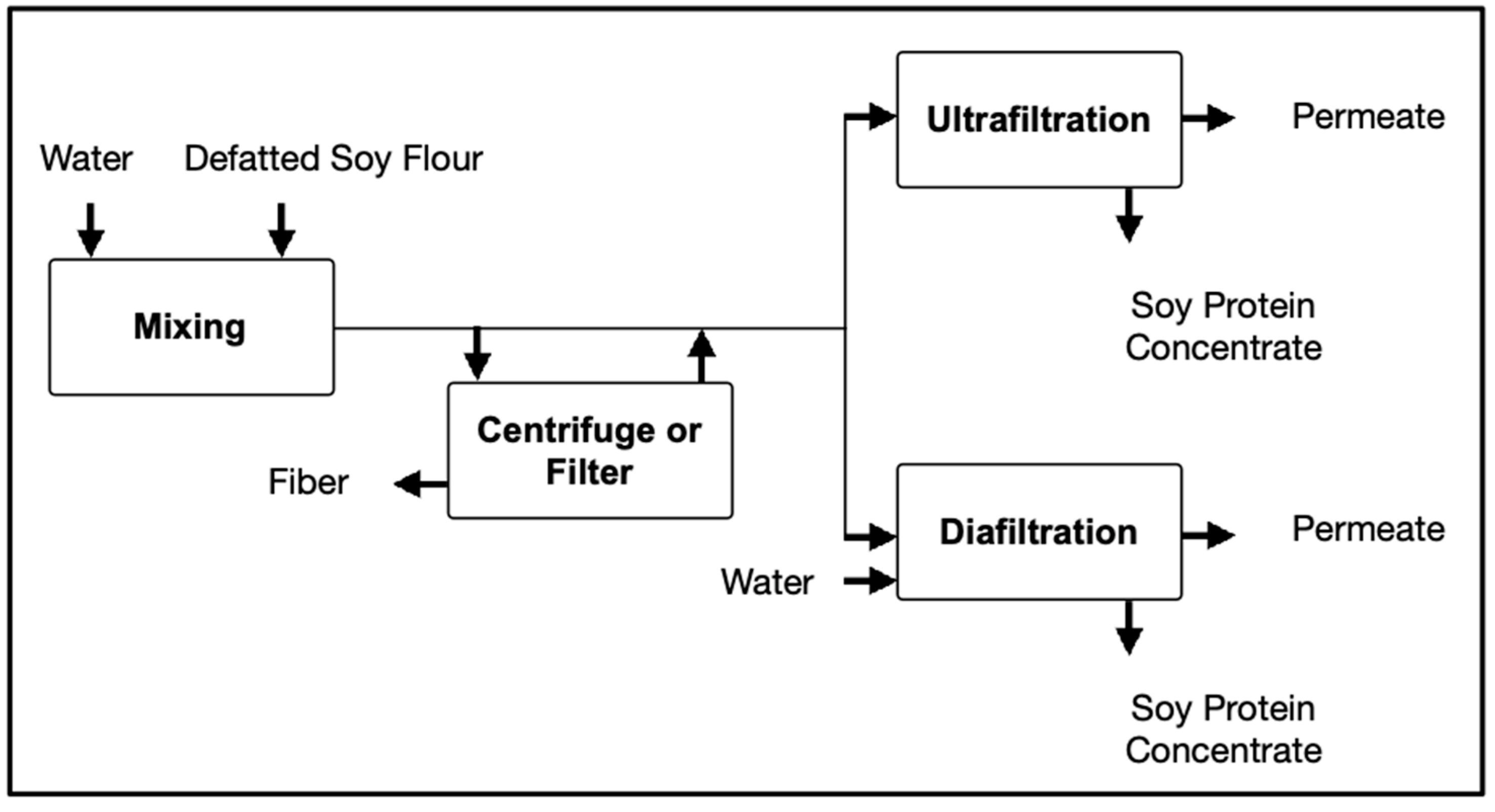
Figure 4. Schematic of ultrafiltration process for soy protein concentrates. UF, ultrafiltration concentration; DF, diafiltration.
Mondor et al. investigated the impact of four different sequences of UF and DF to purify soy protein extract with pH 6. The pH 6 extract was obtained via electro-acidification and the filtration was performed using a polysulfone hollow fibre membrane. The study concluded that the UF/DF sequence had a significant impact on membrane fouling, permeate flux, and protein concentrate properties such as ash and phytic acid content and solubility. Also, the most effective process which yielded a higher protein content was the one in which DF was performed continuously with a more concentrated solution. However, it was also the one more severely affected by membrane fouling [46]. Hernández-Marín et al. [61] used a combination of ultrafiltration and diafiltration (5 kDa membranes) for the purification of Huauzontle seed protein after alkaline extraction. The protein isolation/purification process was completed successfully with about 66% purity for precipitated protein isolate (SPI). To improve purity, they repeated the process with a 10 kDa membrane. A membrane with larger pore sizes was used to separate compounds with higher molecular weights and allow the transport of all proteins to the permeate, resulting in a 78% SPI purity. Taherian et al. compared properties of commercial and membrane-processed pea protein isolates from yellow peas. Four pea protein isolates were obtained using KCl extraction followed by UF and DF. The level of phytic acid was reduced in the range of 28–68% and functional properties were enhanced [62]. UF and DF following alkaline extraction have also been used in the production of protein isolates from Camelina sativa, as reported by Sarv et al., yielding a protein content of 67% in the protein isolate [63]. Boye et al. compared the functional properties of the protein concentrate from pea, chickpea, and lentil using UF/DF and isoelectric precipitation [64]. Figure 5 summarizes the process used in the study to obtain the protein extracts. The membrane separation process yielded products with a higher protein content (69.1–88.6%, w/w) compared to isoelectric precipitation (63.9–81.7%, w/w), and all concentrates presented good functional properties (in terms of solubility, water holding capacity, emulsifying properties, and foam stability) with expected variability among different legumes [64].

Figure 5. Schematic of the process used for the pilot scale production of the pulse protein concentrates.
Membrane dialysis has also been used in protein purification to improve separation efficiency and yield. In a study on protein extraction from soybean, Khan et al. [65] used membrane dialysis to remove the salt from the trypsin inhibitor protein extract. The supernatant from protein extraction was subjected to centrifugation followed by ammonium sulfate precipitation. Membrane dialysis was then used to successfully purify the centrifuged pellets. Hansen et al. [66] also used dialysis for pea protein purification following alkaline solubilization, isoelectric precipitation, and salt solubilization. The results (protein purity, yield, and ash content) were compared with those obtained from purification via ultrafiltration and the combination of dialysis and ultrafiltration (Vivaflow® membrane with 3 kDa). It was found that ultrafiltration did not completely remove salt from the proteinaceous supernatant, leaving a low protein purity and high ash content. In contrast, dialysis increased the protein purity and decreased the ash content (still noticeable). The most favorable results were obtained from the combination of UF and dialysis, such that protein purity, yield, and ash content reached 92.8%, 72%, and 1.56%, respectively.
Plant-based peptides (short chains of amino acids [67]) have been widely used in the food, pharmaceutical, and cosmetic industries [68][69][70]. Membrane technology has made it possible to fractionate/isolate peptides from complex feedstocks based on their electric charge, size, or molecular weight [71][72]. According to Nuchprapha et al. [73] ultrafiltration membranes with 3, 5, and 10 kDa molecular weight cut-offs were employed sequentially to separate the peptides from protein hydrolysates (from longan seeds). Peptides with four different ranges of molecular weight cut-offs were separated (>10 kDa, 5–10 kDa, 3–5 kDa, and <3 kDa). According to their research, ultrafiltration improved the purification efficiency of small peptides which also had the most angiotensin-converting enzyme inhibition (ACEI) activity (ACEI helps in controlling hypertension and promoting cardio protection). The combination of electrodialysis with ultrafiltration membranes (EDUF) proved to be an effective method to fractionate peptide mixtures with charged solutes that have a similar molecular weight. This method has been employed in several studies for bioactive peptide separation [74][75][76][77][78]. In EDUF, electrodialysis and electrophoresis principles are combined using ultrafiltration membranes, which serve as a molecular barrier and separate the components by the electric potential difference [74]. Firdaous et al. [79] employed EDUF for the separation of bioactive peptides from a plant-based (alfalfa) protein hydrolysate. It has been reported that EDUF was able to overcome some of the fouling issues associated with conventional pressure-driven processes and also separate/concentrate charged peptides simultaneously at a transport rate of up to 7.3 g/m2·h. More recently, González-Muñoz et al. [80] used EDUF for the separation of peptides from quinoa. The results showed no significant fouling development, and peptide fractionation from quinoa hydrolysate was proven successful as an antihypertensive and antidiabetic food alternative. In another study conducted by Doyen et al. [81] it was reported that using ultrafiltration facilitated the separation of peptides with lower molecular weights (300–500 Da).
References
- Mistry, K.; Sardar, S.D.; Alim, H.; Patel, N.; Thakur, M.; Jabbarova, D.; Ali, A. Plant Based Proteins: Sustainable Alternatives. Plant Sci. Today 2022, 9, 820–828.
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the Plant Protein Extraction: Mechanism and Recommendations. Food Hydrocoll. 2021, 115, 106595.
- Bilek, S.E. Plant Based Protein Sources and Extraction. Curr. Investig. Agric. Curr. Res. 2018, 2, 169–171.
- Dugardin, C.; Cudennec, B.; Tourret, M.; Caron, J.; Guérin-Deremaux, L.; Behra-Miellet, J.; Lefranc-Millot, C.; Ravallec, R. Explorative Screening of Bioactivities Generated by Plant-Based Proteins after In Vitro Static Gastrointestinal Digestion. Nutrients 2020, 12, 3746.
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent Advances in the Recovery Techniques of Plant-Based Proteins from Agro-Industrial By-Products. Food Rev. Int. 2021, 37, 447–468.
- Munialo, C.D.; Stewart, D.; Campbell, L.; Euston, S.R. Extraction, Characterisation and Functional Applications of Sustainable Alternative Protein Sources for Future Foods: A Review. Futur. Foods 2022, 6, 100152.
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-Based Proteins and Their Multifaceted Industrial Applications. Lebensm.-Wiss. Technol. 2022, 154, 112620.
- Hussain, M.; Qayum, A.; Xiuxiu, Z.; Liu, L.; Hussain, K.; Yue, P.; Yue, S.; Koko, M.Y.; Hussain, A.; Li, X. Potato Protein: An Emerging Source of High Quality and Allergy Free Protein, and Its Possible Future Based Products. Food Res. Int. 2021, 148, 110583.
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional Composition, Anti-Nutritional Factors, Pretreatments-Cum-Processing Impact and Food Formulation Potential of Faba Bean (Vicia faba L.): A Comprehensive Review. Lebensm.-Wiss. Technol. 2021, 138, 110796.
- Liu, C.; Pei, R.; Heinonen, M. Faba Bean Protein: A Promising Plant-Based Emulsifier for Improving Physical and Oxidative Stabilities of Oil-in-Water Emulsions. Food Chem. 2022, 369, 130879.
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A Review of Nutritional Profile and Processing of Faba Bean (Vicia faba L.). Legum. Sci. 2022, 4, e129.
- Purves, R.W.; Khazaei, H.; Vandenberg, A. Quantification of Vicine and Convicine in Faba Bean Seeds Using Hydrophilic Interaction Liquid Chromatography. Food Chem. 2018, 240, 1137–1145.
- Khazaei, H.; Purves, R.W.; Song, M.; Stonehouse, R.; Bett, K.E.; Stoddard, F.L.; Vandenberg, A. Development and Validation of a Robust, Breeder-Friendly Molecular Marker for the vc—Locus in Faba Bean. Mol. Breed. 2017, 37, 140.
- Boukid, F.; Castellari, M. How Can Processing Technologies Boost the Application of Faba Bean (Vicia faba L.) Proteins in Food Production? eFood 2022, 3, e18.
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating Vicine and Convicine, the Main Anti-Nutritional Factors Restricting Faba Bean Usage. Trends Food Sci. Technol. 2019, 91, 549–556.
- Chen, J.; Yu, B.; Cong, H.; Shen, Y. Recent Development and Application of Membrane Chromatography. Anal. Bioanal. Chem. 2022, 415, 45–65.
- Sun, Z.; Chi, Q.; Sun, L.; Liu, Y. Protein Extraction from Microalgae Residue and Nutritional Assessment. Bioprocess Biosyst. Eng. 2022, 45, 1879–1888.
- Geoffroy, T.R.; Bernier, M.E.; Thibodeau, J.; Francezon, N.; Beaulieu, L.; Mikhaylin, S.; Langevin, M.E.; Lutin, F.; Bazinet, L. Semi-Industrial Scale-up of EDUF Technology for the Electroseparation of Bioactive Cationic Peptides: Impact of Process Parameters and Cell Configurations on Eco-Efficiency. J. Memb. Sci. 2022, 641, 119856.
- Schoenbeck, I.; Graf, A.M.; Leuthold, M.; Pastor, A.; Beutel, S.; Scheper, T. Purification of High Value Proteins from Particle Containing Potato Fruit Juice via Direct Capture Membrane Adsorption Chromatography. J. Biotechnol. 2013, 168, 693–700.
- French, D. Advances in Clinical Mass Spectrometry, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 79.
- Nikolov, Z.L.; Woodard, S.L. Downstream Processing of Recombinant Proteins from Transgenic Feedstock. Curr. Opin. Biotechnol. 2004, 15, 479–486.
- Liang, T.; Lu, H.; Ma, J.; Sun, L.; Wang, J. Progress on Membrane Technology for Separating Bioactive Peptides. J. Food Eng. 2023, 340, 111321.
- Gifuni, I.; Lavenant, L.; Pruvost, J.; Masse, A. Recovery of Microalgal Protein by Three-Steps Membrane Filtration: Advancements and Feasibility. Algal Res. 2020, 51, 102082.
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-Based Techniques for the Separation and Purification of Proteins: An Overview. Adv. Colloid Interface Sci. 2009, 145, 1–22.
- Marson, G.V.; Belleville, M.P.; Lacour, S.; Hubinger, M.D. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2021, 11, 23.
- Zaky, A.A.; Abd El-Aty, A.M.; Ma, A.; Jia, Y. An Overview on Antioxidant Peptides from Rice Bran Proteins: Extraction, Identification, and Applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1350–1362.
- Blais, H.N.; Schroën, K.; Tobin, J.T. A Review of Multistage Membrane Filtration Approaches for Enhanced Efficiency during Concentration and Fractionation of Milk and Whey. Int. J. Dairy Technol. 2022, 75, 749–760.
- Ozturk, G.; Liang, N.; Bhattacharya, M.; Robinson, R.C.; Shankar, S.; Huang, Y.-P.; Paviani, B.; Taha, A.Y.; Barile, D. Glycoproteomic and Lipidomic Characterization of Industrially Produced Whey Protein Phospholipid Concentrate with Emphasis on Antimicrobial Xanthine Oxidase, Oxylipins and Small Milk Fat Globules. Dairy 2022, 3, 277–302.
- Sun, Y.; Yi, F.; Li, R.H.; Min, X.; Qin, H.; Cheng, S.Q.; Liu, Y. Inorganic–Organic Hybrid Membrane Based on Pillararene-Intercalated MXene Nanosheets for Efficient Water Purification. Angew. Chemie—Int. Ed. 2022, 61, e202200482.
- Ratnaningsih, E.; Reynard, R.; Khoiruddin, K.; Wenten, I.G.; Boopathy, R. Recent Advancements of UF-Based Separation for Selective Enrichment of Proteins and Bioactive Peptides—A Review. Appl. Sci. 2021, 11, 1078.
- Sá, A.G.A.; Laurindo, J.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Influence of Emerging Technologies on the Utilization of Plant Proteins. Front. Nutr. 2022, 9, 809058.
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane Technologies Assisting Plant-Based and Agro-Food by-Products Processing: A Comprehensive Review. Trends Food Sci. Technol. 2020, 95, 219–232.
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541.
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.N. Fava Bean (Vicia faba L.) for Food Applications: From Seed to Ingredient Processing and Its Effect on Functional Properties, Antinutritional Factors, Flavor, and Color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428.
- Husband, F.A.; Wilde, P.J.; Clark, D.C.; Rawel, H.M.; Muschiolik, G. Foaming Properties of Modified Faba Bean Protein Isolates. Top. Catal. 1994, 8, 455–468.
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522.
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and Functional Properties of Vicia faba Protein Isolates and Related Fractions. Food Chem. 2012, 132, 67–72.
- GEA. Solutions for Vegetable Protein Manufacturing; GEA: Skanderborg, Denmark, 2022; pp. 4–15.
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochem. 2018, 44, 97–105.
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The Use of Ultrasound to Mitigate Membrane Fouling in Desalination and Water Treatment. Desalination 2018, 443, 143–164.
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and Downstream Processing of Plant-Derived Recombinant Proteins. Biotechnol. Adv. 2015, 33, 902–913.
- Herneke, A.; Lendel, C.; Johansson, D.; Newson, W.; Hedenqvist, M.; Karkehabadi, S.; Jonsson, D.; Langton, M. Protein Nanofibrils for Sustainable Food-Characterization and Comparison of Fibrils from a Broad Range of Plant Protein Isolates. ACS Food Sci. Technol. 2021, 1, 854–864.
- Łojewska, E.; Kowalczyk, T.; Olejniczak, S.; Sakowicz, T. Extraction and Purification Methods in Downstream Processing of Plant-Based Recombinant Proteins. Protein Expr. Purif. 2016, 120, 110–117.
- Vishwanathan, K.H.; Govindaraju, K.; Singh, V.; Subramanian, R. Production of Okara and Soy Protein Concentrates Using Membrane Technology. J. Food Sci. 2011, 76, 158–164.
- Kumar, N.S.K.; Yea, M.K.; Cheryan, M. Soy Protein Concentrates by Ultrafiltration. J. Food. Sci. 2003, 7, 2278–2283.
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barredo-Damas, S.; Iborra-Clar, A.; Pascual-Garrido, A.; Iborra-Clar, M.A. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability 2021, 12, 13682.
- Wilken, L.R.; Nikolov, Z.L. Recovery and Purification of Plant-Made Recombinant Proteins. Biotechnol. Adv. 2012, 30, 419–433.
- Di Stefano, E.; Agyei, D.; Njoku, E.N.; Udenigwe, C.C. Plant RuBisCo: An Underutilized Protein for Food Applications. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 1063–1074.
- Pérez-Vila, S.; Fenelon, M.A.; O’Mahony, J.A.; Gómez-Mascaraque, L.G. Extraction of Plant Protein from Green Leaves: Biomass Composition and Processing Considerations. Food Hydrocoll. 2022, 133, 107902.
- Martin, A.H.; Castellani, O.; de Jong, G.A.H.; Bovetto, L.; Schmitt, C. Comparison of the Functional Properties of RuBisCO Protein Isolate Extracted from Sugar Beet Leaves with Commercial Whey Protein and Soy Protein Isolates. J. Sci. Food Agric. 2019, 99, 1568–1576.
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789.
- Wang, Y.; Xiao, T.; Zhang, Z.; Feng, X. Extraction and Concentration of Glutathione from Yeast by Membranes. Can. J. Chem. Eng. 2022, 100, S195–S204.
- Jeon, Y.J.; Byun, H.G.; Kim, S.K. Improvement of Functional Properties of Cod Frame Protein Hydrolysates Using Ultrafiltration Membranes. Process Biochem. 1999, 35, 471–478.
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of Enzymatic Hydrolysis and Ultrafiltration on Physicochemical and Functional Properties of Faba Bean Protein. Cereal Chem. 2019, 96, 725–741.
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811.
- Koros, W.J.; Ma, Y.; Shimidzu, T. Terminology for Membranes and Membrane Processes. J. Memb. Sci. 1996, 120, 149–159.
- Vose, J.R. Production and Functionality of Starches and Protein Isolates from Legume Seeds (Field Peas and Horsebeans). Cereal Chem. 1980, 57, 406–410.
- Jeganathan, B.; Vasanthan, T.; Temelli, F. Isolation of Clean-Label Faba Bean (Vicia faba L.) Proteins: A Comparative Study of Mild Fractionation Methods against Traditional Technologies. Innov. Food Sci. Emerg. Technol. 2023, 84, 103285.
- Koshchuh, W.; Povoden, G.; Thang, V.H.; Kromus, S.; Kulbe, K.D.; Novalin, S.; Krotscheck, C. Production of Leaf Protein Concentrate from Ryegrass (Lolium Perenne x Multiflorum) and Alfalfa (Medicago sativa subsp. Sativa). Comparison between Heat Coagulation/Centrifiguration and Ultrafiltration. Desalination 2004, 163, 253–259.
- Albe-Slabi, S.; Mesieres, O.; Mathé, C.; Ndiaye, M.; Galet, O.; Kapel, R. Combined Effect of Extraction and Purification Conditions on Yield, Composition and Functional and Structural Properties of Lupin Proteins. Foods 2022, 11, 1646.
- Hernandez-Marin, C.R.; Guadarrama-Mendoza, P.C.; Valadez-Blanco, R.; Chen, B.K.; Diosady, L.L. Alkaline Extraction and Purification of Huauzontle (Chenopodium berlandieri subsp. Nuttalliae) Seed Proteins by Ultrafiltration Membranes. In Proceedings of the International Conference on Applied Science and Advanced Technology (iCASAT 2019), Queretaro, Mexico, 27–28 November 2019.
- Taherian, A.R.; Mondor, M.; Labranche, J.; Drolet, H.; Ippersiel, D.; Lamarche, F. Comparative Study of Functional Properties of Commercial and Membrane Processed Yellow Pea Protein Isolates. Food Res. Int. 2011, 44, 2505–2514.
- Sarv, V.; Trass, O.; Diosady, L.L. Preparation and Characterization of Camelina sativa Protein Isolates and Mucilage. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 1279–1285.
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546.
- Khan, S.; Arshad, S.; Arif, A.; Tanveer, R.; Amin, Z.S.; Abbas, S.; Maqsood, A.; Raza, M.; Munir, A.; Latif, A.; et al. Trypsin Inhibitor Isolated from Glycine max (Soya Bean) Extraction, Purification, and Characterization. Dose-Response 2022, 20, 15593258221131462.
- Hansen, L.; Bu, F.; Ismail, B.P. Structure—Function Guided Extraction and Scale—Up of Pea Protein Isolate Production. Food 2022, 11, 3773.
- Mousavi, B.; Azizi, M.H.; Abbasi, S. Antidiabetic Bio-Peptides of Soft and Hard Wheat Glutens. Food Chem. Mol. Sci. 2022, 4, 100104.
- Sadgrove, N.J.; Simmonds, M.S.J. Topical and Nutricosmetic Products for Healthy Hair and Dermal Antiaging Using “Dual-Acting” (2 for 1) Plant-Based Peptides, Hormones, and Cannabinoids. FASEB BioAdv. 2021, 3, 601–610.
- Kumar, M.; Hasan, M.; Choyal, P.; Tomar, M.; Gupta, O.P.; Sasi, M.; Changan, S.; Lorenzo, J.M.; Singh, S.; Sampathrajan, V.; et al. Cottonseed Feedstock as a Source of Plant-Based Protein and Bioactive Peptides: Evidence Based on Biofunctionalities and Industrial Applications. Food Hydrocoll. 2022, 131, 107776.
- Ying, X.; Agyei, D.; Udenigwe, C.; Adhikari, B.; Wang, B. Manufacturing of Plant-Based Bioactive Peptides Using Enzymatic Methods to Meet Health and Sustainability Targets of the Sustainable Development Goals. Front. Sustain. Food Syst. 2021, 5, 769028.
- Mondor, M.; Plamondon, P.; Drolet, H. Valorization of Agri-Food By-Products from Plant Sources Using Pressure-Driven Membrane Processes to Recover Value-Added Compounds: Opportunities and Challenges. Food Rev. Int. 2022, 39, 5761–5785.
- Sosalagere, C.; Adesegun Kehinde, B.; Sharma, P. Isolation and Functionalities of Bioactive Peptides from Fruits and Vegetables: A Reviews. Food Chem. 2022, 366, 130494.
- Nuchprapha, A.; Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Two Novel ACE Inhibitory Peptides Isolated from Longan Seeds: Purification, Inhibitory Kinetics and Mechanisms. RSC Adv. 2020, 10, 12711–12720.
- Dlask, O.; Václavíková, N. Electrodialysis with Ultrafiltration Membranes for Peptide Separation. Chem. Pap. 2018, 72, 261–271.
- Poulin, J.F.; Amiot, J.; Bazinet, L. Simultaneous Separation of Acid and Basic Bioactive Peptides by Electrodialysis with Ultrafiltration Membrane. J. Biotechnol. 2006, 123, 314–328.
- Poulin, J.F.; Amiot, J.; Bazinet, L. Improved Peptide Fractionation by Electrodialysis with Ultrafiltration Membrane: Influence of Ultrafiltration Membrane Stacking and Electrical Field Strength. J. Memb. Sci. 2007, 299, 83–90.
- Roblet, C.; Doyen, A.; Amiot, J.; Pilon, G.; Marette, A.; Bazinet, L. Enhancement of Glucose Uptake in Muscular Cell by Soybean Charged Peptides Isolated by Electrodialysis with Ultrafiltration Membranes (EDUF): Activation of the AMPK Pathway. Food Chem. 2014, 147, 124–130.
- Cecile Urbain Marie, G.; Perreault, V.; Henaux, L.; Carnovale, V.; Aluko, R.E.; Marette, A.; Doyen, A.; Bazinet, L. Impact of a High Hydrostatic Pressure Pretreatment on the Separation of Bioactive Peptides from Flaxseed Protein Hydrolysates by Electrodialysis with Ultrafiltration Membranes. Sep. Purif. Technol. 2019, 211, 242–251.
- Firdaous, L.; Dhulster, P.; Amiot, J.; Gaudreau, A.; Lecouturier, D.; Kapel, R.; Lutin, F.; Vézina, L.P.; Bazinet, L. Concentration and Selective Separation of Bioactive Peptides from an Alfalfa White Protein Hydrolysate by Electrodialysis with Ultrafiltration Membranes. J. Memb. Sci. 2009, 329, 60–67.
- González-Muñoz, A.; Valle, M.; Aluko, R.E.; Bazinet, L.; Enrione, J. Production of Antihypertensive and Antidiabetic Peptide Fractions from Quinoa (Chenopodium quinoa Willd.) by Electrodialysis with Ultrafiltration Membranes. Food Sci. Hum. Wellness 2022, 11, 1650–1659.
- Doyen, A.; Udenigwe, C.C.; Mitchell, P.L.; Marette, A.; Aluko, R.E.; Bazinet, L. Anti-Diabetic and Antihypertensive Activities of Two Flaxseed Protein Hydrolysate Fractions Revealed Following Their Simultaneous Separation by Electrodialysis with Ultrafiltration Membranes. Food Chem. 2014, 145, 66–76.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
19 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




