Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yogitha Sai Vempati | -- | 1487 | 2024-01-19 01:48:45 | | | |
| 2 | Rita Xu | Meta information modification | 1487 | 2024-01-19 02:44:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sobel, J.D.; Vempati, Y.S. Bacterial Vaginosis and Vulvovaginal Candidiasis. Encyclopedia. Available online: https://encyclopedia.pub/entry/54073 (accessed on 08 March 2026).
Sobel JD, Vempati YS. Bacterial Vaginosis and Vulvovaginal Candidiasis. Encyclopedia. Available at: https://encyclopedia.pub/entry/54073. Accessed March 08, 2026.
Sobel, Jack D., Yogitha Sai Vempati. "Bacterial Vaginosis and Vulvovaginal Candidiasis" Encyclopedia, https://encyclopedia.pub/entry/54073 (accessed March 08, 2026).
Sobel, J.D., & Vempati, Y.S. (2024, January 19). Bacterial Vaginosis and Vulvovaginal Candidiasis. In Encyclopedia. https://encyclopedia.pub/entry/54073
Sobel, Jack D. and Yogitha Sai Vempati. "Bacterial Vaginosis and Vulvovaginal Candidiasis." Encyclopedia. Web. 19 January, 2024.
Copy Citation
Among the infectious causes of vulvovaginal symptoms, bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) dominate. Apart from infrequent mixed infections, both are considered independent and caused by unrelated pathogenic mechanisms.
bacterial vaginosis
vulvovaginal candidiasis
fluconazole resistance
1. Introduction
Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) are the two most common varieties of vaginal infection. Each presents a unique spectrum of clinical manifestations, and they are viewed as separate entities of markedly different etiopathogenesis and causation. Although infrequent, mixed infections with simultaneous characteristic expression of both BV and VVC do occur. Little is known about linkage and disease interaction. In a study of women with RVVC in Argentina, an association with BV was found in 35% and intermediate vaginal microbiota in an additional 33.2% of women [1]. For clinicians, the only well-recognized link is the development of VVC in the following days or few weeks after a symptomatic episode of BV treated with appropriate antibiotics (Figure 1). A reasonable causal explanation is that antibacterial agents impact the already pathologic microbiome of BV and remove any residual bacterial restraints or “brakes”, facilitating vaginal yeast proliferation, leading to symptomatic VVC. The treatment of the BV episode rather than the BV infection itself is thought to result in the consecutive episode of VVC. This phenomenon is well known to both patients and practitioners, so much so that women with this highly predictable complication of BV treatment routinely request a simultaneous prophylactic oral antifungal drug, usually fluconazole, when receiving antibacterial agents. Prophylactic fluconazole is highly effective in this context, but its use is largely unvalidated. This relationship between BV and VVC has therefore been limited to a drug-induced VVC attack. Little thought or enquiry into other linkages has been explored, nor are data available on the frequency of this occurrence or the clinical and therapeutic implications. BV is widely recognized as predisposing to multiple sexually transmitted infections, but little attention has been directed at VVC as a consequence of BV [2].
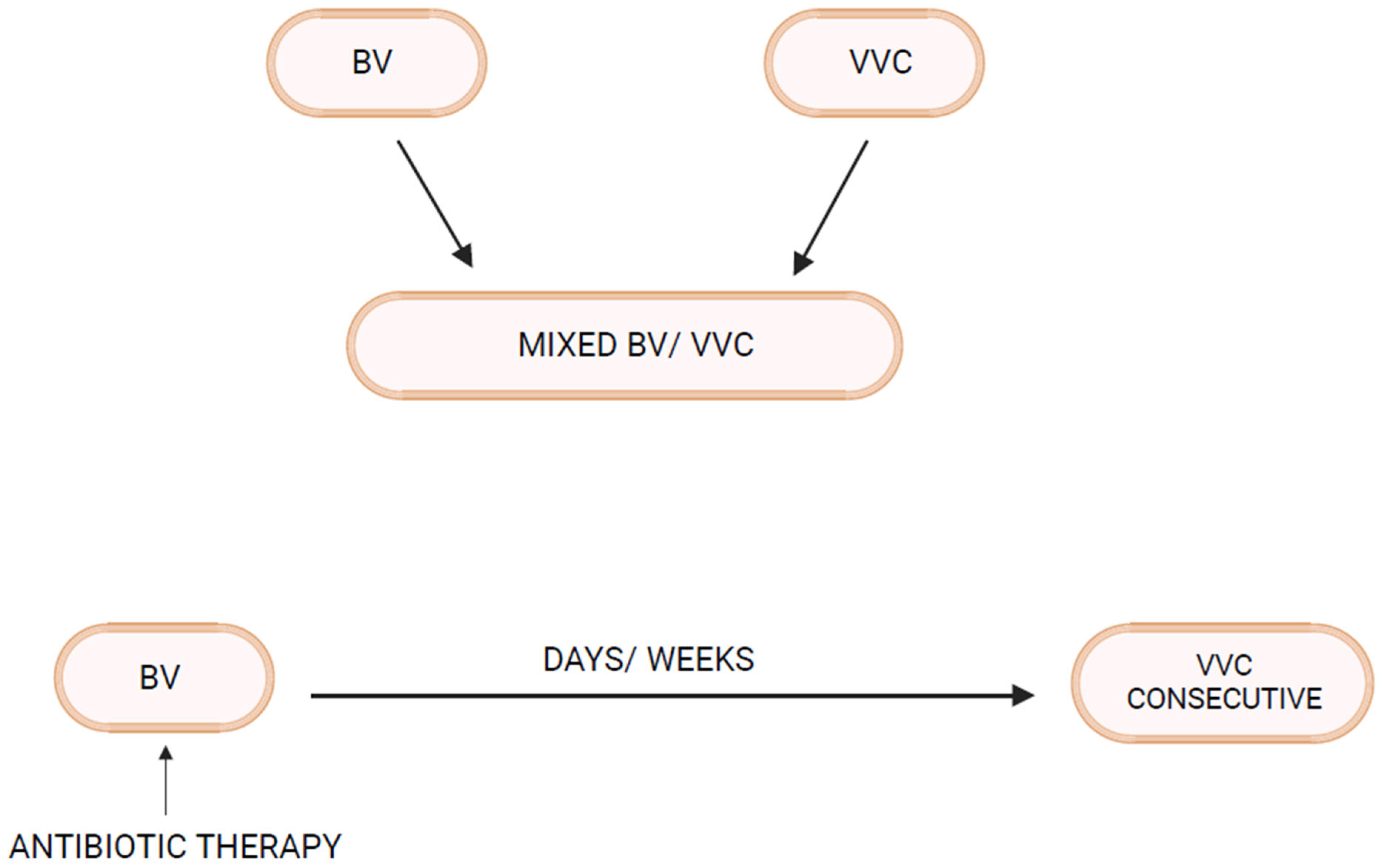
Figure 1. Bacterial Vaginosis–Vulvovaginal Candidiasis interrelationship.
As clinicians in a tertiary care vaginitis clinic, researchers evaluate women referred for management of either recurrent BV (RBV) or RVVC, with more than 80% of women referred for RBV declaring a history of RVVC. Vaginal yeast infections are viewed by patients as the non-dominant problem in terms of personal suffering and only viewed as an unavoidable collateral effect of needed antibiotic therapy for BV with no thoughts by practitioners of an alternate biologic association. In the authors’ experience, dealing with a predominantly African American population served in Detroit, MI, although a variety of unrelated causes and triggers of RVVC are described, researchers propose that RBV is the dominant contributor to RVVC [3][4].
2. Pathogenesis of Vulvovaginal Candidiasis (Abbreviated Summary)
The primary pathogens in VVC are Candida species, likely originating from the gastrointestinal tract and achieving initial asymptomatic vaginal colonization as commensals. Candida microorganisms enjoy a saprophytic existence in a moderated non-adversarial environment created by vagino-protective microbiota. In particular, organic acids, both acetic and lactic acid, contribute to vaginal tolerance of Candida spp. [5]. The presence of a tolerant cytokine atmosphere emphasizes the critical role of the vaginal mucosa and its expressed defensive function [2][3][4]. The local effect of estrogen plays a dominant role in the healthy lower genital tract microbiome in allowing Candida commensalism. Vaginal yeast colonization can be long term and is influenced by host genetic influences together with acquired colonizing promoting factors, both behavioral and biologic [3][4]. The outcome of benign asymptomatic colonization as well as Candida vaginitis reflects the interplay of three factors: yeast, vaginal microbiota, and host mucosal immune factors. A detailed description of human vaginal mucosa, host immunity, and the role of vaginal microbiota in the pathogenesis of VVC has been reviewed by several investigators [3][4][5][6][7][8][9][10]. A critical role of host local innate immunity, in both defense and pathogenesis of VVC, is widely proposed with resultant symptoms and signs the consequence of host no-longer protective inflammatory response [3][4][5][6][7][8][9][10][11][12][13][14].
Acute symptomatic Candida vulvovaginitis represents a dramatic change triggered by multiple factors but always requiring prior vaginal yeast colonization and characterized by proliferation of yeast blastospores and hyphae formation with expression of multiple fungal virulence factors, and these microbiome changes result in superficial vaginal epithelial surface invasion and consequent vaginal epithelial cell proinflammatory reaction. The accompanying myriad signs and symptoms evident in acute vulvovaginitis soon follow. Both IL-1β and IL-6 are increased as part of the proinflammatory mucosal response [3][4][7][8]. Risk factors for acute VVC include vaginal dysbiosis after antimicrobials, increased estrogen, and uncontrolled diabetes, all superimposed upon genetic susceptibility consisting largely of single nucleotide polymorphisms [3][15]. All members of the triad contribute to the risk and expression of disease. VVC may be acute, short-lived, chronic, or recurrent. Unlike oral candidiasis, immunosuppression is not a prerequisite for VVC.
3. Pathogenesis of Bacterial Vaginosis (Abbreviated Summary)
Unlike VVC, with a single or mono-pathogen pathogenesis, BV represents a severe polymicrobial vaginal dysbiosis with loss or disappearance of what is considered “healthy” protective Lactobacillus species and overgrowth of multiple largely strict anaerobic and facultative species, creating a more diverse bacterial abundance. This includes multiple G. vaginalis species, Fannyhessea vaginae, Mobiluncus spp., and BVAB (BV-associated bacteria 1–3) and Bacteroides spp., Clostridiale spp., Prevotella spp., Zozaya spp., and others [16][17]. Whether the overgrowth of these pathogenic species contributes to the reduction or elimination of Lactobacillus species or alternatively follows their disappearance has not been conclusively established. Many investigators favor the introduction or emergence of the pathologic consortium of anaerobes as the primary process, possibly related to sexual transmission from a male or female partner [18]. Some virulent Gardnerella spp. are thought to be a key factor in BV pathogenesis, displacing vaginal Lactobacillus spp. and adhering to vaginal epithelial cells. Considerable evidence indicates the role of sexual transmission to explain multiple recurring BV episodes in women followed longitudinally, although sexual transmission or pathogen reintroduction is clearly not the only mechanism of frequent recurrence or relapses [19].
More recently, the recognition and appreciation of the BV biofilm coating the surface of the vaginal mucosa has contributed to understanding the pathogenesis of BV, especially RBV, and has improved treatment of BV. Virulent strains of Gardnerella spp. are thought to initiate biofilm production and are often the predominant species present in the biofilm [19]. It is hypothesized that certain Gardnerella species act to lower the oxidation reduction potential of the vaginal micro-environment as well as elaborating essential substrates (NH3), allowing growth of strict anaerobic bacteria. The microbiome of BV has undergone extensive investigation in the last decade, significantly enhanced by the availability of PCR and next-generation sequencing, leading to advances in diagnosis, but it has not, to date, contributed to treatment advantage [20][21][22][23]. A conceptual model of BV pathogenesis including initial invasion with G. vaginalis and Prevotella bivia, followed by a second wave of colonizers including Atopobium vaginae and Sneathia spp., has been proposed [23].
Turning to the third component of the causal triad, the role of the host immune system in the pathogenesis of BV and expressed in the vaginal mucosa has been extensively investigated. BV has long been considered a “non-inflammatory” condition, hence the term “vaginosis” and not “vaginitis” [24]. This early definition or designation was largely driven by lack of typical clinical signs of vaginal and vulvar inflammation including pain, soreness, or dyspareunia. This decision was reinforced by the striking absence of polymorphonuclear leukocytes (PMNs) in the vaginal exudate or discharge on saline microscopy. However, this assumption did not match later immunologic scrutiny. The vaginal environment in BV is profoundly proinflammatory, as confirmed in multiple studies [20][25][26][27]. Cytokine and chemokine increase are evident with increase in IL-2, type 1 interferon. The lack of PMNs appropriately reflects the effect of chemokine or chemotaxic inhibitors preventing their accumulation. The role of vaginal inflammatory mediators in the pathogenesis of BV is largely unknown and is perhaps crucial to the loss of protective Lactobacillus species as well as to explaining clinical BV recurrence and resistance to probiotic therapy.
4. Linking BV and VVC
As mentioned above, not all women with RVVC have associated BV or RBV. In fact, many women with RVVC have no such association. On the other hand, in some women prone to RBV, clinical experience reveals that RVVC is an enormous and predictable additional problem [28]. Invariably, it is BV that precedes and appears to trigger RVVC episodes. The first question to be addressed is whether vaginal Candida colonization rates are increased in women with BV. In fact, several vaginal microbiota studies in women with BV reveal increased rates of Candida vaginal colonization [28]. Similarly, Candida colonization rates in the clinic in women with acute BV, even in the absence of positive microscopy for yeast elements, are approximately 30–35%, significantly higher than in matched women serving as normal controls (12–15%). This is not surprising given the higher vaginal pH characteristic of BV reflecting loss of lactic acid and bacteriocin-producing protective Lactobacillus species. This is particularly evident in L. iners-dominant communities characteristic of BV, both during acute attacks of symptomatic BV as well as following successful treatment of BV with metronidazole [29][30]. As a general principle, therefore, vaginal dysbiosis facilitates Candida colonization [2][6].
References
- Arechavala, A.; Negroni, R.; Santiso, G.; Depardo, R.; Bonvehí, P. Chronic recurrent vulvovaginitis is not only due to Candida. Rev. Iberoam. Micol. 2021, 38, 132–137.
- Dabee, S.; Passmore, J.-A.S.; Heffron, R.; Jaspan, H.B. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect. Immun. 2021, 89, e00487-20.
- Jafarzadeh, L.; Ranjbar, M.; Nazari, T.; Eshkaleti, M.N.; Gharehbolagh, S.A.; Sobel, J.D.; Mahmoudi, S. Vulvovaginal candidiasis: An overview of mycological, clinical, and immunological aspects. J. Obstet. Gynaecol. Res. 2022, 48, 1546–1560.
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5.
- Lourenço, A.; Pedro, N.A.; Salazar, S.B.; Mira, N.P. Effect of Acetic Acid and Lactic Acid at Low pH in Growth and Azole Resistance of Candida albicans and Candida glabrata. Front. Microbiol. 2019, 9, 3265.
- Bojang, E.; Ghuman, H.; Kumwenda, P.; Hall, R.A. Immune Sensing of Candida albicans. J. Fungi 2021, 7, 119.
- Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Icely, P.A.; García, L.N.; Peinetti, N.; Maldonado, C.A.; Riera, F.O.; Caeiro, J.P.; Sotomayor, C.E. Contribution of TLR2 pathway in the pathogenesis of vulvovaginal candidiasis. Pathog. Dis. 2017, 75, ftx096.
- Balakrishnan, S.N.; Yamang, H.; Lorenz, M.C.; Chew, S.Y.; Than, L.T.L. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens 2022, 11, 618.
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27.
- Gaziano, R.; Sabbatini, S.; Monari, C. The interplay between Candida albicans, vaginal mucosa, host immunity and resident microbiota in health and disease: An overview and future perspective. Microorganisms 2023, 11, 1211.
- Papon, N.; Van Dijck, P. A Complex Microbial Interplay Underlies Recurrent Vulvovaginal Candidiasis Pathobiology. mSystems 2021, 6, e0106621.
- Rosati, D.; Bruno, M.; Jaeger, M.; Ten Oever, J.; Netea, M.G. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 2020, 8, 144.
- Ardizzoni, A.; Wheeler, R.T.; Pericolini, E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front. Microbiol. 2021, 12, 692491.
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220.
- Smeekens, S.P.; van de Veerdonk, F.L.; Kullberg, B.J.; Netea, M.G. Genetic susceptibility to Candida infections. EMBO Mol. Med. 2013, 5, 805–813.
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res. Hum. Retrovir. 2019, 35, 219–228.
- Fredricks, D.N.; Fiedler, T.L.; Thomas, K.K.; Oakley, B.B.; Marrazzo, J.M. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J. Clin. Microbiol. 2007, 45, 3270–3276.
- Vodstrcil, L.A.; Muzny, C.A.; Plummer, E.L.; Sobel, J.D.; Bradshaw, C.S. Bacterial vaginosis: Drivers of recurrence and challenges and opportunities in partner treatment. BMC Med. 2021, 19, 194.
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106 Pt 1, 1013–1023.
- Murphy, K.; Mitchell, C.M. The Interplay of Host Immunity, Environment and the Risk of Bacterial Vaginosis and Associated Reproductive Health Outcomes. J. Infect. Dis. 2016, 214 (Suppl. S1), S29–S35.
- Chacra, L.A.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 2022, 11, 672429.
- Vitali, B.; Pugliese, C.; Biagi, E.; Candela, M.; Turroni, S.; Bellen, G.; Donders, G.G.G.; Brigidi, P. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Appl. Environ. Microbiol. 2007, 73, 5731–5741.
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host–vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65.
- Forsum, U.; Holst, E.; Larsson, P.G.; Vasquez, A.; Jakobsson, T.; Mattsby-Baltzer, I. Bacterial vaginosis—A microbiological and immunological enigma. Apmis 2005, 113, 81–90.
- Gardner, J.K.; Łaniewski, P.; Knight, A.; Haddad, L.B.; Swaims-Kohlmeier, A.; Herbst-Kralovetz, M.M. Interleukin-36γ Is Elevated in Cervicovaginal Epithelial Cells in Women with Bacterial Vaginosis and In Vitro after Infection with Microbes Associated With Bacterial Vaginosis. J. Infect. Dis. 2019, 221, 983–988.
- Mitchell, C.; Marrazzo, J. Bacterial vaginosis and the cervicovaginal immune response. Am. J. Reprod. Immunol. 2014, 71, 555–563.
- Lennard, K.; Dabee, S.; Barnabas, S.L.; Havyarimana, E.; Blakney, A.; Jaumdally, S.Z.; Botha, G.; Mkhize, N.N.; Bekker, L.-G.; Lewis, D.A.; et al. Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females. Infect. Immun. 2017, 86, e00410-17.
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019, 134, 103599.
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9.
- Holm, J.B.; Carter, K.A.; Ravel, J.; Brotman, R.M. Lactobacillus iners and genital health: Molecular clues to an enigmatic vaginal species. Curr. Infect. Dis. Rep. 2023, 25, 67–75.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
516
Revisions:
2 times
(View History)
Update Date:
19 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





