1. Introduction
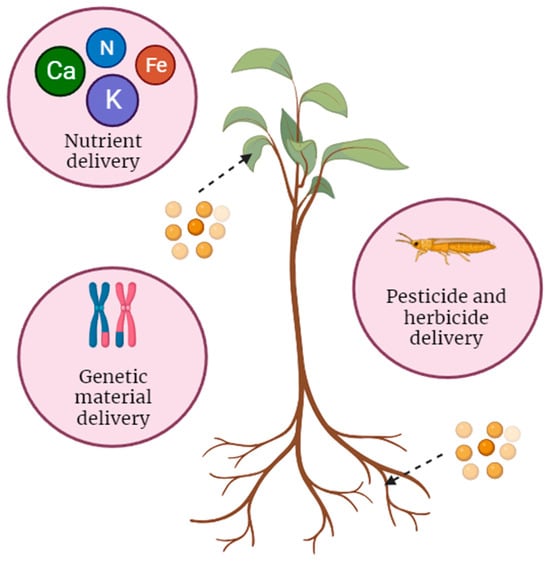
Understanding the transport of nanoparticles into plants is crucial for harnessing their potential in agriculture and biotechnology. The journey of nanoparticles from external environments to internal plant tissues involves intricate processes influenced by both the properties of the nanoparticles and the characteristics of plant structures. Nanoparticles can be designed to transport specific materials, compounds, or genetic information within plants, acting as carriers to deliver these payloads to target locations. The controlled delivery of nanoparticles can be advantageous for precise and efficient plant applications. Nanoparticles can be used for fertilizer or nutrient delivery, genetic material delivery, and pesticide or herbicide delivery (
Figure 1). Nanoparticles can be used to transport essential nutrients, including micronutrients such as iron, zinc, and other trace elements, into plant cells
[1][2][3]. Tombuloglu et al. successfully synthesized composites of micronutrient nanoparticles (NPs) and applied them to
Hordeum vulgare L.
[3]. They demonstrated the effective incorporation of these micronutrients into plant tissue. The transportation of these NPs significantly increased the quantity of elements in both the root and leaf tissues. Specifically, the contents of Fe, Zn, and Cu were raised to approximately 5, 3, and 18 times higher than the control, respectively
[3]. This approach is known as nanoparticle-mediated nutrient delivery and is aimed at enhancing the nutrient uptake and utilization of plants. Nanoparticles, due to their small size, can be taken up by plant roots or leaves. The nanoparticles may enter plant cells through various mechanisms, such as diffusion, endocytosis, or direct uptake through ion channels and transporters
[4][5]. Once inside the plant cells, the nanoparticles release the encapsulated nutrients. This controlled release ensures that the nutrients are made available within the plant at a rate that matches the plant’s needs
[3]. By improving nutrient uptake and utilization, nanoparticle-mediated nutrient delivery can reduce the number of traditional fertilizers needed, minimizing the environmental impact associated with excess nutrient runoff and leaching.
Figure 1. Nanoparticles used as carriers for nutrient (fertilizer), genetic material, and pesticide delivery into plants.
Nanoparticles can also serve as vehicles for delivering genetic material, such as DNA, RNA, or small interfering RNA (siRNA), into plant cells
[6][7]. This technology, often referred to as “nanoparticle-mediated gene delivery”, has several applications in plant biotechnology and agriculture. Nanoparticles are engineered to encapsulate, bind, or complex with genetic material. These nanoparticles are designed to protect the genetic material from degradation and facilitate its delivery into plant cells
[8]. Wang et al. used several types of NPs (CS, PEI, protamine, CQD, PAMAM, and CSC) to deliver dsRNA against rice sheath blight (
Rhizoctonia solani) in
Oryza sativa L.
[9]. These nanoparticles could protect dsRNA from degradation by nucleases. Nanoparticles are introduced to plant tissues through various methods, such as direct injection into plant cells, root soaking, or foliar spray. Once inside the plant cells, the nanoparticles release the encapsulated genetic material. This can be achieved through controlled release mechanisms or by breaking down the nanoparticle complexes under specific conditions. Nanoparticles can be used to transport pesticides or herbicides to specific target areas within plants
[10]. This approach can enhance the precision and efficiency of pesticide application and reduce the environmental impact associated with conventional spray applications. Nanoparticles can be designed to target specific plant tissues or cell types, ensuring that the pesticides or herbicides are delivered directly to the intended areas, such as the leaves, stem, or root system
[10][11]. A water-soluble chitosan (CS) derivative (N-(2-hydroxyl)propyl-3-trimethyl ammonium CS chloride, HTCC) was successfully capped on the surface of pyraclostrobin-loaded MSNs by Cao et al.
[12]. This particle directly targeted
Phomopsis asparagi (Sacc.) and had fungicidal activity against it. By improving the targeted delivery of pesticides and herbicides, nanoparticle-mediated delivery can reduce the amount of chemicals needed, minimize off-target effects, and reduce environmental pollution and contamination.
Nanoparticles have also shown potential impacts on plant development and growth, and their applications in agriculture are an active area of research. Nanoparticles can influence seed germination rates and early plant growth
[13][14]. They may enhance seedling vigor and promote healthier plant establishment. Previous study showed that the use of SiO
2 NPs could improve tomato (
Solanum lycopersicum var. Momotaro) seed germination
[14]. Some nanoparticles have been studied for their potential to enhance plant stress tolerance, such as resistance to drought, salinity, or heavy metal toxicity
[15]. These nanoparticles may act as stress mitigators and improve overall plant health. The presence of nanoparticles in soil can also influence microbial communities and soil health
[16]. The impact depends on the type of nanoparticles used and their interactions with soil microorganisms. Some nanoparticles may exhibit phytotoxic effects. This can result in stunted growth, reduced photosynthesis, or other negative impacts on plant health
[17]. The fate of nanoparticles in the environment, including their persistence and potential for leaching into water sources, is an important consideration for sustainable agricultural practices. Balancing the potential benefits with environmental and human health considerations is crucial for the sustainable integration of nanotechnology in agriculture.
2. Types of Nanoparticles Used for Transport
Various types of nanoparticles are used for transport in plants, depending on the specific application and the payload (substance being transported). Different nanoparticles possess unique properties that make them suitable for diverse purposes in plant biology and agriculture. The choice of nanoparticle type in plant-related applications depends on several critical factors, as listed in Table 1. The careful consideration of these factors is essential when choosing the appropriate nanoparticle type for specific plant-related applications. The goal is to ensure that the selected nanoparticles align with the objectives of the application, provide benefits, and minimize potential risks to plants and the environment.
Nanoparticles can be categorized into commercial (engineered) and biogenic (naturally occurring) based on their origin
[18]. Commercial nanoparticles, also referred to as engineered nanoparticles, are particles that are intentionally designed, synthesized, and produced with specific characteristics for various industrial, technological, agricultural, or consumer applications such as metal nanoparticles, metal oxide nanoparticles, carbon nanotubes, quantum dots, nanocomposites, and polymeric nanoparticles
[18][19]. These nanoparticles are created through engineering processes to achieve desired properties at the nanoscale, typically ranging from 1 to 100 nanometers. The intentional manipulation of the size, shape, surface properties, and composition distinguishes engineered nanoparticles from naturally occurring nanoparticles. On the other hand, biogenic nanoparticles refer to nanoparticles that are naturally formed or synthesized by living organisms or natural processes without human intervention. Unlike engineered nanoparticles, which are intentionally produced by humans for specific purposes, biogenic nanoparticles occur naturally because of biological and environmental processes
[20]. These nanoparticles can be found in various natural sources and are often associated with specific organisms or geological phenomena such as bacterial nanoparticles, fungal nanoparticles, volcanic ash, clay minerals, and wildfire ash
[20].
Table 1. Factors influencing nanoparticle selection for plant transport-related applications.
There are numerous types of nanoparticles, and the field of nanotechnology continues to evolve, leading to the development of new types and functionalities. Examples of nanoparticles used for transport in plants are presented in Table 2 along with their introduction site. Metals and metal oxides are types of nanoparticles that are commonly used in various plant transportation applications.
Table 2. Types of nanoparticles used for transport in plants and their places of introduction.
Research on the interaction of nanomaterials with plants has mainly focused on phytotoxicology. At the same time, less research has been conducted on positive effects such as increasing crop productivity and enhancing plant resistance, and research on beneficial effects on plants is still incomplete. The entry of nanoparticles into plants, especially at high concentrations, can lead to phytotoxicity. Geisler-Lee et al. showed that Ag NPs could exert detrimental effects on
A. thaliana, but the phytotoxic effect of Ag NPs could not be fully explained by the released silver ions
[31]. Another study also indicated that the internalization and upward translocation of ZnO NPs by
Lolium perenne could significantly reduce the biomass and cause damage to root epidermal and cortical cells
[35]. Some nanoparticles, such as certain metal and metal oxide nanoparticles, can generate reactive oxygen species (ROS) when they enter plant cells
[64]. Elevated ROS levels can damage cell structures, disrupt cellular functions, and cause oxidative stress, leading to plant injury or even cell death
[64][65]. High nanoparticle concentrations can disrupt the integrity of plant cell membranes
[66]. This can lead to the leakage of cellular contents and negatively impact cell viability. Rossi et al. stated that NPs could also influence plant symplastic pathways by altering ion transport activity or root cell membrane integrity
[67]. Excessive nanoparticle accumulation can interfere with the uptake and distribution of essential nutrients, disrupting nutrient balance in plants and causing nutrient deficiencies or toxicities. High nanoparticle concentrations may also physically obstruct the plant’s nutrient and water transport systems, impeding the movement of substances throughout the plant
[5]. Plants exposed to high nanoparticle concentrations may activate stress responses, diverting resources away from growth and development and reducing overall plant health
[27][68]. The phytotoxicity of nanoparticles can vary depending on factors such as nanoparticle type, size, shape, surface properties, and the plant species involved. Therefore, selecting the appropriate concentration of nanoparticles is a critical decision, as it can significantly impact the performance and effectiveness of nanoparticle transport.
Nanoparticles, whether naturally occurring or engineered, can have various negative effects on plants. The impact of nanoparticles on plants depends on factors such as the type of nanoparticles, their concentration, exposure duration, and the specific plant species
[69]. Nanoparticles can exhibit phytotoxic effects, leading to damage being caused to the plant cells, tissues, and overall plant structure. This can result in stunted growth, reduced biomass, and compromised plant health
[17]. Exposure to certain nanoparticles can alter the root morphology and function. This includes changes in the root length, surface area, and the structure of root hairs, which can impact nutrient and water uptake
[70]. Nanoparticles can induce oxidative stress in plants by generating reactive oxygen species (ROS). Elevated ROS levels can lead to the damage of cellular components, such as membranes, proteins, and DNA, affecting plant health
[71]. Some nanoparticles may persist in the environment, leading to long-term exposure for plants. Additionally, there is a concern about the potential bioaccumulation of nanoparticles in plant tissues, which can have implications for organisms higher up the food chain
[72].
3. Modes of Transport of Nanoparticles into Plants
Nanoparticle transport in plants can be categorized based on two main mechanisms, i.e., assisted delivery and passive delivery. When referring to assisted delivery in the context of transporting nanoparticles to the plant body, it means that external power or forces are applied to facilitate the transport of nanoparticles into the plant tissues
[73]. This external power or force assists in the delivery process, often overcoming barriers that would hinder passive transport
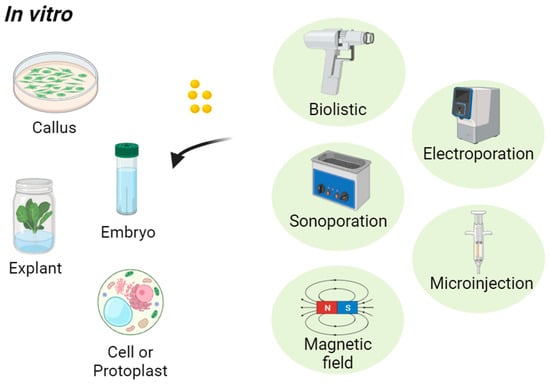
[74]. Examples of assisted delivery techniques in plant nanoparticle applications are shown in
Figure 2 and described briefly in
Table 3. The biolistic (gene gun) is a device that uses an external force, such as compressed gas or helium, to propel nanoparticles
[75][76]. Rajkumari et al. reported the use of Ag NPs as gene carriers, replacing Au microcarriers for biolistic gene delivery in
Nicotiana tabacum L., and showed that the transformation efficiency was significantly higher with Ag NPs than Au microparticles as carriers
[76]. Sonoporation involves the application of ultrasound waves to create temporary pores in plant cell membranes, allowing nanoparticles to enter the cells
[77]. The external force of ultrasound assists in the delivery process. Zolghadrnasab et al. showed that ultrasonic treatment provides an economical and straightforward approach for poly-ethyleneimine (PEI)-coated mesoporous silica nanoparticles (MSNs) transferring into plant cells without any need for complicated devices and without concerns about safety issues
[78]. Magnetic nanoparticles can be guided to specific plant tissues using external magnetic fields, effectively assisting in the targeted delivery of these nanoparticles
[79]. Characterizing the intrinsic magnetic properties of nanoparticles involves understanding their behavior at the bulk level as well as at the level of individual molecules. Various techniques are employed for both bulk and single-molecule investigations. Bulk techniques include vibrating sample magnetometry (VSM), superconducting quantum interference device (SQUID), magnetic resonance imaging (MRI), Mössbauer spectroscopy, and X-ray magnetic circular dichroism (XMCD)
[80][81]. Single-molecule techniques include magnetic force microscopy (MFM), scanning tunneling microscopy (STM), electron magnetic circular dichroism (EMCD), single-particle magnetometry, and fluorescence-based techniques
[82][83]. Electroporation involves applying external electric fields to plant cells, creating temporary pores in the cell membranes
[84]. This method uses an external electrical power source to facilitate nanoparticle entry. Microinjection, combined with external manipulation, can be used to introduce nanoparticles into specific plant cells
[85]. Most of the assisted delivery methods are used for transporting nanoparticles in vitro (outside living organisms, typically in a controlled laboratory setting) as indicated in
Figure 2. Nanoparticles can be introduced to plant embryos cultivated in a controlled laboratory setting
[86]. Plant embryos, which are the earliest stages of plant development, offer a convenient point of entry for introducing nanoparticles that can influence the growth and characteristics of the resulting plant. Nanoparticles can also be introduced to plant callus cultures, which are undifferentiated masses of plant cells grown in vitro
[87]. Plant callus cultures are typically initiated from explants, such as leaf pieces, stem segments, or immature embryos. These explants are sterilized and placed on a suitable culture medium containing plant growth regulators to induce callus formation. Introducing nanoparticles to plant protoplasts is also a technique that is commonly used in plant biotechnology. Protoplasts are plant cells that have had their cell walls enzymatically removed, leaving behind the cell membrane and cytoplasm
[88].
Figure 2. Assisted delivery techniques for introducing nanoparticles into plants in vitro.
Assisted delivery methods can facilitate the delivery of relatively larger particles due to the external forces involved, as indicated in
Table 3. These methods are valuable for overcoming natural barriers and transporting particles efficiently. However, there are still many big issues related to this transport method, especially regarding their scalability to larger scales. Therefore, passive delivery still has advantages over it. In a controlled laboratory setting, it is easier to maintain uniform conditions for assisted delivery, ensuring consistency in nanoparticle uptake
[89]. On larger scales, achieving uniformity across a field or agricultural area becomes more challenging. Variability in environmental conditions, soil composition, and plant physiology can affect the effectiveness of delivery methods
[90]. Assisted delivery in vitro also allows for precise control over factors such as temperature, humidity, and nutrient levels. However, real-world conditions vary widely in agricultural settings. Adapting in vitro strategies to diverse environments becomes complex, and factors like wind, rainfall, and temperature fluctuations can impact the efficacy of nanoparticle delivery
[91]. Implementing these assisted delivery methods on a large scale also requires significant resources and may not be economically feasible. Factors such as the cost of materials, equipment, and labor must be considered for practical application in agriculture. The large-scale implementation of assisted delivery may face regulatory challenges related to environmental impact, safety, and ethical considerations
[92]. Demonstrating the safety and environmental compatibility of nanoparticle delivery systems becomes essential.
Table 3. Examples of assisted delivery techniques for introducing nanoparticles into plants.
In contrast to assisted delivery, passive delivery in the context of nanoparticle transport in plants typically refers to the movement of nanoparticles through natural routes without the assistance of external forces or active mechanisms. It encompasses various methods through which nanoparticles enter the plant body, often via sites such as roots and leaves. In summary, assisted delivery and passive delivery are different concepts. Assisted delivery involves an intervention to enhance nanoparticle uptake, while passive delivery relies on natural processes, without external assistance. These approaches cater to different research goals and applications in the field of nanotechnology for plant science. These passive delivery routes include root uptake and foliar uptake
[10]. Plants naturally absorb water and dissolved substances, and nanoparticles can enter the plant through the roots as they take up water and nutrients
[5][10]. This is a common method for delivering nanoparticles to plants in vivo. When discussing in vivo delivery in the context of plants, it typically involves the intact, living plant. In vivo experiments aim to study the interaction between nanoparticles and the entire living organism, considering the complexities of the plant’s biological systems
[101]. Nanoparticles can also be sprayed or applied to the leaves of plants
[5][102]. Lian et al. showed that the response of
Zea mays L. to Cd and TiO
2 NPs was highly dependent on the exposure mode. They reported that leaf exposure provided more benefits than root exposure
[102]. Some nanoparticles, like those used in foliar fertilizers or pesticides, can be taken up by the plant through the stomata (small openings on leaf surfaces) or through the leaf cuticle
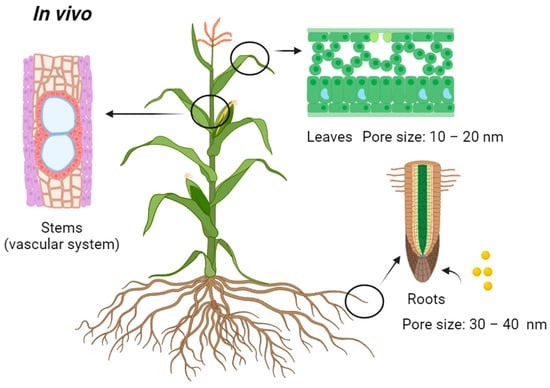
[10]. Examples of these two methods are shown in
Table 1 above, which use the in vivo nanoparticle transport route (
Figure 3). After nanoparticles enter the roots or leaves of a plant, they can be transported throughout the plant’s vascular system, which includes the xylem and phloem in the stem
[103]. The vascular system plays a crucial role in the distribution of water, nutrients, and various substances, including nanoparticles, to different parts of the plant.
Figure 3. Passive delivery introduction sites for in vivo transport of nanoparticles into plants.
4. Challenges during Nanoparticle Transport
One of the primary challenges of using assisted delivery techniques is the potential for plant cell damage
[104]. Assisted delivery methods, especially those involving physical forces or electrical pulses, can cause physical stress or damage to the target plant cells
[105]. This may result in cell death, reduced viability, or altered cell function. Each assisted delivery method often requires the careful optimization of parameters. Finding the optimal parameters for different applications can be labor-intensive and may involve trial and error. Some assisted delivery methods may raise safety concerns due to potential unintended biological effects. Some assisted delivery methods may also be less suitable for in vivo applications due to challenges related to safety, depth of penetration, and the potential for systemic effects. A description of the advantages and disadvantages of each assisted delivery method can be seen in
Table 4. Some of the disadvantages associated with assisted delivery methods are precisely what make passive delivery methods preferable in certain situations. Passive delivery methods are often favored when conducting studies in living plants, particularly when the goal is to minimize risks to plant health and create conditions that more closely resemble real-world scenarios.
Table 4. Advantages and disadvantages of using assisted delivery methods to introduce nanoparticles into plants.
Passive delivery methods in plants have several advantages over assisted delivery methods, especially in certain applications and contexts. Passive delivery methods do not require the application of external forces, such as mechanical or electrical forces, which can potentially damage plant cells or tissues
[8][104]. This results in less invasive effects on the plant, reducing the risk of cell damage or stress. Characterizing the mechanical and electrical properties of nanoparticles is essential for understanding their behavior and performance, especially when they interact with plant tissues
[117]. Mechanical properties at the bulk level are characterized by atomic force microscopy (AFM) and dynamic mechanical analysis (DMA)
[118]. In contrast, AFM-based nanoindentation and scanning probe microscopy (SPM) are the techniques commonly used to characterize at the nanoscale level
[119], and techniques used to characterize electrical properties at the bulk level are impedance spectroscopy and four-point probe measurement
[120]. On the other hand, techniques used to characterize at the nanoscale level are conductive AFM (cAFM), Kelvin probe force microscopy (KPFM), and transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDS)
[121]. Passive delivery methods are often simpler and more straightforward to implement. They do not require specialized equipment or complex optimization processes, making them accessible to a wider range of researchers and practitioners. Passive delivery methods are generally more cost-effective because they do not necessitate the use of expensive equipment or consumables. However, there are several challenges, particularly when it comes to particle size limitations. Passive delivery methods are often constrained by the physical characteristics of the nanoparticles being transported
[122]. In the case of roots and leaves, the size of particles that can be transported is limited by the size of pores, channels, or structures within the plant tissue
[123]. Large particles may encounter limitations in passing through these structures, thereby constraining the efficacy of passive transport. The examples presented in
Table 1 indicate that the majority of the particle sizes utilized are below 40 nm. Particles with sizes above 40 nm may not be accommodated through passive delivery via roots and leaves. Plant cells have rigid cell walls, and the pore size diameter ranges from less than 10 nm in most pores to a rare maximum size of 40 nm
[124][125][126]. McCann et al. observed that intact cell walls of
Allium cepa var. Jumbo generally measure 10–20 nm
[124]. The average cell wall size was 30 nm in width, which was visualized using spectroscopic methods
[127]. The same is true for foliar uptake, where a waxy cuticle in leaves acts as a barrier for particles entering the plant
[128]. Many particles, particularly larger ones, will not be able to pass through the stomata (microscopic openings) on the leaf surface, as these typically have diameters of less than 10 nm to 20 nm
[129]. Passive delivery methods often involve incorporating nanoparticles into the growth medium, soil, or other components of the plant’s environment during specific developmental stages, such as seed germination or seedling growth, as indicated in
Table 5. Passive delivery via root uptake and foliar uptake has its own set of advantages and disadvantages, as also listed in
Table 5. Exploring alternative introduction sites for passive transport in plants is a worthwhile direction for future research and development. It allows for the customization of delivery methods and the potential to overcome limitations associated with roots and stems.
Table 5. Advantages and disadvantages of using existing passive delivery methods and plant developmental stages to introduce nanoparticles into plants.