
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manas Gartia | -- | 7331 | 2024-01-17 16:46:00 | | | |
| 2 | Lindsay Dong | + 4 word(s) | 7335 | 2024-01-19 03:21:35 | | |
Video Upload Options
Biomarkers are vital in healthcare as they provide valuable insights into disease diagnosis, prognosis, treatment response, and personalized medicine. They serve as objective indicators, enabling early detection and intervention, leading to improved patient outcomes and reduced costs. Biomarkers also guide treatment decisions by predicting disease outcomes and facilitating individualized treatment plans. They play a role in monitoring disease progression, adjusting treatments, and detecting early signs of recurrence.
1. Introduction
2. Challenges Associated with Detecting Early-Stage Tumors
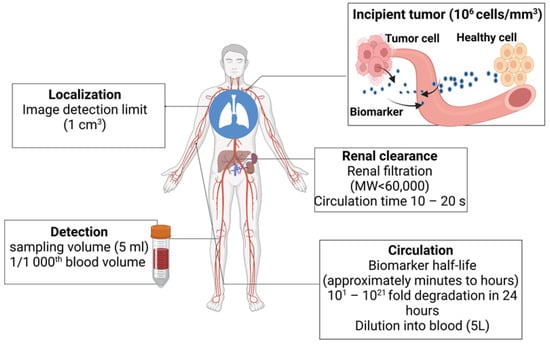
3. Biomarkers in Cancer Detection, Diagnosis, and Prognosis
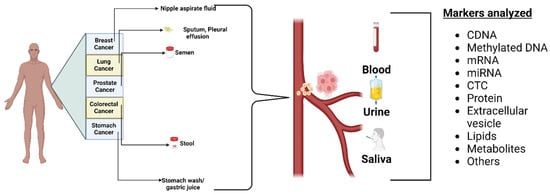
3.1. Biofluid Biomarkers
3.2. Imaging Biomarkers
3.3. Needle Biopsy
3.4. Tissue Imaging
4. Types of Cancer Biomarkers
4.1. Genetic Biomarkers
4.1.1. Mutations and Gene Alterations
4.1.2. Gene Expression Profiles
4.1.3. DNA as a Cancer Biomarker
4.1.4. RNA as a Cancer Biomarker
4.1.5. Epigenetics as a Cancer Biomarker
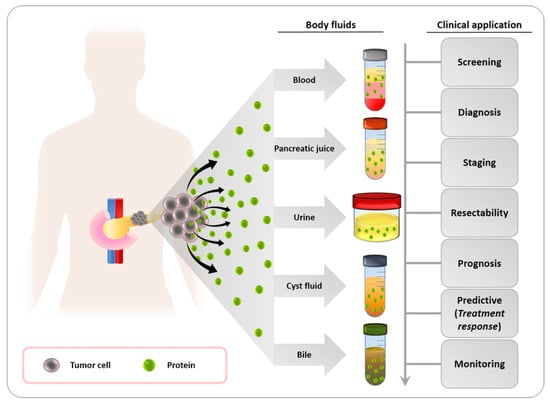
4.2. Protein Biomarkers
Proteins as Cancer Biomarkers
4.3. Metabolic Biomarkers
4.3.1. Metabolites and Metabolic Pathways
4.3.2. Metabolic Imaging Techniques
4.3.3. Molecular Probes and Contrast Agents
4.4. Cells as Cancer Biomarkers
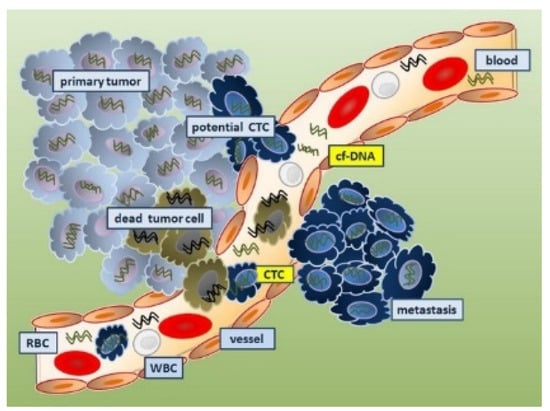
4.4.1. Circulating Tumor Cells as Cancer Biomarkers
4.4.2. Immune Cells as Cancer Biomarkers
4.4.3. Cancer Stem Cells as Cancer Biomarkers
4.5. Lamins as Cancer Biomarkers
4.6. Galectins as Cancer Biomarkers
4.7. Carbohydrate Antigens as Cancer Biomarkers
4.8. Viruses as Cancer Biomarkers
4.9. Exosomes as a Cancer Biomarker
4.10. Lipids as Cancer Biomarkers
Jiang and colleagues reported the lipid species that could be utilized as markers for early detection of breast cancer. In comparison to healthy controls, researchers noticed higher amounts of Phytosterol Diosgenin (DG), and Phosphatidylcholines (PC) in breast cancer samples. The level of Phosphatidylethanolamine (PE) was shown to be lower in breast cancer samples [27]. Prostate cancer patients have a 2.7-fold elevation in Lysophosphatidylcholine (LPC) relative to healthy subjects, according to Zhou and colleagues [28].
5. Emerging Technologies and Techniques
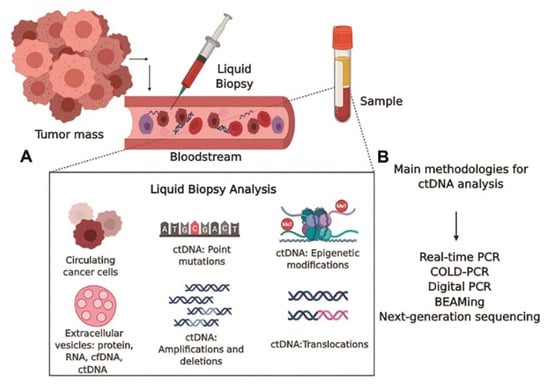
5.1. Liquid Biopsy
5.2. Single-Cell Analysis
5.3. Artificial Intelligence and Machine Learning
References
- Allegra, C.J.; Jessup, J.M.; Somerfield, M.R.; Hamilton, S.R.; Hammond, E.H.; Hayes, D.F.; McAllister, P.K.; Morton, R.F.; Schilsky, R.L. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti–epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009, 27, 2091–2096.
- Burstein, H.J.; Mangu, P.B.; Somerfield, M.R.; Schrag, D.; Samson, D.; Holt, L.; Zelman, D.; Ajani, J.A. American Society of Clinical Oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J. Clin. Oncol. 2011, 29, 3328–3330.
- Carlson, R.W.; Allred, D.C.; Anderson, B.O.; Burstein, H.J.; Carter, W.B.; Edge, S.B.; Erban, J.K.; Farrar, W.B.; Forero, A.; Giordano, S.H. Invasive breast cancer. J. Natl. Compr. Cancer Netw. 2011, 9, 136–222.
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791.
- Freidlin, B.; McShane, L.M.; Korn, E.L. Randomized clinical trials with biomarkers: Design issues. J. Natl. Cancer Inst. 2010, 102, 152–160.
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81.
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 370, 1287–1297.
- Serganova, I.; Blasberg, R.G. Molecular imaging with reporter genes: Has its promise been delivered? J. Nucl. Med. 2019, 60, 1665–1681.
- Gilad, A.A.; Shapiro, M.G. Molecular imaging in synthetic biology, and synthetic biology in molecular imaging. Mol. Imaging Biol. 2017, 19, 373–378.
- Condeelis, J.; Weissleder, R. In vivo imaging in cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003848.
- Cho, W.C. Contribution of oncoproteomics to cancer biomarker discovery. Mol. Cancer 2007, 6, 25.
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548.
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406.
- Bardelli, A.; Pantel, K. Liquid biopsies, what we do not know (yet). Cancer Cell 2017, 31, 172–179.
- Tan, G.H.; Nason, G.; Ajib, K.; Woon, D.T.S.; Herrera-Caceres, J.; Alhunaidi, O.; Perlis, N. Smarter screening for prostate cancer. World J. Urol. 2019, 37, 991–999.
- Gahan, P.; Stroun, M. The biology of circulating nucleic acids in plasma and serum (CNAPS). In Extracellular Nucleic Acids; Springer: Berlin/Heidelberg, Germany, 2010; pp. 167–189.
- Porto-Mascarenhas, E.C.; Assad, D.X.; Chardin, H.; Gozal, D.; Canto, G.D.L.; Acevedo, A.C.; Guerra, E.N.S. Salivary biomarkers in the diagnosis of breast cancer: A review. Crit. Rev. Oncol./Hematol. 2017, 110, 62–73.
- Lawrence, H.P. Salivary markers of systemic disease: Noninvasive diagnosis of disease and monitoring of general health. J.-Can. Dent. Assoc. 2002, 68, 170–175.
- Zhang, L.; Farrell, J.J.; Zhou, H.; Elashoff, D.; Akin, D.; Park, N.H.; Chia, D.; Wong, D.T. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology 2010, 138, 949–957.e7.
- Gao, S.; Chen, L.-Y.; Wang, P.; Liu, L.-M.; Chen, Z. MicroRNA expression in salivary supernatant of patients with pancreatic cancer and its relationship with ZHENG. BioMed Res. Int. 2014, 2014, 756347.
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary microRNA in pancreatic cancer patients. PLoS ONE 2015, 10, e0130996.
- Lau, C.; Kim, Y.; Chia, D.; Spielmann, N.; Eibl, G.; Elashoff, D.; Wei, F.; Lin, Y.-L.; Moro, A.; Grogan, T. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J. Biol. Chem. 2013, 288, 26888–26897.
- Zhang, L.; Xiao, H.; Zhou, H.; Santiago, S.; Lee, J.M.; Garon, E.B.; Yang, J.; Brinkmann, O.; Yan, X.; Akin, D. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell. Mol. Life Sci. 2012, 69, 3341–3350.
- Wu, Z.-Z.; Wang, J.-G.; Zhang, X.-L. Diagnostic model of saliva protein finger print analysis of patients with gastric cancer. World J. Gastroenterol. WJG 2009, 15, 865.
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7.
- Landgren, O.; Porwit MacDonald, A.; Tani, E.; Czader, M.; Grimfors, G.; Skoog, L.; Ost, A.; Wedelin, C.; Axdorph, U.; Svedmyr, E. A prospective comparison of fine-needle aspiration cytology and histopathology in the diagnosis and classification of lymphomas. Hematol. J. 2004, 5, 69–76.
- Barker, A.; Kettle, J.G.; Nowak, T.; Pease, J.E. Expanding medicinal chemistry space. Drug Discov. Today 2013, 18, 298–304.
- Blagg, J.; Workman, P. Chemical biology approaches to target validation in cancer. Curr. Opin. Pharmacol. 2014, 17, 87–100.
- Atlas, A.B.-R. Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013.
- Edge, S.B.; Cancer, A.J.C.o. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7.
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247.
- Reid, D.M.; Doughty, J.; Eastell, R.; Heys, S.D.; Howell, A.; McCloskey, E.V.; Powles, T.; Selby, P.; Coleman, R.E. Guidance for the management of breast cancer treatment-induced bone loss: A consensus position statement from a UK Expert Group. Cancer Treat. Rev. 2008, 34, S3–S18.
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.–Cardiovasc. Imaging 2014, 15, 1063–1093.
- Tzogani, K.; Skibeli, V.; Westgaard, I.; Dalhus, M.; Thoresen, H.; Slot, K.B.; Damkier, P.; Hofland, K.; Borregaard, J.; Ersbøll, J. The European Medicines Agency approval of axitinib (Inlyta) for the treatment of advanced renal cell carcinoma after failure of prior treatment with sunitinib or a cytokine: Summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2015, 20, 196–201.
- U.S. Food & Drug Administration. Novel Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 (accessed on 18 December 2023).
- Bergström, M.; Hargreaves, R.J.; Burns, H.D.; Goldberg, M.R.; Sciberras, D.; Reines, S.A.; Petty, K.J.; Ögren, M.; Antoni, G.; Långström, B. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol. Psychiatry 2004, 55, 1007–1012.
- Willett, C.G.; Boucher, Y.; Di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V. Erratum: Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer (Nature Medicine (2004) 10 (145-147)). Nat. Med. 2004, 10, 649.
- Avril, N.; Propper, D. Functional PET imaging in cancer drug development. Future Med. 2007, 3, 215–228.
- O’connor, J.P.; Jackson, A.; Parker, G.J.; Roberts, C.; Jayson, G.C. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat. Rev. Clin. Oncol. 2012, 9, 167–177.
- Lassau, N.; Chapotot, L.; Benatsou, B.; Vilgrain, V.; Kind, M.; Lacroix, J.; Cuinet, M.; Taieb, S.; Aziza, R.; Sarran, A. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: The French multicenter Support for Innovative and Expensive Techniques Study. Investig. Radiol. 2012, 47, 711–716.
- Gupta, R.; Naran, S.; Lallu, S.; Fauck, R. The diagnostic value of fine needle aspiration cytology (FNAC) in the assessment of palpable supraclavicular lymph nodes: A study of 218 cases. Cytopathology 2003, 14, 201–207.
- Ben-Yehuda, D.; Polliack, A.; Okon, E.; Sherman, Y.; Fields, S.; Lebenshart, P.; Lotan, H.; Libson, E. Image-guided core-needle biopsy in malignant lymphoma: Experience with 100 patients that suggests the technique is reliable. J. Clin. Oncol. 1996, 14, 2431–2434.
- Pappa, V.; Hussain, H.; Reznek, R.; Whelan, J.; Norton, A.; Wilson, A.; Love, S.; Lister, T.; Rohatiner, A. Role of image-guided core-needle biopsy in the management of patients with lymphoma. J. Clin. Oncol. 1996, 14, 2427–2430.
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood J. Am. Soc. Hematol. 2011, 117, 5019–5032.
- Tatli, S.; Gerbaudo, V.H.; Mamede, M.; Tuncali, K.; Shyn, P.B.; Silverman, S.G. Abdominal masses sampled at PET/CT-guided percutaneous biopsy: Initial experience with registration of prior PET/CT images. Radiology 2010, 256, 305–311.
- Lawson, M.H.; Rassl, D.M.; Cummings, N.M.; Russell, R.; Morjaria, J.B.; Brenton, J.D.; Murphy, G.; Rintoul, R.C. Tissue banking of diagnostic lung cancer biopsies for extraction of high quality RNA. J. Thorac. Oncol. 2010, 5, 956–963.
- de Kerviler, E.; Guermazi, A.; Zagdanski, A.M.; Meignin, V.; Gossot, D.; Oksenhendler, E.; Mariette, X.; Brice, P.; Frija, J. Image-guided core-needle biopsy in patients with suspected or recurrent lymphomas. Cancer 2000, 89, 647–652.
- Demharter, J.; Müller, P.; Wagner, T.; Schlimok, G.; Haude, K.; Bohndorf, K. Percutaneous core-needle biopsy of enlarged lymph nodes in the diagnosis and subclassification of malignant lymphomas. Eur. Radiol. 2001, 11, 276–283.
- Loubeyre, P.; McKee, T.; Copercini, M.; Rosset, A.; Dietrich, P. Diagnostic precision of image-guided multisampling core needle biopsy of suspected lymphomas in a primary care hospital. Br. J. Cancer 2009, 100, 1771–1776.
- Amador-Ortiz, C.; Chen, L.; Hassan, A.; Frater, J.L.; Burack, R.; Nguyen, T.T.; Kreisel, F. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. Am. J. Clin. Pathol. 2011, 135, 516–524.
- Shaw, J.; Rumball, E. Complications and local recurrence following lymphadenectomy. Br. J. Surg. 1990, 77, 760–764.
- de Kerviler, E.; de Bazelaire, C.; Mounier, N.; Mathieu, O.; Brethon, B.; Brière, J.; Marolleau, J.-P.; Brice, P.; Gisselbrecht, C.; Frija, J. Image-guided core-needle biopsy of peripheral lymph nodes allows the diagnosis of lymphomas. Eur. Radiol. 2007, 17, 843–849.
- Sklair-Levy, M.; Amir, G.; Spectre, G.; Lebensart, P.; Applbaum, Y.; Agid, R.; Lieberman, S.; Ben-Yehuda, D.; Sherman, Y.; Libson, E. Image-guided cutting-edge-needle biopsy of peripheral lymph nodes and superficial masses for the diagnosis of lymphoma. J. Comput. Assist. Tomogr. 2005, 29, 369–372.
- Tangella, L.P.; Clark, M.E.; Gray, E.S. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma-A mini review. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129736.
- Passaro, A.; Mok, T.; Peters, S.; Popat, S.; Ahn, M.-J.; De Marinis, F. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J. Thorac. Oncol. 2021, 16, 764–773.
- Zenonos, K.; Kyprianou, K. RAS signaling pathways, mutations and their role in colorectal cancer. World J. Gastrointest. Oncol. 2013, 5, 97.
- Pujol, P.; Barberis, M.; Beer, P.; Friedman, E.; Piulats, J.M.; Capoluongo, E.D.; Foncillas, J.G.; Ray-Coquard, I.; Penault-Llorca, F.; Foulkes, W.D. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur. J. Cancer 2021, 146, 30–47.
- Amisha, F.; Malik, P.; Saluja, P.; Gautam, N.; Patel, T.H.; Roy, A.M.; Singh, S.R.; Malapati, S.J. A Comprehensive Review on the Role of Human Epidermal Growth Factor Receptor 2 (HER2) as a Biomarker in Extra-Mammary and Extra-Gastric Cancers. Onco 2023, 3, 96–124.
- Ma, R.; de Pennington, N.; Hofer, M.; Blesing, C.; Stacey, R. Diagnostic and prognostic markers in gliomas—An update. Br. J. Neurosurg. 2013, 27, 311–315.
- Bernhardt, S.M.; Dasari, P.; Wrin, J.; Raymond, W.; Edwards, S.; Walsh, D.; Townsend, A.R.; Price, T.J.; Ingman, W.V. Discordance in 21-gene recurrence scores between paired breast cancer samples is inversely associated with patient age. Breast Cancer Res. 2020, 22, 90.
- Haan, J.C.; Bhaskaran, R.; Ellappalayam, A.; Bijl, Y.; Griffioen, C.J.; Lujinovic, E.; Audeh, W.M.; Penault-Llorca, F.; Mittempergher, L.; Glas, A.M. MammaPrint and BluePrint comprehensively capture the cancer hallmarks in early-stage breast cancer patients. Genes Chromosomes Cancer 2022, 61, 148–160.
- Verma, M.; Kumar, D. Application of mitochondrial genome information in cancer epidemiology. Clin. Chim. Acta 2007, 383, 41–50.
- Sidransky, D. Emerging molecular markers of cancer. Nat. Rev. Cancer 2002, 2, 210–219.
- Leon, S.; Shapiro, B.; Sklaroff, D.; Yaros, M. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977, 37, 646–650.
- Verma, M.; Srivastava, S. Epigenetics in cancer: Implications for early detection and prevention. Lancet Oncol. 2002, 3, 755–763.
- Verma, M.; Kagan, J.; Sidransky, D.; Srivastava, S. Proteomic analysis of cancer-cell mitochondria. Nat. Rev. Cancer 2003, 3, 789–795.
- Jin, H.; Ma, Y.; Shen, Q.; Wang, X. Circulating methylated DNA as biomarkers for cancer detection. In Methylation: From DNA, RNA and Histones to Diseases and Treatment; InTech: London, UK, 2012; p. 137.
- Velculescu, V.E.; Zhang, L.; Vogelstein, B.; Kinzler, K.W. Serial analysis of gene expression. Science 1995, 270, 484–487.
- Bartels, C.L.; Tsongalis, G.J. MicroRNAs: Novel biomarkers for human cancer. Clin. Chem. 2009, 55, 623–631.
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866.
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838.
- Kasinski, A.L.; Slack, F.J. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864.
- Stahlhut, C.; Slack, F.J. MicroRNAs and the cancer phenotype: Profiling, signatures and clinical implications. Genome Med. 2013, 5, 111.
- Manterola, L.; Guruceaga, E.; Pérez-Larraya, J.G.; González-Huarriz, M.; Jauregui, P.; Tejada, S.; Diez-Valle, R.; Segura, V.; Samprón, N.; Barrena, C. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-Oncol. 2014, 16, 520–527.
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529.
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856.
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338.
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691.
- Zhu, X.; Wang, X.; Wei, S.; Chen, Y.; Chen, Y.; Fan, X.; Han, S.; Wu, G. hsa_circ_0013958: A Circular RNA and Potential Novel Biomarker for Lung Adenocarcinoma; Wiley Online Library: Hoboken, NJ, USA, 2017; Volume 284, pp. 2170–2182.
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054.
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463.
- Belinsky, S.A. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat. Rev. Cancer 2004, 4, 707–717.
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867.
- Colantonio, D.A.; Chan, D.W. The clinical application of proteomics. Clin. Chim. Acta 2005, 357, 151–158.
- Jimenez-Luna, C.; Torres, C.; Ortiz, R.; Dieguez, C.; Martinez-Galan, J.; Melguizo, C.; Prados, J.C.; Caba, O. Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget 2018, 9, 16573.
- Sandfeld-Paulsen, B.; Jakobsen, K.R.; Bæk, R.; Folkersen, B.H.; Rasmussen, T.R.; Meldgaard, P.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol. 2016, 11, 1701–1710.
- Nedjadi, T.; Benabdelkamal, H.; Albarakati, N.; Masood, A.; Al-Sayyad, A.; Alfadda, A.A.; Alanazi, I.O.; Al-Ammari, A.; Al-Maghrabi, J. Circulating proteomic signature for detection of biomarkers in bladder cancer patients. Sci. Rep. 2020, 10, 10999.
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47.
- Nava, G.M.; Madrigal Perez, L.A. Metabolic profile of the Warburg effect as a tool for molecular prognosis and diagnosis of cancer. Expert Rev. Mol. Diagn. 2022, 22, 439–447.
- Cardaci, S.; Ciriolo, M.R. TCA cycle defects and cancer: When metabolism tunes redox state. Int. J. Cell Biol. 2012, 2012, 161837.
- Minton, D.R.; Fu, L.; Chen, Q.; Robinson, B.D.; Gross, S.S.; Nanus, D.M.; Gudas, L.J. Analyses of the transcriptome and metabolome demonstrate that HIF1α mediates altered tumor metabolism in clear cell renal cell carcinoma. PLoS ONE 2015, 10, e0120649.
- Mori, N.; Wildes, F.; Takagi, T.; Glunde, K.; Bhujwalla, Z.M. The tumor microenvironment modulates choline and lipid metabolism. Front. Oncol. 2016, 6, 262.
- Wu, X.; Qin, L.; Fako, V.; Zhang, J.-T. Molecular mechanisms of fatty acid synthase (FASN)-mediated resistance to anti-cancer treatments. Adv. Biol. Regul. 2014, 54, 214–221.
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30.
- Aird, K.M.; Zhang, R. Nucleotide metabolism, oncogene-induced senescence and cancer. Cancer Lett. 2015, 356, 204–210.
- Mankoff, D.A.; Eary, J.F.; Link, J.M.; Muzi, M.; Rajendran, J.G.; Spence, A.M.; Krohn, K.A. Tumor-specific positron emission tomography imaging in patients: fluorodeoxyglucose and beyond. Clin. Cancer Res. 2007, 13, 3460–3469.
- Glunde, K.; Artemov, D.; Penet, M.-F.; Jacobs, M.A.; Bhujwalla, Z.M. Magnetic resonance spectroscopy in metabolic and molecular imaging and diagnosis of cancer. Chem. Rev. 2010, 110, 3043–3059.
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C. Hyperpolarized 13C MRI: Path to clinical translation in oncology. Neoplasia 2019, 21, 1–16.
- Vallabhajosula, S.; Killeen, R.P.; Osborne, J.R. Altered biodistribution of radiopharmaceuticals: Role of radiochemical/pharmaceutical purity, physiological, and pharmacologic factors. Semin. Nucl. Med. 2010, 40, 220–241.
- Barrett, T.; Brechbiel, M.; Bernardo, M.; Choyke, P.L. MRI of tumor angiogenesis. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 26, 235–249.
- Koyama, Y.; Barrett, T.; Hama, Y.; Ravizzini, G.; Choyke, P.L.; Kobayashi, H. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia 2007, 9, 1021–1029.
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application. Int. J. Mol. Med. 2016, 38, 1319–1326.
- Barwick, T.; Bencherif, B.; Mountz, J.M.; Avril, N. Molecular PET and PET/CT imaging of tumour cell proliferation using F-18 fluoro-L-thymidine: A comprehensive evaluation. Nucl. Med. Commun. 2009, 30, 908–917.
- Klibanov, A.L. Ligand-carrying gas-filled microbubbles: Ultrasound contrast agents for targeted molecular imaging. Bioconjugate Chem. 2005, 16, 9–17.
- Bu, L.; Shen, B.; Cheng, Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv. Drug Deliv. Rev. 2014, 76, 21–38.
- Ring, A.; Smith, I.E.; Dowsett, M. Circulating tumour cells in breast cancer. Lancet Oncol. 2004, 5, 79–88.
- Cristofanilli, M.; Budd, G.; Ellis, M.; Stopeck, A.; Matera, J.; Miller, M.; Doyle, G.; Allard, W.; Terstappen, L.; Hayes, D. Presence of circulating tumor cells (CTC) in metastatic breast cancer (MBC) predicts rapid progression and poor prognosis. J. Clin. Oncol. 2005, 23, 524.
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964.
- Halama, N.; Braun, M.; Kahlert, C.; Spille, A.; Quack, C.; Rahbari, N.; Koch, M.; Weitz, J.; Kloor, M.; Zoernig, I. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin. Cancer Res. 2011, 17, 678–689.
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016, 44, 698–711.
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734.
- Kather, J.N.; Poleszczuk, J.; Suarez-Carmona, M.; Krisam, J.; Charoentong, P.; Valous, N.A.; Weis, C.-A.; Tavernar, L.; Leiss, F.; Herpel, E. In silico modeling of immunotherapy and stroma-targeting therapies in human colorectal cancer. Cancer Res. 2017, 77, 6442–6452.
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306.
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E. Topography of cancer-associated immune cells in human solid tumors. Elife 2018, 7, e36967.
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C. Towards the introduction of the ‘Immunoscore’in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209.
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241.
- Lee, S.; de Boer, W.B.; Fermoyle, S.; Platten, M.; Kumarasinghe, M.P. Human epidermal growth factor receptor 2 testing in gastric carcinoma: Issues related to heterogeneity in biopsies and resections. Histopathology 2011, 59, 832–840.
- Haikal, A.; Borba, E.; Khaja, T.; Doolittle, G.; Schmidt, P. Nivolumab-induced new-onset seronegative rheumatoid arthritis in a patient with advanced metastatic melanoma: A case report and literature review. Avicenna J. Med. 2018, 8, 34–36.
- Liyanage, U.K.; Moore, T.T.; Joo, H.-G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002, 169, 2756–2761.
- Siddiqui, S.A.; Frigola, X.; Bonne-Annee, S.; Mercader, M.; Kuntz, S.M.; Krambeck, A.E.; Sengupta, S.; Dong, H.; Cheville, J.C.; Lohse, C.M. Tumor-infiltrating Foxp3− CD4+ CD25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 2075–2081.
- Woo, E.Y.; Yeh, H.; Chu, C.S.; Schlienger, K.; Carroll, R.G.; Riley, J.L.; Kaiser, L.R.; June, C.H. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 2002, 168, 4272–4276.
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949.
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.L.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009, 69, 5996–6004.
- Xue, D.; Xia, T.; Wang, J.; Chong, M.; Wang, S.; Zhang, C. Role of regulatory T cells and CD8+ T lymphocytes in the dissemination of circulating tumor cells in primary invasive breast cancer. Oncol. Lett. 2018, 16, 3045–3053.
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344.
- Clarke, M.F.; Fuller, M. Stem cells and cancer: Two faces of eve. Cell 2006, 124, 1111–1115.
- Takaishi, S.; Okumura, T.; Wang, T.C. Gastric cancer stem cells. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2876.
- Xin, H.-W.; Hari, D.M.; Mullinax, J.E.; Ambe, C.M.; Koizumi, T.; Ray, S.; Anderson, A.J.; Wiegand, G.W.; Garfield, S.H.; Thorgeirsson, S.S. Tumor-initiating label-retaining cancer cells in human gastrointestinal cancers undergo asymmetric cell division. Stem Cells 2012, 30, 591–598.
- Marquardt, J.U.; Thorgeirsson, S.S. Stem cells in hepatocarcinogenesis: Evidence from genomic data. Semin. Liver Dis. 2010, 30, 26–34.
- Langan, R.C.; Mullinax, J.E.; Ray, S.; Raiji, M.T.; Schaub, N.; Xin, H.-W.; Koizumi, T.; Steinberg, S.M.; Anderson, A.; Wiegand, G. A pilot study assessing the potential role of non-CD133 colorectal cancer stem cells as biomarkers. J. Cancer 2012, 3, 231.
- Papailiou, J.; Bramis, K.J.; Gazouli, M.; Theodoropoulos, G. Stem cells in colon cancer. A new era in cancer theory begins. Int. J. Color. Dis. 2011, 26, 1–11.
- Lim, S.-C.; Oh, S.-H. The role of CD24 in various human epithelial neoplasias. Pathol.-Res. Pract. 2005, 201, 479–486.
- Weichert, W.; Denkert, C.; Burkhardt, M.; Gansukh, T.; Bellach, J.; Altevogt, P.; Dietel, M.; Kristiansen, G. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin. Cancer Res. 2005, 11, 6574–6581.
- Akamine, T.; Tagawa, T.; Ijichi, K.; Toyokawa, G.; Takamori, S.; Hirai, F.; Okamoto, T.; Oda, Y.; Maehara, Y. The significance of CD44 variant 9 in resected lung adenocarcinoma: Correlation with pathological early-stage and EGFR mutation. Ann. Surg. Oncol. 2019, 26, 1544–1551.
- Lau, W.M.; Teng, E.; Chong, H.S.; Lopez, K.A.P.; Tay, A.Y.L.; Salto-Tellez, M.; Shabbir, A.; So, J.B.Y.; Chan, S.L. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014, 74, 2630–2641.
- Shimi, T.; Pfleghaar, K.; Kojima, S.-i.; Pack, C.-G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T. The A-and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008, 22, 3409–3421.
- Burke, B.; Stewart, C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24.
- Meng, E.; Mitra, A.; Tripathi, K.; Finan, M.A.; Scalici, J.; McClellan, S.; da Silva, L.M.; Reed, E.; Shevde, L.A.; Palle, K. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS ONE 2014, 9, e107142.
- Tripathi, K.; Mani, C.; Barnett, R.; Nalluri, S.; Bachaboina, L.; Rocconi, R.P.; Athar, M.; Owen, L.B.; Palle, K. Gli1 protein regulates the S-phase checkpoint in tumor cells via Bid protein, and its inhibition sensitizes to DNA topoisomerase 1 inhibitors. J. Biol. Chem. 2014, 289, 31513–31525.
- Dings, R.P.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as molecular targets for therapeutic intervention. Int. J. Mol. Sci. 2018, 19, 905.
- Zhang, H.; Liang, X.; Duan, C.; Liu, C.; Zhao, Z. Galectin-3 as a marker and potential therapeutic target in breast cancer. PLoS ONE 2014, 9, e103482.
- Koo, J.S.; Jung, W. Clinicopathlogic and immunohistochemical characteristics of triple negative invasive lobular carcinoma. Yonsei Med. J. 2011, 52, 89–97.
- Cindolo, L.; Benvenuto, G.; Salvatore, P.; Pero, R.; Salvatore, G.; Mirone, V.; Prezioso, D.; Altieri, V.; Bruni, C.B.; Chiariotti, L. Galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer 1999, 84, 39–43.
- Gillenwater, A.; Xu, X.C.; El-Naggar, A.K.; Clayman, G.L.; Lotan, R. Expression of galectins in head and neck squamous cell carcinoma. Head Neck J. Sci. Spec. Head Neck 1996, 18, 422–432.
- Kuo, P.-L.; Hung, J.-Y.; Huang, S.-K.; Chou, S.-H.; Cheng, D.-E.; Jong, Y.-J.; Hung, C.-H.; Yang, C.-J.; Tsai, Y.-M.; Hsu, Y.-L. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J. Immunol. 2011, 186, 1521–1530.
- Langbein, S.; Brade, J.; Badawi, J.K.; Hatzinger, M.; Kaltner, H.; Lensch, M.; Specht, K.; André, S.; Brinck, U.; Alken, P. Gene-expression signature of adhesion/growth-regulatory tissue lectins (galectins) in transitional cell cancer and its prognostic relevance. Histopathology 2007, 51, 681–690.
- Szöke, T.; Kayser, K.; Baumhäkel, J.-D.; Trojan, I.; Furak, J.; Tiszlavicz, L.; Horvath, A.; Szluha, K.; Gabius, H.-J.; Andre, S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology 2005, 69, 167–174.
- Tang, C.-E.; Tan, T.; Li, C.; Chen, Z.-C.; Ruan, L.; Wang, H.-H.; Su, T.; Zhang, P.-F.; Xiao, Z.-Q. Identification of Galectin-1 as a novel biomarker in nasopharyngeal carcinoma by proteomic analysis. Oncol. Rep. 2010, 24, 495–500.
- von Klot, C.-A.; Kramer, M.W.; Peters, I.; Hennenlotter, J.; Abbas, M.; Scherer, R.; Herrmann, T.R.; Stenzl, A.; Kuczyk, M.A.; Serth, J. Galectin-1 and Galectin-3 mRNA expression in renal cell carcinoma. BMC Clin. Pathol. 2014, 14, 15.
- Chiariotti, L.; Berlingieri, M.T.; Battaglia, C.; Benvenuto, G.; Martelli, M.L.; Salvatore, P.; Chiappettaxy, G.; Bruni, C.B.; Fusco, A. Expression of galectin-1 in normal human thyroid gland and in differentiated and poorly differentiated thyroid tumors. Int. J. Cancer 1995, 64, 171–175.
- Torres-Cabala, C.; Bibbo, M.; Panizo-Santos, A.; Barazi, H.; Krutzsch, H.; Roberts, D.D.; Merino, M.J. Proteomic identification of new biomarkers and application in thyroid cytology. Acta Cytol. 2006, 50, 518–528.
- Xu, X.-C.; El-Naggar, A.K.; Lotan, R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815.
- Čada, Z.; Smetana Jr, K.; Lacina, L.; Plzáková, Z.; Štork, J.; Kaltner, H.; Russwurm, R.; Lensch, M.; André, S.; Gabius, H. Immunohistochemical Fingerprinting of the Network of Seven Adhe sion/Growth-Regulatory Lectins in Human Skin and De-tection of Distinct Tumour-Associated Alterations. Folia Biol. 2009, 55, 145–152.
- Mathieu, V.; De Lassalle, E.M.; Toelen, J.; Mohr, T.; Bellahcene, A.; Van Goietsenoven, G.; Verschuere, T.; Bouzin, C.; Debyser, Z.; De Vleeschouwer, S. Galectin-1 in melanoma biology and related neo-angiogenesis processes. J. Investig. Dermatol. 2012, 132, 2245–2254.
- Choufani, G.; Nagy, N.; Saussez, S.; Marchant, H.; Bisschop, P.; Burchert, M.; Danguy, A.; Louryan, S.; Salmon, I.; Gabius, H.J. The levels of expression of galectin-1, galectin-3, and the Thomsen–Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1999, 86, 2353–2363.
- Makino, K.; Kawamura, K.; Sato, W.; Kawamura, N.; Fujimoto, T.; Terada, Y. Inhibition of uterine sarcoma cell growth through suppression of endogenous tyrosine kinase B signaling. PLoS ONE 2012, 7, e41049.
- Chung, H.; Kim, B.; Jung, S.-H.; Won, K.-J.; Jiang, X.; Lee, C.-K.; Lim, S.D.; Yang, S.-K.; Song, K.H.; Kim, H.S. Does phosphorylation of cofilin affect the progression of human bladder cancer? BMC Cancer 2013, 13, 45.
- Kim, S.-J.; Hwang, J.-A.; Ro, J.Y.; Lee, Y.-S.; Chun, K.-H. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget 2013, 4, 1461.
- Zhu, X.; Ding, M.; Yu, M.-L.; Feng, M.-X.; Tan, L.-J.; Zhao, F.-K. Identification of galectin-7 as a potential biomarker for esophageal squamous cell carcinoma by proteomic analysis. BMC Cancer 2010, 10, 290.
- Demers, M.; Rose, A.A.; Grosset, A.-A.; Biron-Pain, K.; Gaboury, L.; Siegel, P.M.; St-Pierre, Y. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am. J. Pathol. 2010, 176, 3023–3031.
- Rorive, S.; Eddafali, B.; Fernandez, S.; Decaestecker, C.; André, S.; Kaltner, H.; Kuwabara, I.; Liu, F.-T.; Gabius, H.-J.; Kiss, R. Changes in galectin-7 and cytokeratin-19 expression during the progression of malignancy in thyroid tumors: Diagnostic and biological implications. Mod. Pathol. 2002, 15, 1294–1301.
- Cada, Z.; Chovanec, M.; Smetana Jr, K.; Betka, J.; Lacina, L.; Plzák, J.; Kodet, R.; Stork, J.; Lensch, M.; Kaltner, H. Galectin-7, will the lectinrsquos activity establish clinical correlations in head and neck squamous cell and basal cell carcinomas? Histol. Histopathol. 2009, 24, 41–48.
- Sakaki, M.; Oka, N.; Nakanishi, R.; Yamaguchi, K.; Fukumori, T.; Kanayama, H.-o. Serum level of galectin-3 in human bladder cancer. J. Med. Investig. 2008, 55, 127–132.
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.-G. Serum galectin-2,-4, and-8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 2011, 17, 7035–7046.
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.-G. Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin. Cancer Res. 2013, 19, 1693–1704.
- Iacovazzi, P.A.; Notarnicola, M.; Caruso, M.G.; Guerra, V.; Frisullo, S.; Altomare, D.F.; Correale, M. Serum levels of galectin-3 and its ligand 90k/mac-2bp in colorectal cancer patients. Immunopharmacol. Immunotoxicol. 2010, 32, 160–164.
- Watanabe, M.; Takemasa, I.; Kaneko, N.; Yokoyama, Y.; Matsuo, E.-I.; Iwasa, S.; Mori, M.; Matsuura, N.; Monden, M.; Nishimura, O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol. Rep. 2011, 25, 1217–1226.
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, e232.
- De Caceres, I.I.; Battagli, C.; Esteller, M.; Herman, J.G.; Dulaimi, E.; Edelson, M.I.; Bergman, C.; Ehya, H.; Eisenberg, B.L.; Cairns, P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004, 64, 6476–6481.
- Chen, C.J.; Wang, L.Y.; Yu, M.W. Epidemiology of hepatitis B virus infection in the Asia–Pacific region. J. Gastroenterol. Hepatol. 2000, 15, E3–E6.
- Kirk, G.D.; Bah, E.; Montesano, R. Molecular epidemiology of human liver cancer: Insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis 2006, 27, 2070–2082.
- Shukla, S.; Bharti, A.C.; Mahata, S.; Hussain, S.; Kumar, R.; Hedau, S.; Das, B.C. Infection of human papillomaviruses in cancers of different human organ sites. Indian J. Med. Res. 2009, 130, 222–233.
- Kreimer, A.R.; Clifford, G.M.; Snijders, P.J.; Castellsagué, X.; Meijer, C.J.; Pawlita, M.; Viscidi, R.; Herrero, R.; Franceschi, S. HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int. J. Cancer 2005, 115, 329–332.
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334.
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296.
- Masaoutis, C.; Mihailidou, C.; Tsourouflis, G.; Theocharis, S. Exosomes in lung cancer diagnosis and treatment. From the translating research into future clinical practice. Biochimie 2018, 151, 27–36.
- Whiteside, T.L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2016, 74, 103–141.
- Suchorska, W.M.; Lach, M.S. The role of exosomes in tumor progression and metastasis. Oncol. Rep. 2016, 35, 1237–1244.
- Kimura, H.; Yamamoto, H.; Harada, T.; Fumoto, K.; Osugi, Y.; Sada, R.; Maehara, N.; Hikita, H.; Mori, S.; Eguchi, H. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin. Cancer Res. 2019, 25, 1936–1947.
- Schwarzenbach, H.; Gahan, P.B. Predictive value of exosomes and their cargo in drug response/resistance of breast cancer patients. Cancer Drug Resist. 2020, 3, 63.
- Sohn, W.; Kim, J.; Kang, S.H.; Yang, S.R.; Cho, J.-Y.; Cho, H.C.; Shim, S.G.; Paik, Y.-H. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 2015, 47, e184.
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41.
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol./Hematol. 2020, 155, 103109.




