Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Azher Hassan | -- | 3591 | 2024-01-17 12:26:31 | | | |
| 2 | Lindsay Dong | + 42 word(s) | 3633 | 2024-01-19 03:02:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Memon, M.A.; Jiang, Y.; Hassan, M.A.; Ajmal, M.; Wang, H.; Liu, Y. Heterogeneous Catalysts for Carbon Dioxide Methanation. Encyclopedia. Available online: https://encyclopedia.pub/entry/53973 (accessed on 07 February 2026).
Memon MA, Jiang Y, Hassan MA, Ajmal M, Wang H, Liu Y. Heterogeneous Catalysts for Carbon Dioxide Methanation. Encyclopedia. Available at: https://encyclopedia.pub/entry/53973. Accessed February 07, 2026.
Memon, Mazhar Ahmed, Yanan Jiang, Muhammad Azher Hassan, Muhammad Ajmal, Hong Wang, Yuan Liu. "Heterogeneous Catalysts for Carbon Dioxide Methanation" Encyclopedia, https://encyclopedia.pub/entry/53973 (accessed February 07, 2026).
Memon, M.A., Jiang, Y., Hassan, M.A., Ajmal, M., Wang, H., & Liu, Y. (2024, January 17). Heterogeneous Catalysts for Carbon Dioxide Methanation. In Encyclopedia. https://encyclopedia.pub/entry/53973
Memon, Mazhar Ahmed, et al. "Heterogeneous Catalysts for Carbon Dioxide Methanation." Encyclopedia. Web. 17 January, 2024.
Copy Citation
CO2 methanation offers a promising route for converting CO2 into valuable chemicals and energy fuels at the same time as hydrogen is stored in methane, so the development of suitable catalysts is crucial. Among the noble metal-based catalysts (Ru, Rh, and Pd), Ru-based catalysts show the best catalytic performance. In the non-noble metal catalysts, Ni-based catalysts are the best among Ni-, Co-, and Fe-based catalysts. The factors predominantly affecting catalytic performance are the dispersion of the active metal; the synergy of the active metal with support; and the addition of dopants.
carbon dioxide
methanation
hydrogen
heterogeneous catalyst
1. Introduction
Fossil fuels have been considered the world’s principal energy resource since the early 1970s [1][2][3]. After the industrial revolution, using different energy sources led to global warming and climate change [4][5]. Oil industries, power production plants, cement factories, steel, building constructions, and iron manufacturers are regarded as major contributors to the rise in the emission of carbon dioxide (CO2), which is a well-known greenhouse gas [6][7][8][9]. The sixth report of the Intergovernmental Panel on Climate Change (IPCC) states that global net anthropogenic emissions include a considerable amount of CO2, equivalent to 75%, which is the byproduct of the world energy sector [10]. Moreover, anthropogenic CO2 is a promoter of many other pollutants and a predominant global warming precursor [5][11][12]. Fossil fuels, industrial processes, land use change, and forestry are significantly responsible for releasing CO2 into the environment, as shown in Figure 1a. Region-wise global cumulative net anthropogenic CO2 is shown in Figure 1b [13]. The cumulative net anthropogenic analysis reveals North America is the lead emitter, releasing 23% of CO2 into the environment from 1850 to 2019.
CO2 emissions into the atmosphere are increasing every second, directly affecting global warming and climate change [6][10]. A record increase in CO2 was reported at 424 parts per million (ppm) on a global average in May 2023, reported by the Mauna Loa Observatory, as shown in Figure 1c. If this ongoing upward trend persists, it could have a detrimental impact on the global average temperature [14]. The recorded average growth in CO2 concentration in the last decade was 2 ppm per year [15]. The current and projected trends are identical, as many underdeveloped countries still produce 65% of their energy from the combustion of fossil fuels [16]. It is a harsh reality that we—mankind—are solely responsible for climate change and global warming [3]. Suppose that considerable actions are not taken right now to regulate and stabilize the emission of CO2 into the atmosphere. In that case, a disastrous threat will come from ecosystem destruction due to heavy rain spells, tornadoes, and storms; flooding from low coastal glaciers melting; and a rise in sea levels [3][10].
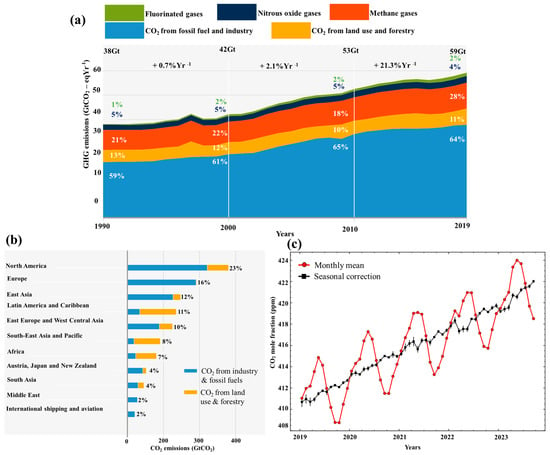
Figure 1. Global trend in CO2 emissions from 1850 to 2019: (a) net anthropogenic emissions [10], (b) net anthropogenic emissions per region [13], (c) global monthly CO2 measured by Mauna Loa Observatory [17].
Several international conferences, treaties, and summits have been held to propose a solution to mitigate this situation, such as the 1997 International Agreement on Climate Change and the 2005 Kyoto Protocol in collaboration with the UN, which was held to reduce CO2 emissions [18]. At the UN Climate Change Conference in Paris in 2017, an agreement was reached to limit the global temperature rise to within 2 °C by minimizing CO2 emissions [19]. In 2019, the Climate Change Action Summit committed to zero carbon emissions by 2050 [20].
Numerous strategies have been proposed in the literature to reduce CO2 emissions and combat climate change [21][22][23][24][25]. There are two prominent available routes: (i) carbon capture, utilization, and storage (CCUS) and (ii) substituting conventional fuels with renewable energy sources. Both routes can be combined by using CO2 with renewable power sources, i.e., solar and wind to produce fuel [23][26]. Wind and solar energy are intermittent and variable energy sources, posing challenges to long-term storage. However, renewable energy can produce hydrogen via water electrolysis, for which the storage of hydrogen is also a crucial task. Thus, to address these issues effectively and promote environmentally friendly solutions, utilizing CO2 alongside renewable energy for fuel production, such as methane (CH4), emerges as a compelling green alternative.
CH4 can be produced by utilizing CO2 and hydrogen (H2) through CO2 methanation, also known as the Sabatier process, which is an efficient and appropriate procedure for CO2 utilization [27][28][29]. The extant natural gas infrastructure may be used for the CH4 produced from CO2 methanation. Thus, this strategy has the potential for large-scale deployment. By integrating CO2 capture, H2 from renewable energy, and CO2 methanation, we can make significant strides in reducing CO2 emissions, transitioning to a low-carbon economy, and mitigating the impacts of climate change [27][30][31].
2. Reaction Mechanism and Thermodynamic Equilibrium
2.1. CO2 Methanation Reaction
In 1872, renowned scientist Brodie demonstrated the reduction of CO2 into CH4 [16]. Paul Sabatier and Jean Baptiste Senderens analyzed the same results using a heterogeneous catalyst in 1902. Later, in 1912, the Nobel Prize was awarded to Sabatier for CO2 hydrogenation into CH4 (the Sabatier reaction) with a well-dispersed catalyst [22][27][32].
The Sabatier reaction is a highly exothermic reaction with eight electron processes with considerable kinetic limitations [33][34]. Catalysts are necessary to overcome the activation barriers and achieve maximum CO2 conversions at low enough temperatures, which is recommended based on the equilibrium conditions [33][34].
The CO2 methanation reaction, characterized by several elementary steps, displays varying reaction mechanisms that can be investigated through diverse in situ spectroscopic techniques and DFT theoretical calculations, contingent on the catalyst type. The process initiates with CO2 adsorption onto the carrier, leading to interactions with hydroxyl groups, forming bicarbonate species.
The first pathway involves the further reduction of formate into adsorbed CO, aided by H in facilitating CO dissociation, thereby forming the (HCOad) intermediate. This intermediate is then subjected to a series of consecutive hydrogenation reactions, culminating in CH4 formation.
The second pathway is analogous to the first in its initial steps and diverges as (COad) dissociates directly into (Cad) and O, with (Cad) undergoing direct hydrogenation into CH4, bypassing other intermediates. This is known as the CO pathway. Xinyu et al. [35] noted that the active sites on the Ni catalyst facilitate H2 molecule dissociation into H atoms. They proposed that, on Ni/ZrO2, CO2 is activated by O2 vacancies and transformed into adsorbed CO, which, along with H2 activation on Ni, leads to CH4 generation.
2.2. Thermodynamic Equilibrium Conversion and Selectivity
Several thermodynamic studies have examined the effects of reaction parameters on CO2 methanation [33][36][37][38][39][40].
Understanding the CO2 methanation reaction significantly relies on thermodynamic equilibrium. The CO2 methanation reaction is a combination of the deceleration of exothermic (CO methanation) and endothermic (water gas shift) reactions, as shown in Equations (1)–(3) [41][42].
Lower temperatures are more favorable for the methanation reaction, leading to an increase in CO2 conversion and an improvement in CH4 selectivity [43][44]. The CO2 methanation process exhibits the highest productivity at low temperatures, resulting in nearly 100% CO2 conversion and CH4 selectivity [45].
In CO2 methanation, the number of molecules decreases from five for reactants to three for products; therefore, the rise in pressure has a positive impact on CO2 conversion, mainly when operating within temperature ranges of 200–500 °C [46]. High pressure is also favorable for CH4 selectivity [43].
3. The Catalytic Performance of Noble Metal Catalysts in CO2 Methanation
3.1. Ruthenium-Based Catalysts
The catalytic performance of alumina-supported ruthenium (Ru/Al2O3) was investigated by Garbarino et al. [47], who observed that supported Ru catalysts are more active and stable than other metal-based catalysts. The supported Ru catalysts show excellent activity, especially at low temperatures, while the selection of the best catalyst preparation method and activation need to be carefully managed [48]. Ru/Al2O3 demonstrates high catalytic activity and good stability at 375 °C for 100 h, exhibiting 91% conversion and standing at 91% CH4 selectivity. The superior activity and stability were attributed to the high dispersion of Ru nanoparticles (NPs) and interactions between Ru NPs and the Al2O3 support. The consistent and stable high activity observed after different shutdown and start-up sequences suggests that the catalyst retains its effectiveness even under intermittent operation [47].
Ru doped with ceria is one of the most promising methanation catalysts owing to the essential nature of the O2 vacancies on the surface of CeO2, which can activate CO2. For Ru/Al3O2, adding ceria can improve the activity [49]. For Ce-doped Ru/Al3O2 catalysts, initial Ru NPs can be redispersed during oxidative pretreatment into atomically distributed RuOx species owing to interactions between Ru and ceria.
Besides CeO2 and Al2O3, TiO2 is also a promising support for loading Ru [50]. Researchers have demonstrated that TiO2-supported Ru NP catalysts possess good stability, which is attributable to the unique interaction between metal Ru and support TiO2. The CO2 conversion can maintain stability for 34 h running, with 68% of CO2 conversion and 98% selectivity to CH4 at 290 °C and 1 atm. For Ru/TiO2 catalysts, the catalytic performance relies on metal–support interactions, the loading amount of Ru, and the particle size of Ru NPs [51][52][53][54][55].
3.2. Rhodium-Based Catalysts
For Rh-based catalysts, the best performance is shown on Rh/TiO2 with 1 wt.% of Rh loading, which exhibits 90% CO2 conversion and 96% selectivity to CH4 with only 3 h of stability running under reaction conditions of a GHSV/WHSV of 12,000 h−1 (mL.g−1h−1), 370 °C, and 2 bar [56]. Considering the high reaction temperature and pressure, the activity of Rh is inferior to that of Ru; furthermore, investigations on the stability of Rh-based catalysts for CO2 methanation have not been extensively covered in the literature.
Moreover, the activity of Rh-based catalysts has been improved by adding other active metals, such as Ru-Rh/Al2O3, showing better activity than mono Rh [57], and Ni-Rh/Al2O3 also exhibits better activity than Rh/Al2O3 [58].
Catalytic performance may be affected by the O2 presence; O2 in a low percentage boosts the catalyst’s performance, whereas a higher concentration leads to a negative effect [59][60].
TiO2 is one of the best options for supporting Rh catalysts. The effects of Rh/TiO2 on catalytic performance at low temperatures were investigated by Alejandro et al. [61], who found that large Rh particle sizes have more active sites and weak CO intermediates, which also affects the order of CO2 methanation reaction and activation energies. Notably, a slight variation in the activation energy of CO dissociation with Rh particle size was observed. Higher response orders in H2 were also seen for smaller particles, indicative of reduced H2 coverage. CH4 selectivity gradually increased with a change in particle size (from 2 nm to 7 nm), while, beyond this particle size, there was no discernible difference [61].
It has been observed that Rh/TiO2 catalysts have higher CH4 selectivity as compared with Rh/Al2O3 and Rh/SiO2. The literature suggests that the breakdown of the C-O bond could be aided by electron interactions between metal and supports or interactions between CO absorbed by a catalyst and Ti 3+ ions positioned at the border metal support [56][61].
Research on Rh/TiO2 by Martnez et al. [52] showed that changes in activity depend on interactions between Rh NPs and the support. The average particle size after H2 reduction at a high temperature is substantially smaller than the size of calcined Ru in the presence of synthetic air and then further heated at 300 °C, which was confirmed with TEM and XRD [52]. The interaction can make Rh re-disperse, which leads to the high dispersion state of Rh NPs, resulting in higher CO2 conversion.
3.3. Palladium-Based Catalysts
For Pd-based catalysts, the best catalytic performance was shown on Pd@FeO with a Pd loading amount of 5.2 wt.%, showing 98% CO2 conversion and 100% selectivity to CH4 at a low temperature of 180 °C, and the catalytic performance was maintained for 20 rounds of cyclic running [62]. The high activity was attributed to the face-centered tetragonal structure of the Pd-Fe intermetallic nanocrystal, and it was proposed that Pd-Fe intermetallic nanocrystals aided in maintaining metallic Fe species during CO2 methanation via a reversible oxidation-reduction mechanism; thus, adding metallic Fe facilitated the direct conversion of CO2. This study was efficient on a laboratory scale, but for practical applications, the loading amount of Pd is too high, and the stability needs to be investigated.
The bimetallic catalysts of Ni-Pd supported on Al2O3 show better activity with 0.5 wt.% and 10 wt.% loadings of Pd and Ni, respectively. In one study, a CO2 conversion of 91% and 99% CH4 selectivity was achieved under reaction conditions of a GHSV/WHSV of 57,000 (mL.g−1h−1), 300 °C, and 1 atm, and it remained stable for 4 h [58].
3.4. Summary of Performance of Noble Metal Catalysts
Studies on noble metal catalysts for CO2 methanation have mainly concentrated on Ru-, Rh-, and Pd-based catalysts, among them Ru based catalysts exhibited the best catalytic performance. For all catalysts, selectivity to CH4 can be very good, especially at low reaction temperatures, so the challenge is to enhance the activity at low temperatures. When the loading amount of noble metal is as high as 3–6 wt.%, much higher CO2 conversion can be achieved, although the high price means it has promise in practical applications. Ru-based catalysts have shown comparatively good stability in maintaining stability for 300 h running, but stability investigations of Rh- and Pd-based catalysts are needed.
Research on noble metal catalysts for CO2 methanation has predominantly focused on Ru-, Rh-, and Pd-based catalysts; among them, Ru-based catalysts demonstrate superior catalytic performance. These catalysts generally exhibit high CH4 selectivity, particularly at low reaction temperatures. However, enhancing activity at low temperatures remains a challenge.
4. The Catalytic Performance of Non-Noble Metal Catalysts in CO2 Methanation
4.1. Nickel-Based Catalysts
Ni-based catalysts have gained significant attention in CO2 methanation. The research emphasis has been on enhancing its activity in low reaction temperatures and improving the sintering resistance of Ni NPs because of the inert nature of CO2 and the strong exothermic activity of the methanation reaction. In designing a promising catalyst for industrial applications, the critical factors of catalytic performance are the properties of Ni NPs, including dispersion and its chemical state, metal–support interactions, and additive materials. Supports such as Al2O3 [47][63], SiO2 [49][64], TiO2 [65][66], ZrO2 [67][68], CeO2 [69][70], and the solid solution Ce-Zr-O [71] are predominantly utilized for loading Ni NPs. The additives mostly employed are alkaline earth metals, such as La, Y, and Ce; the basic element Mg; the transition element Mn; and so on. Catalyst synthesis methods are diverse, with the principal objectives being to increase Ni NP dispersion and/or adjust interactions between Ni NPs and the support or additives.
Al2O3 is the most frequently applied catalyst support for CO2 methanation [72][73]. Al2O3 is extensively used owing to its high surface area, adjustable porous structure, and complex chemistry properties [74][75][76]. Therefore, Al2O3 is not suitable for loading Ni NPs for CO2 methanation, but reports on Ni/Al2O3 for CO2 methanation are significant [77][78]. An issue with using Al2O3 as a support for the methanation reaction is that it tends to sinter when exposed to water (a byproduct of the process) at high temperatures [74].
The activity of Ni/Al2O3 is not good enough; with a loading of 12.5 wt.% Ni on Al2O3, CO2 conversion could reach 71% at a high temperature of 500 °C [79], and the reason for the poor activity can likely be attributed to the poor CO2 activation ability of Al2O3. When adding an additive to activate CO2, such as ceria, the activity could be improved effectively over Ni/CeO2-Al2O3; 71% CO2 conversion and 99% selectivity to CH4 could be achieved at a low temperature of 350 °C, at reaction conditions of a GHSV/WHSV of 15,000 (mL.g−1h−1) and 1 atm [80].
Silica (SiO2) is another popular choice since it has a large surface area and may adjust its pore diameter to suit a given application [81][82][83]. Ni and SiO2 form metal–support interactions, which are antagonistic to the growth of Ni carbide, which leads to the improved catalyst’s resistance to coke production and Ni sintering. [84][85]. In the literature, CO2 methanation with a SiO2-supported catalyst has shown a CO2 conversion efficiency of only about 60% to 90% [86][87][88][89][90], which may be due to the inertness of SiO2; therefore, the additive addition or surface modification of the support needs to be further investigated [91][92].
Similar to studies that used alumina-supported Ni catalysts, Li et al. used Mg as a promoter for Ni/SiO2; the resultant catalyst showed obviously improved activity with 82% CO2 conversion and 99% selectivity to CH4 under reaction conditions of a GHSV/WHSV of 60,000 (mL.g−1h−1), 250 °C, and 1 atm [93]. SiO2 with a high surface area and a mesoporosity of MCM-41 is sufficient support for CO2 methanation. A high surface area favors the high dispersion of Ni NPs, and mesoporosity is beneficial for reactant transfer [73][94].
The distinctive catalytic performance of SiO2 with a high surface area loaded with Ni NPs has naturally inspired researchers to investigate zeolite as a support for CO2 methanation, as it is known that a key characteristic of silica zeolite is a high surface area. Chen et al. [95] used a conventional Ni/SiO2 as a precursor-prepared core–shell catalyst for Ni@HZSM-5 via the hydrothermal method. Compared with traditionally prepared Ni/SiO2 and Ni/HZSM-5 catalysts, Ni@HZSM-5 exhibited superior performance, preserving its Ni content and the structure of the active Ni after a 40 h CO2 methanation reaction. A key feature of this catalyst is the interaction between the Ni active phase and zeolite, with the former donating more electrons to the latter, thus preventing sintering and enhancing the activity of Ni. At 400 °C, the Ni@HZSM-5 catalyst demonstrated a CO2 conversion of 64% and near 100% CH4 selectivity under reaction conditions of a GHSV/WHSV of 36,000 (mL.g−1h−1), 400 °C, and 1 atm [96].
4.2. Cobalt-Based and Iron-Based Catalysts
Co-based catalysts are the most well-studied active component of Fischer–Tropsch synthesis (FTS), which generates hydrocarbons from syngas (a gas mixture of CO and H2), as CO2 can be converted into CO via reverse water gas shift reactions; therefore, studying Co-based catalysts for CO methanation is expected [97][98].
In another study, Co/ZrO2 showed good catalytic performance with 92% CO2 conversion and 99% selectivity to CH4, and this performance was maintained for 300 h under reaction conditions of a GHSV/WHSV of 36,000 (mL.g−1h−1), 400 °C, and 30 atm [99]. Considering the low space velocity, high pressure, and comparatively high temperature in these reaction conditions, the activity is very good but not excellent compared with Ni-based catalysts, although the stability seems very good.
In a study by Moghaddam et al. [100], Ni/Al2O3 catalysts were prepared using the one-pot sol–gel method, with small amounts of additional elements such as Fe, Co, Cu, Zr, and La added. The catalyst containing Fe demonstrated exceptional performance, achieving 71% CO2 conversion and nearly 99% selectivity for CH4 at 350 °C, a GHSV/WHSV of 9000 (mL.g−1h−1), and 1 atm. This improvement can be attributed to the presence of a Ni-Fe alloy, which enhanced the adsorption of H2 and the dissociation of CO2. Interestingly, increasing the Fe content from 5 to 7 wt.% resulted in enhanced activity at lower temperatures and maintained stability over a 10 h period.
4.3. Summary of Performance of Non-Noble Catalysts
Non-noble catalysts for CO2 methanation include Ni-, Co-, and Fe-based catalysts; among all catalyst systems, Ni-based catalysts show the best catalytic performance. The selectivity to CH4 is generally high (close to 100%), especially at low reaction temperatures (lower than 400 °C), and they have revealed very good activity. Excellent Ni-based catalysts can reach or come close to equilibrium conversion at around 350 °C and 1 atm with a WHSV of 10,000 mL.g−1h−1 or even higher, and the stability generally extends to several hundred hours. For Co- and Fe-based catalysts, some show very high activity at low temperatures, though the stability and selectivity compared with the corresponding catalyst is possibly not good enough. Co- and Fe-based catalysts are well known for being used as FTS catalysts, which means that hydrocarbons besides CH4 can be easily generated, thus leading to decreased selectivity to CH4.
In this study, the catalytic performance of noble metal catalysts was found to be dependent on metal dispersion, metal–support interactions, and additive doping. Various catalyst configurations, including supports made of single-oxide supports (Al2O3, TiO2, ZrO2, and CeO2), composite oxide supports (Ce-Zr-O), basic oxide promoters, and bimetallic systems, were investigated to determine their impact on catalytic activity and stability. These results suggest that Ni-based catalysts are the most promising.
5. Conclusions
In combatting the challenges posed by CO2 emissions, using renewable energy to produce green hydrogen and then hydrogenating CO2 into valuable chemicals is a promising route, including through the production of CH4 via CO2 methanation. Given the thermodynamic equilibrium of the reaction, to convert CO2 completely into CH4, low reaction temperatures and high pressure are favorable, and meeting the requirements of developing catalysts is critical. The catalysts that have been extensively reported include noble metal-based catalysts and non-noble metal-based catalysts.
Among the noble metal catalysts, Ru shows the best catalytic performance. In excellent Ru-based catalysts, CO2 conversion can be achieved close to equilibrium (98%) and with 100% selectivity to CH4, with 300 h of stability obtained at 24,000 mL.g−1h−1, 230 °C, and 1 atm. Excellent noble metal-based catalysts feature the high dispersion of the noble metal, the strong metal-support interaction with noble metal NPs, and doped with a transitional metal: Ni, Co, or Fe. The disadvantages of noble metal-based catalysts are the high price and high loading, and the stability needs to be improved.
Non-noble metal catalysts include Ni-, Co-, and Fe-based catalysts. Ni-based catalysts show the best catalytic performance and are well studied, while studies on Co- and Fe-based catalysts are much fewer, possibly because other hydrocarbons besides CH4 can be formed over Co and Fe catalysts. With excellent Ni-based catalysts, CO2 conversion close to equilibrium (around 90%) and 100% selectivity to CH4 can be obtained at around 350 °C a WHSV of 10,000 (mL.g−1h−1) or higher, and 1 atm with good stability. Excellent Ni-based catalysts feature high Ni NP dispersion; a support and/or additive to activate or help activate CO2; and interactions between Ni NPs and the support that resist sintering. Bimetals (Ni-Fe, Ni-Co) are favorable for improving catalytic performance.
References
- Ahn, J.; Chung, W. A Study on Activity of Coexistent CO Gas during the CO2 Methanation Reaction in Ni-Based Catalyst. Processes 2023, 11, 628.
- Asim, M.; Maryam, B.; Zhang, S.; Sajid, M.; Kurbanov, A.; Pan, L.; Zou, J.J. Synergetic effect of Au nanoparticles and transition metal phosphides for enhanced hydrogen evolution from ammonia-borane. J. Colloid Interface Sci. 2023, 638, 14–25.
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. CO2 Methanation: Principles and Challenges, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 178, ISBN 9780444641274.
- Asim, M.; Zhang, S.; Maryam, B.; Xiao, J.; Shi, C.; Pan, L.; Zou, J.J. Pt loading to promote hydrogen evolution from ammonia-borane hydrolysis of Ni2P under visible light. Appl. Surf. Sci. 2023, 620, 156787.
- Hassan, M.A.; Mehmood, T.; Liu, J.; Luo, X.; Li, X.; Tanveer, M.; Faheem, M.; Shakoor, A.; Dar, A.A.; Abid, M. A review of particulate pollution over Himalaya region: Characteristics and salient factors contributing ambient PM pollution. Atmos. Environ. 2023, 294, 119472.
- Mehmood, T.; Hassan, M.A.; Li, X.; Ashraf, A.; Rehman, S.; Bilal, M.; Obodo, R.M.; Mustafa, B.; Shaz, M.; Bibi, S. Mechanism behind sources and sinks of major anthropogenic greenhouse gases. In Climate Change Alleviation for Sustainable Progression; CRC Press: Boca Raton, FL, USA, 2022; pp. 114–150.
- Bai, C.; Chen, Z.; Wang, D. Transportation carbon emission reduction potential and mitigation strategy in China. Sci. Total Environ. 2023, 873, 162074.
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100.
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751.
- IPCC. Summary for Policymakers Sixth Assessment Report (WG3); IPCC: Geneva, Switzerland, 2022; ISBN 9781107415416.
- Hassan, M.A.; Mehmood, T.; Lodhi, E.; Bilal, M.; Dar, A.A.; Liu, J. Lockdown Amid COVID-19 Ascendancy over Ambient Particulate Matter Pollution Anomaly. Int. J. Environ. Res. Public Health 2022, 19, 3540.
- Hassan, M.A.; Faheem, M.; Mehmood, T.; Yin, Y.; Liu, J. Assessment of meteorological and air quality drivers of elevated ambient ozone in Beijing via machine learning approach. Environ. Sci. Pollut. Res. 2023, 30, 104086–104099.
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123.
- Dlugokencky, E.; Tans, P. Co2_Trend_Gl.Pdf. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 5 November 2023).
- Warsi, Y.; Kabanov, V.; Zhou, P.; Sinha, A. Novel Carbon Dioxide Utilization Technologies: A Means to an End. Front. Energy Res. 2020, 8, 574147.
- Raheem, I.; Mubarak, N.M.; Karri, R.R.; Manoj, T.; Ibrahim, S.M.; Mazari, S.A.; Nizamuddin, S. Forecasting of energy consumption by G20 countries using an adjacent accumulation grey model. Sci. Rep. 2022, 12, 13417.
- Monthly Average Mauna Loa CO2 Monthly Average Mauna Loa CO2.pdf. Available online: https://www.gml.noaa.gov/ccgg/trends/ (accessed on 21 November 2022).
- Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts 2022, 12, 300.
- UNFCCC. UN Climate Change ANNUAL REPORT 2017; United Nations Framework Convention on Climate Change: Bonn, Germany, 2017; ISBN 9789292191757.
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794.
- Saravanan, A.; Senthil kumar, P.; Vo, D.V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515.
- Feng, X.; Wang, K.; Zhou, M.; Li, F.; Liu, J.; Zhao, M.; Zhao, L.; Song, X.; Zhang, P.; Gao, L. Metal organic framework derived Ni/CeO2 catalyst with highly dispersed ultra-fine Ni nanoparticles: Impregnation synthesis and the application in CO2 methanation. Ceram. Int. 2021, 47, 12366–12374.
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic conversions of CO2 to help mitigate climate change: Recent process developments. Process Saf. Environ. Prot. 2021, 145, 172–194.
- Liu, M.; Yi, Y.; Wang, L.; Guo, H.; Bogaerts, A. Hydrogenation of carbon dioxide to value-added chemicals by heterogeneous catalysis and plasma catalysis. Catalysts 2019, 9, 275.
- Asim, M.; Zhang, S.; Wang, Y.; Maryam, B.; Sajid, M.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.J. Self-supporting NiCoP for hydrogen generation via hydrolysis of ammonia borane. Fuel 2022, 318, 123544.
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756.
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2020, 264, 118494.
- Varvoutis, G.; Lampropoulos, A.; Oikonomou, P.; Andreouli, C.D.; Stathopoulos, V.; Lykaki, M.; Marnellos, G.E.; Konsolakis, M. Fabrication of highly active and stable Ni/CeO2-nanorods wash-coated on ceramic NZP structured catalysts for scaled-up CO2 methanation. J. CO2 Util. 2023, 70, 102425.
- Varvoutis, G.; Karakoulia, S.A.; Lykaki, M.; Stefa, S.; Binas, V.; Marnellos, G.E.; Konsolakis, M. Support-induced modifications on the CO2 hydrogenation performance of Ni/CeO2: The effect of ZnO doping on CeO2 nanorods. J. CO2 Util. 2022, 61, 102057.
- Refaat, Z.; El Saied, M.; El Naga, A.O.A.; Shaban, S.A.; Hassan, H.B.; Shehata, M.R.; Kady, F.Y.E. Efficient CO2 methanation using nickel nanoparticles supported mesoporous carbon nitride catalysts. Sci. Rep. 2023, 13, 4855.
- Gholami, S.; Alavi, S.M.; Rezaei, M. Preparation of highly active and stable nanostructured Ni-Cr2O3 catalysts for hydrogen purification via CO2 methanation reaction. J. Energy Inst. 2021, 95, 132–142.
- Zhou, G.; Wu, T.; Xie, H.; Zheng, X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrogen Energy 2013, 38, 10012–10018.
- Nieß, S.; Armbruster, U.; Dietrich, S.; Klemm, M. Recent Advances in Catalysis for Methanation of CO2 from Biogas. Catalysts 2022, 12, 374.
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2020, 265, 118538.
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.j. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169.
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776.
- Ridzuan, N.D.M.; Shaharun, M.S.; Anawar, M.A.; Ud-Din, I. Ni-Based Catalyst for Carbon Dioxide Methanation: A Review. Catalysts 2022, 12, 469.
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19.
- Martínez, J.; Hernández, E.; Alfaro, S.; Medina, R.L.; Aguilar, G.V.; Albiter, E.; Valenzuela, M.A. High selectivity and stability of nickel catalysts for CO2 Methanation: Support effects. Catalysts 2019, 9, 24.
- Liang, C.; Zhang, L.; Zheng, Y.; Zhang, S.; Liu, Q.; Gao, G.; Dong, D.; Wang, Y.; Xu, L.; Hu, X. Methanation of CO2 over nickel catalysts: Impacts of acidic/basic sites on formation of the reaction intermediates. Fuel 2020, 262, 116521.
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic methanation of CO and CO2 in coke oven gas over Ni-Co/ZrO2-CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256.
- Li, S.; Tang, H.; Gong, D.; Ma, Z.; Liu, Y. Loading Ni/La2O3 on SiO2 for CO methanation from syngas. Catal. Today 2017, 297, 298–307.
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368.
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027.
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170.
- Yarbaş, T.; Ayas, N. A detailed thermodynamic analysis of CO2 hydrogenation to produce methane at low pressure. Int. J. Hydrogen Energy 2023, 49, 1134–1144.
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182.
- Park, S.J.; Wang, X.; Ball, M.R.; Proano, L.; Wu, Z.; Jones, C.W. CO2 methanation reaction pathways over unpromoted and NaNO3-promoted Ru/Al2O3 catalysts. Catal. Sci. Technol. 2022, 12, 4637–4652.
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531.
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by a polygonal barrel-sputtering method. Energy Environ. Sci. 2009, 2, 315–321.
- Hatzisymeon, M.; Petala, A.; Panagiotopoulou, P. Carbon Dioxide Hydrogenation over Supported Ni and Ru Catalysts. Catal. Lett. 2021, 151, 888–900.
- Martinez T, L.M.; Muñoz, A.; Pérez, A.; Laguna, O.H.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. The effect of support surface hydroxyls on selective CO methanation with Ru based catalysts. Appl. Catal. A Gen. 2022, 641, 118678.
- Loccufier, E.; Watson, G.; Zhao, Y.; Meledina, M.; Denis, R.; Derakhshandeh, P.G.; Van Der Voort, P.; Leus, K.; Debecker, D.P.; De Buysser, K.; et al. CO2 methanation with Ru@MIL-101 nanoparticles fixated on silica nanofibrous veils as stand-alone structured catalytic carrier. Appl. Catal. B Environ. 2023, 320, 121972.
- Bobadilla, L.F.; Muñoz-Murillo, A.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. Does shaping catalysts modify active phase sites? A comprehensive in situ FTIR spectroscopic study on the performance of a model Ru/Al2O3 catalyst for the CO methanation. Chem. Eng. J. 2019, 357, 248–257.
- Fechete, I.; Vedrine, J.C. Nanoporous materials as new engineered catalysts for the synthesis of green fuels. Molecules 2015, 20, 5638–5666.
- Jiang, Y.; Lang, J.; Wu, X.; Hu, Y.H. Electronic structure modulating for supported Rh catalysts toward CO2 methanation. Catal. Today 2020, 356, 570–578.
- Meloni, E.; Cafiero, L.; Renda, S.; Martino, M.; Pierro, M. Ru- and Rh-Based Catalysts for CO2 Methanation Assisted by Non-Thermal Plasma. Catalysts 2023, 13, 488.
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299.
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic biogas upgrading based on the Sabatier process: Thermodynamic and dynamic process simulation. Bioresour. Technol. 2015, 178, 323–329.
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59.
- Karelovic, A.; Ruiz, P. Improving the hydrogenation function of Pd/γ-Al2O3 catalyst by Rh/γ-Al2O3 Addition in CO2 methanation at low temperature. ACS Catal. 2013, 3, 2799–2812.
- Luo, L.; Wang, M.; Cui, Y.; Chen, Z.; Wu, J.; Cao, Y.; Luo, J.; Dai, Y.; Li, W.X.; Bao, J.; et al. Surface Iron Species in Palladium–Iron Intermetallic Nanocrystals that Promote and Stabilize CO2 Methanation. Angew. Chem.-Int. Ed. 2020, 59, 14434–14442.
- Lee, S.M.; Lee, Y.H.; Moon, D.H.; Ahn, J.Y.; Nguyen, D.D.; Chang, S.W.; Kim, S.S. Reaction Mechanism and Catalytic Impact of Ni/CeO2−x Catalyst for Low-Temperature CO2 Methanation. Ind. Eng. Chem. Res. 2019, 58, 8656–8662.
- Wu, H.C.; Chen, T.C.; Wu, J.H.; Pao, C.W.; Chen, C.S. Influence of sodium-modified Ni/SiO2 catalysts on the tunable selectivity of CO2 hydrogenation: Effect of the CH4 selectivity, reaction pathway and mechanism on the catalytic reaction. J. Colloid Interface Sci. 2021, 586, 514–527.
- Yan, Z.; Liu, Q.; Liang, L.; Ouyang, J. Surface hydroxyls mediated CO2 methanation at ambient pressure over attapulgite-loaded Ni-TiO2composite catalysts with high activity and reuse ability. J. CO2 Util. 2021, 47, 101489.
- Zhou, R.; Rui, N.; Fan, Z.; Liu, C. jun Effect of the structure of Ni/TiO2 catalyst on CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 22017–22025.
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly Dispersed Ni Catalyst on Metal-Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12, 17436–17442.
- Foraita, S.; Fulton, J.L.; Chase, Z.A.; Vjunov, A.; Xu, P.; Baráth, E.; Camaioni, D.M.; Zhao, C.; Lercher, J.A. Impact of the oxygen defects and the hydrogen concentration on the surface of tetragonal and monoclinic ZrO2 on the reduction rates of stearic acid on Ni/ZrO2. Chem.-A Eur. J. 2015, 21, 2423–2434.
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219.
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252.
- Sun, H.; Wang, H.; Liu, X.; Zhang, Z.; Zhang, S.; Wang, X.; Liu, Y. Stable and Highly Dispersed Nickel Catalysts on Ce-Zr-O Solid Solutions for CO2 Methanation. ChemistrySelect 2022, 7, 2–10.
- Takano, H.; Kirihata, Y.; Izumiya, K.; Kumagai, N.; Habazaki, H.; Hashimoto, K. Highly active Ni/Y-doped ZrO2 catalysts for CO2 methanation. Appl. Surf. Sci. 2016, 388, 653–663.
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184.
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced investigation of CO methanation over Ni/Al2O3 catalysts for synthetic natural gas production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886.
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395.
- Liu, Y.; Zong, X.; Patra, A.; Caratzoulas, S.; Vlachos, D.G. Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110). ACS Catal. 2023, 13, 2802–2812.
- Cai, M.; Wen, J.; Chu, W.; Cheng, X.; Li, Z. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324.
- Dias, Y.R.; Bernardi, F.; Perez-Lopez, O.W. Improving Low-Temperature CO2 Methanation by Promoting Ni-Al LDH-Derived Catalysts with Alkali Metals. ChemCatChem 2023, 202300834.
- Riani, P.; Garbarino, G.; Lucchini, M.A.; Canepa, F.; Busca, G. Unsupported versus alumina-supported Ni nanoparticles as catalysts for steam/ethanol conversion and CO2 methanation. J. Mol. Catal. A Chem. 2014, 383–384, 10–16.
- Kim, M.J.; Youn, J.R.; Kim, H.J.; Seo, M.W.; Lee, D.; Go, K.S.; Lee, K.B.; Jeon, S.G. Effect of surface properties controlled by Ce addition on CO2 methanation over Ni/Ce/Al2O3 catalyst. Int. J. Hydrogen Energy 2020, 45, 24595–24603.
- Liu, Y.; McGill, C.J.; Green, W.H.; Deshlahra, P. Effects of surface species and homogeneous reactions on rates and selectivity in ethane oxidation on oxide catalysts. AIChE J. 2021, 67, e17483.
- Velty, A.; Corma, A. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 2023, 52, 1773–1946.
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698.
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541.
- Li, S.; Guo, S.; Gong, D.; Kang, N.; Fang, K.G.; Liu, Y. Nano composite composed of MoOx-La2O3–Ni on SiO2 for storing hydrogen into CH4 via CO2 methanation. Int. J. Hydrogen Energy 2019, 44, 1597–1609.
- Park, J.N.; McFarland, E.W. A highly dispersed Pd-Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97.
- Ma, H.; Ma, K.; Ji, J.; Tang, S.; Liu, C.; Jiang, W.; Yue, H.; Liang, B. Graphene intercalated Ni-SiO2/GO-Ni-foam catalyst with enhanced reactivity and heat-transfer for CO2 methanation. Chem. Eng. Sci. 2019, 194, 10–21.
- Guo, M.; Lu, G. The difference of roles of alkaline-earth metal oxides on silica-supported nickel catalysts for CO2 methanation. RSC Adv. 2014, 4, 58171–58177.
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Catal. Commun. 2014, 54, 55–60.
- Gac, W.; Zawadzki, W.; Słowik, G.; Sienkiewicz, A.; Kierys, A. Nickel catalysts supported on silica microspheres for CO2 methanation. Microporous Mesoporous Mater. 2018, 272, 79–91.
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113–114, 19–30.
- Rossetti, I.; Biffi, C.; Bianchi, C.L.; Nichele, V.; Signoretto, M.; Menegazzo, F.; Finocchio, E.; Ramis, G.; Di Michele, A. Ni/SiO2 and Ni/ZrO2 catalysts for the steam reforming of ethanol. Appl. Catal. B Environ. 2012, 117–118, 384–396.
- Li, Y.; Lu, G.; Ma, J. Highly active and stable nano NiO-MgO catalyst encapsulated by silica with a core-shell structure for CO2 methanation. RSC Adv. 2014, 4, 17420–17428.
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368.
- Chen, Y.; Qiu, B.; Liu, Y.; Zhang, Y. An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl. Catal. B Environ. 2020, 269, 118801.
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Saad, M.W.A. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764.
- ten Have, I.C.; Kromwijk, J.J.G.; Monai, M.; Ferri, D.; Sterk, E.B.; Meirer, F.; Weckhuysen, B.M. Uncovering the reaction mechanism behind CoO as active phase for CO2 hydrogenation. Nat. Commun. 2022, 13.
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170.
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408.
- Valinejad Moghaddam, S.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Carbon dioxide methanation over Ni-M/Al2O3 (M: Fe, CO, Zr, La and Cu) catalysts synthesized using the one-pot sol-gel synthesis method. Int. J. Hydrogen Energy 2018, 43, 16522–16533.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
19 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




