Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monika Michalak | -- | 3326 | 2024-01-17 08:09:47 | | | |
| 2 | Lindsay Dong | Meta information modification | 3326 | 2024-01-19 02:10:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Encyclopedia. Available online: https://encyclopedia.pub/entry/53939 (accessed on 08 February 2026).
Michalak M. Plant Extracts as Skin Care and Therapeutic Agents. Encyclopedia. Available at: https://encyclopedia.pub/entry/53939. Accessed February 08, 2026.
Michalak, Monika. "Plant Extracts as Skin Care and Therapeutic Agents" Encyclopedia, https://encyclopedia.pub/entry/53939 (accessed February 08, 2026).
Michalak, M. (2024, January 17). Plant Extracts as Skin Care and Therapeutic Agents. In Encyclopedia. https://encyclopedia.pub/entry/53939
Michalak, Monika. "Plant Extracts as Skin Care and Therapeutic Agents." Encyclopedia. Web. 17 January, 2024.
Copy Citation
Natural ingredients have been used for centuries for skin treatment and care. Interest in the health effects of plants has recently increased due to their safety and applicability in the formulation of pharmaceuticals and cosmetics. Long-known plant materials as well as newly discovered ones are increasingly being used in natural products of plant origin.
plants
skin
photoprotection
wound healing
anti-aging
cosmetics
1. Introduction
The skin consists of the epidermis and the dermis, below which lies subcutaneous tissue. The five-layer epidermis consists of keratinocytes—cells taking part in keratinization, melanocytes—pigment cells, Langerhans cells, mastocytes and Merkel cells. The dermis is composed of connective tissue and consists of a papillary layer and a reticular layer. It contains fibroblasts, which are responsible for the production of collagen, elastin and glycosaminoglycans (GAG), as well as numerous blood vessels, nerve endings and appendages, including hair follicles and sweat and sebaceous glands. The skin performs multiple complex functions; it takes part in metabolic and homeostatic processes and is responsible for the excretion, selective absorption and storage of substances. In addition, it protects against biological (e.g., microbes), physical (e.g., UV radiation) and chemical factors [1][2].
Botanical ingredients are one of the main sources of materials that are used in the cosmetics and pharmaceutical industries. Recent years have seen increasing interest in dermocosmetics and cosmeceuticals produced from plant materials, and thus, there has been greater interest in plant-based products with skin care properties. Plant materials can be applied topically for skin care purposes, as well as for the treatment of numerous skin diseases [2] (Figure 1). Their advantage is that they are gentle but effective, safe and non-toxic, without side effects. Cosmetics fortified with bioactive compounds are ideally suited to the needs of the skin and are more environmentally friendly than conventional cosmetics. A group of natural ingredients widely used in cosmetics is plant extracts, which are a rich source of biologically active substances significantly affecting human skin. They may exhibit a wide range of properties, both medicinal (in certain skin disorders, including inflammatory disorders such as acne, psoriasis or atopic dermatitis) and for use in skin care (e.g., antioxidant, antibacterial, astringent, moisturizing, regenerating, cleansing, smoothing or lightening) [3][4]. Plant extracts are obtained via extraction from various parts of raw plants, e.g., using an appropriately chosen solvent, such as water, ethyl alcohol, glycerine, glycols or vegetable oil. Plant extracts are obtained from whole plants or parts of plants (fruits, leaves, roots, bark, stems, branches, seeds or flowers). The composition and properties of plant extracts, which can be found in the formulas of natural cosmetics, depend on a variety of factors, including cultivation and harvest conditions, how and to what extent the material is broken up, or drying and extraction methods. Extracts from whole plants as well as individual chemical substances contained in them are used in cosmetics. Active plant substances are divided into primary and secondary metabolites. The former are basic substances that are essential to the plant for life, constituting building materials and energy sources. They include sugars, fats, proteins, amino acids and enzymes. Secondary metabolites include terpenes, steroids, saponins, tannins, alkaloids, volatile oils, resins, vitamins and phenolics [1][4].
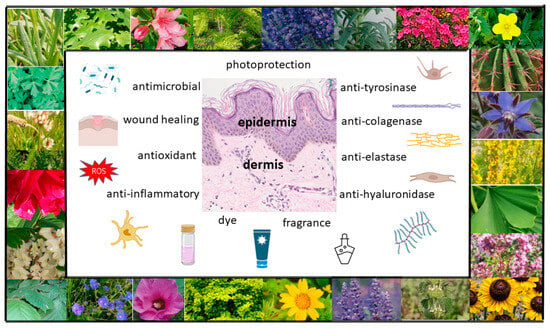
Figure 1. Possible uses of plants in skin care and treatment (own work; photos: M. Michalak).
2. Plants as Photoprotective Agents against Ultraviolet-Radiation-Induced Inflammation and Skin Damage
Selected plant extracts and single compounds with antioxidant, anti-inflammatory and immunomodulatory effects play an important role in the photoprotection of the skin. Phytochemicals have shown the ability to act as free radical scavengers, radical chain reaction inhibitors, metal chelators, oxidative enzyme inhibitors and antioxidant enzyme cofactors. Some studies have reported that plant extracts promote endogenous antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), which protect the skin against increasing ROS levels under oxidative stress. Moreover, plant materials can modulate the expression and activation of a wide variety of cytokines, such as TNF-α IL-1β, IL-6 and IL-8. Botanicals have also shown the ability to regulate the expression of various pro-inflammatory genes and inhibit the activity of pro-inflammatory enzymes such as inducible nitric oxide synthase (iNOS), COX-2 and lipoxygenase (LOX) [5][6][7].
Plant extracts and natural compounds from plants have been reported in the earlier literature to possess photoprotective properties. These include phytochemicals such as ferulic, caffeic, cinnamic, rosmarinic acid, quercetin, apigenin, rutin, luteolin, chrysin, hesperidin, dihydromyricetin, chrysanthemin, curcumin, genistein, resveratrol, carnosic, ursolic, ellagic, asiatic acid, zerumbone, astaxanthin, β-carotene, lycopene, zeaxantin, lutein and L-ergothioneine, as well as extracts from plants such as Opuntia humifusa [8], Camellia sinensis [9], Punica granatum [10], Hibiscus furcatus, Atalantia ceylanica, Mollugo cerviana, Leucas zeylanica, Ophiorrhiza mungos, Olax zeylanica [11] Silybum marianum [12], Polypodium leucotomos [13], Vaccinium myrtillus [14], Lonicera caerulea [15], Thymus vulgaris [16], Opuntia ficus-indica [17], Morinda citrifolia [18], Galinsoga parviflora, Galinsoga quadriradiata [19], Coffea arabica [20], Amaranthus cruentus, Moringa oleifera, Malaxis acuminata, Schinus terebinthifolius [21], Schinopsis brasiliensis [22], Crataegus pentagyna [23], Sambucus nigra, Helichrysum arenarium, Crataegus monogyna [24], Capnophyllum peregrinum [25], Dalbergia monetaria [26], Baccharis antioquensis [27], Juglans regia [28], Dimorphandra gardneriana and Lippia microphylla [29].
Some plants that are effective UV filters may be potential sunscreen ingredients [30]. These include plant extracts such as Astragalus gombiformis with an SPF value of 38 [31], Sloanea calva with an SPF value of 35.4 [32], Hylocereus polyrhizus with an SPF value of 35.02 [33] or Rosa centifolia with SPF values of 32 [34]. Moreover, plant extracts, through their synergistic effects with some physical or chemical UV filters (e.g., benzophenone-3 (BP-3), octyl methoxycinnamate (OMC) or titanium dioxide (TiO2)), may also play a role as cosmetic components that enhance the SPF of sunscreen formulations [30]. This effect has been shown for extracts from Sanionia uncinata [35][36], Vitis vinifera [37], Nephelium lappaceum [38], Psidium guajava [39], Campomanesia adamantium and Campomanesia xanthocarpa [40], as well as moss extracts from Leucobryum spp. and Holomitriopsis laevifolia [41].
3. Plants as Regenerative and Wound-Healing Agents
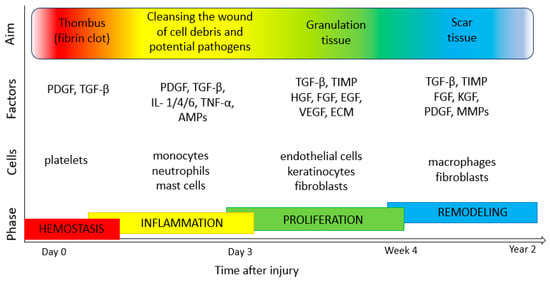
The process of the regeneration and healing of the skin involves interactions between many types of cells, including endothelial cells, inflammatory cells, keratinocytes and fibroblasts. It consists of stages such as coagulation (haemostasis, fibrin clot formation and activation of the clotting cascade by platelets), inflammation (neutrophil and monocyte migration, phagocytosis of bacteria and the release of proteolytic enzymes to debride the wound), proliferation (angiogenesis by endothelial cells, granulation tissue formation by fibroblasts and reepithelialization by keratinocytes) and tissue maturation (collagen/ECM remodeling by fibroblasts) [42][43][44] (Figure 2). An important step in tissue formation, repair and the maintenance of good skin conditions is proper cell proliferation and migration processes. These depend on many factors, such as biochemical communication, adhesion strength and mechanical flexibility, as well as organization of the cellular cytoskeleton [45][46][47]. Numerous regulators take part in keratinocyte migration and proliferation, including epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), angiopoietin-related growth factor (AGF), vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF) and platelet derived-endothelial cell growth factor (PD-ECGF). In addition, cytokines (e.g., IL-1, IL-6 and TNF-α), neuropeptides (G protein-coupled receptor (GCRP), vasoactive intestinal peptide (VIP) and substance P (SP)), MMPs and extracellular macromolecules also play various roles in the regulation of skin cell motility and proliferation [48][49].

Figure 2. Phases of wound healing (own work based on [42][49][50][51]). TGF-β, transforming growth factor β; PDGF, platelet-derived growth factor; IL-1, 4, 6, interleukin-1, -4, -6); TNF-α, tumor necrosis factor α; AMPs, antimicrobial peptides; TIMP, tissue inhibitors of metalloproteinase; HGF, hepatocyte growth factor; FGF, fibroblast growth factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; ECM, extracellular matrix; MMPs, matrix metalloproteinases.
A wound is an injury involving a breach of the integrity of the skin. A chronic wound may lead to complications, such as bacterial infections. Bacterial infections also delay the wound-healing process, prolonging inflammation. The surface of human skin is colonized by commensal bacteria with low virulence, such as coagulase-negative staphylococci and non-pathogenic corynebacteria and cutibacteria, but also by opportunistic pathogenic microbes (such as Candida spp., Malassezia spp. or Staphylococcus aureus) and bacteria with high pathogenic potential (e.g., Streptococcus pyogenes). The skin of hospitalized patients who have undergone antibiotic treatment may be colonized by Gram-negative non-fermenting bacteria (Pseudomonas aeruginosa or Acinetobacter baumannii) or yeasts, including the opportunistic pathogen Candida auris. The choice of treatment for skin and wound infections depends on various factors (e.g., the severity of the disease or host factors), but plants and drugs of natural origin can undoubtedly have broad applications alongside topical synthetic antibiotics and antiseptic agents [52][53][54].
Botanicals have been used topically for decades for skin regeneration and the treatment of dermatological problems, such as chronic diabetic wounds, ulcers, bedsores, burns and non-healing wounds. Numerous plants and drugs of natural origin support the normal repair systems of the skin and therefore show great therapeutic potential in skin regeneration and wound treatment by various mechanisms. These include effects on keratinocyte migration and proliferation rates, modulation of the release of various growth factors, cytokines, chemokines or neuropeptides by skin cells, increasing the formation of capillary vessels and increasing fibroblast activity. Another important group of raw materials comprises plants with astringent and antimicrobial properties, which contribute to wound contraction and increase the rate of epithelialization [43][44][55]. The scientific literature points to the important effects of plants (e.g., Achiella millefolium [56], Aloe vera [57], Althaea officinalis [58], Calendula officinalis [59], Curcuma longa [60], Eucalyptus globulus [61], Simmondsia chinensis [62], Pinus sylvestris [63] and Camellia sinensis [64]) and phytochemicals (e.g., triterpenes, alkaloids and flavonoids) on tissues and their potential to amplify skin regeneration and accelerate the process of wound repair and healing [44][55].
4. Plants as Anti-Aging Agents
Over the centuries, the search for new substances to slow down the aging process and restore the skin’s young appearance has not diminished. Bioactive substances with anti-aging properties include moisturizers, which influence the hydrolipid barrier and minimize destructive lesions occurring in the stratum corneum. The skin may be hydrated through the external supply of water from moisturizing agents or via the application of agents forming an occlusive lipid film to slow down water loss from the skin. An important group of anti-aging agents comprises bioactive substances, which take part in the synthesis and metabolism of skin components (e.g., proteins and essential unsaturated fatty acids) and also exhibit collagenase, elastase and hyaluronidase inhibitory activity [1][65]. Collagenase is an enzyme belonging to the family of matrix metalloproteinases (MMP), which can degrade collagen, the fibrous component of the extracellular matrix (ECM) and the major structural protein in human skin. Elastase is a proteolytic enzyme involved in the degradation of elastin, a protein responsible for skin elasticity. Hyaluronidase is an enzyme (an endoglycosidase) responsible for the hydrolysis of hyaluronic acid, a skin glycosaminoglycan, which is a major component of ECM [66][67].
Botanicals that support the health, texture and integrity of the skin are widely used in cosmetic formulations for dry and mature skin. Plant extracts and natural products are recommended because they increase skin hydration, reduce TEWL, display skin-barrier-reinforcing properties, inhibit the degradation of skin components and help to maintain the integrity of the skin’s structure. These are promising approaches to preventing skin aging using products derived from plants. Plants can be a very interesting source of ingredients with potential anti-aging properties, as confirmed by the results of in vitro studies. However, further research is needed to confirm the efficacy of plant-derived materials in vivo, as the most important factor determining the effectiveness of active ingredients of natural origin is their bioavailability. In some studies, plants have been shown to exert notable in vivo anti-aging properties. According to the literature, skin parameters associated with skin aging, such as skin hydration (measured with a corneometer and tewameter), skin elasticity (measured with a cutometer and elastometer) or facial wrinkles (measured with a skin visiometer and camera for skin analysis) have been evaluated following the application of cosmetic formulations based on various plant extracts, alone or in combination [68][69][70][71].
5. Plants as Anti-Tyrosinase Agents
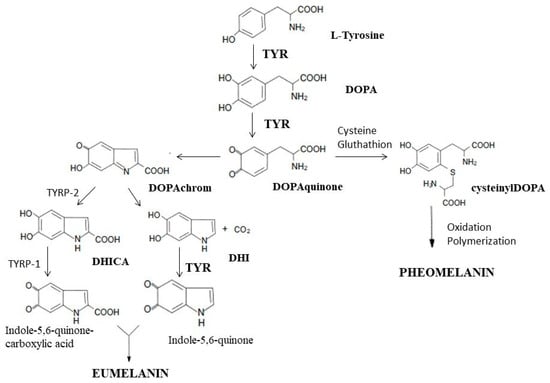
Tyrosinase is an enzyme that is widely distributed in the cells of animals, plants and microorganisms. It is a key enzyme in the biosynthesis of melanin, responsible for the catalysis of the first two synthesis reactions, i.e., the hydroxylation of tyrosine to DOPA and the oxidation of DOPA to dopaquinone. At the stage of dopaquinone formation, the eumelanin and pheomelanin pathways are separated. When thiol compounds (cysteine and glutathione) are present, they attach to dopaquinone, and the biosynthesis pathway is redirected toward pheomelanin. When the L-tyrosine concentration is low and that of cysteine is high, cysteine attaches to dopaquinone, and cysteinyldopa isomers are formed [72][73]. In the absence of thiol compounds, highly reactive dopaquinone easily undergoes intracellular cyclization, oxidation and transformation to dopachrome [72][73][74][75]. In the presence of TYRP2 (tyrosinase-related protein 2, also called dopachrome tautomerase—DCT) or metal cations (Cu2+, Zn2+, Fe2+, Co2+ or Ni2+), dopachrome may be converted to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) [72][75]. In the absence of DCT, dopachrome is converted to 5,6-dihydroxyindole (DHI) by nonenzymatic decarboxylation [72]. TYRP1 (tyrosinase-related protein 1) causes the oxidation of DHICA to indole-5,6-quinone-2-carboxylic acid, and TYR causes the oxidation of DHI to indole-5,6-quinone. The polymerization of the resulting monomers (indole and quinone) leads to the formation of eumelanin [73][75] (Figure 3).

Figure 3. Participation of tyrosinase in the synthesis of melanins: eumelanin and pheomelanin. TYR, tyrosinase; DOPA, dihydroxyphenylalanine; TYRP2, tyrosinase-related protein 2; TYRP1, tyrosinase-related protein 1; DHICA, 5,6-dihydroxyindole-2-carboxylic acid; DHI, 5,6-dihydroksyindol (own work based on [72][74][76]).
As a metalloenzyme, tyrosinase has two copper atoms in its active site, determining its catalytic function. Substances belonging to the group of tyrosinase inhibitors inhibit melanin synthesis by interacting with copper ions in the active site of tyrosinase, thereby reducing the activity of the enzyme [77][78].
In recent years, anti-tyrosinase agents have attracted the attention of researchers searching for substances that can whiten the skin and also treat skin pigmentation disorders. Ongoing research indicates that many plant extracts and plant-derived chemicals are strong tyrosinase inhibitors and prevent the overproduction of melanin in the epidermal layers. At the same time, importantly, they inhibit melanogenesis without exerting cytotoxic or mutagenic effects on melanocytes [77][79][80][81]. Constituents of plant extracts with depigmenting properties resulting from the inhibition of tyrosinase activity include arbutin (found in, e.g., Pyrus pyrifolia peel (3.35 mg/g) [82], Origanum majorana herbs (51.3 mg/g) [83], Arctostaphylos uva-ursi leaves (6.4%) [84], Vaccinium vitis idaeae leaves (46.78 mg/g) [85] or Bergenia crassifolia leaves (22.59%) [86]), coumaric acid (present in, e.g., Artocapus altilis fruits (11.85 mg/100 g) [87][88]), ellagic acid (occurs in, e.g., Juglans regia leaves (16.25%), Castanea sativa stem bark (2.75%) or Eucalyptus camaldulensis leaves (0.28%) [89]), aloesin (isolated from the Aloe vera leaves (64 mg/L) [90]), baicalein (present in Scutellaria baicalensis roots (16.61 mg/g) [91][92]) and glabridin (found in Glycyrrhiza glabra roots (22.87 mg/g) [93]).
6. Plants as Aromatic Agents
Over the centuries, the aromatic applications of plant extracts have gained importance. Plant essential oils, considered to be those with an oil content above 0.01% of the fresh weight of the plant, are of particular importance. Some plant materials may contain even 20% essential oils (EOs) [94][95][96]. EOs are mainly obtained from plants of the Apiaceae, Asteraceae, Lamiaceae, Lauraceae, Myrtaceae, Rutaceae, Verbenaceae and Geraniaceae families [95][97]. EOs can be found in all parts of the plant, i.e., the flowers (rose, lavender, jasmine or ylang-ylang), leaves (eucalyptus, peppermint, geranium, rosemary or tea tree), herbs (basil, hyssop and lemon balm), roots (ginger and vetiver), wood (cedarwood, camphor and sandalwood), bark (cinnamon and myrtle), seeds (anise, cumin, cardamom and fennel) and fruits (pepper, nutmeg and juniper). They are obtained from raw plant materials via distillation (water, steam or dry distillation), extraction (microwave, ultrasound, solvent extraction, maceration or enfleurage) or mechanical or cold pressing. EOs are mixtures of volatile substances, mostly colorless or light yellow, with an intense odor and an oily consistency, and they are soluble in liquid fats, alcohol, ether or chloroform. The biological activity and fragrance of EOs are determined according to their chemical composition. Their composition depends on numerous factors, including the origin of the plant materials or the conditions of plant growth. EOs are not chemically homogeneous. They may contain up to several hundred chemical compounds, including terpene hydrocarbons and their oxygen derivatives, alcohols, aldehydes, ketones, organic acids, esters and ethers [94][95][97][98].
Cosmetic aromatherapy utilizes EOs for skin, body, face and hair products. EOs are added to skincare and bath cosmetics or massage preparations as substances providing fragrance and as active ingredients. Smell is an important criterion in purchasing cosmetic products. A wide range of essential oils is available, and their marketing potential is enormous. Fragrance composition is an important element of the formulation of new cosmetic preparations. Fragrances also play an important role in masking unpleasant aromas from fatty acids, oils and surfactants used in cosmetic formulations [94][96][98].
EOs and their constituents, in addition to their aromatic effects, are also used in modern cosmetics and dermocosmetics as absorption promoters and preservatives [96]. The absorption of active substances by the skin can also be increased by EOs, such as eucalyptus, peppermint or terpentine oil, as well as by components of essential oils, such as menthol, limonene, carvacrol, linalool, α-pinene or terpineol [96][97]. Due to their antimicrobial action, EOs can act as natural preservatives to prolong the durability of cosmetics, e.g., essential oils from lavender (Lavandula angustifolia) [99], thyme (Thymus vulgaris) [100], peppermint (Mentha piperita) [101], cajuput (Melaleuca cajuputi), cinnamon (Cinnamomum zeylanicum) [102], clove (Syzygium aromaticum) [103], eucalyptus (Eucalyptus globulus) [104], sage (Salvia officinalis) [105] and tea tree (Melaleuca alternifolia) [106].
7. Plants as Colorants and Dye Agents
Plant dyes, which are varied in terms of chemical structure, are a group of compounds that are present in plant parts such as flowers, fruits and leaves. Plant pigments include quinones, polyphenols, chlorophylls, carotenoids and betalains [107][108][109][110][111].
Quinones are compounds whose color ranges from yellow to orange to red to brown. Quinones, which include benzoquinones, naphthoquinones and anthraquinones, are a large group of pigments. Anthraquinones are anthracene derivatives that are widespread in the plant world. They can be found among plants of the Polygonaceae, Rubiaceae, Rhamnaceae, Scrophulariaceae, Liliaceae, Hypericaceae and Fabaceae families.
A wealth of flavonoids can be found in plants of the Apiaceae, Asteraceae, Betulaceae, Polygonaceae, Brassicaceae, Ericaceae, Fabaceae, Hypericaceae, Primulaceae, Lamiaceae, Rosaceae, Rubiaceae, Rutaceae and Scrophulariaceae families. Apart from their role in skin care, flavonoids are used in cosmetics as natural plant dyes, including flavonols (intense yellow), flavones (light yellow and cream-colored), chalcones (light yellow) and aurones (intense yellow) [107][108][109].
Anthocyanins are widespread plant dyes, the most common of which include red pelargonidin (geranium and dahlia), blue-to-red peonidin (elderberry and peony) and cyanidin (cornflower, chokeberry, cranberry and cherry), purple malvidin (mallow and grapes), petunidin (petunia) and delphinidin (grape, elderberry and cranberry). Tannins are broadly distributed in the plant kingdom and are generally classified into two types: hydrolysable tannins (e.g., gallotannins and ellagitannins) and condensed tannins (catechins and leucoanthocyanidins). Plants supplying brown, gray or sometimes rust-colored tannin dyes include the species Uncaria gambir, Galla chinensis (Chinese gallnut), Acacia catechu, Schinopsis balansae, Pteropcarpus marspinum, Eucalyptus rostrata, Quercus infectoria, Quercus robur, Quercus sessilis, Potentilla erecta, Alchemilla vulgaris, Sanguisorba officinalis and Polygonum bistorta [107][108][109][112].
Chlorophylls are a pigment that is present in all green plants (in the stems, leaves, flowers, fruits or seeds), e.g., Urtica dioica, Medicago sativa, spinach, lettuce and broccoli. Among the known plant chlorophylls, two have significance as dyes: chlorophyll a (blue-green) and chlorophyll b (yellow-green). Chemically, chlorophyll is an ester (magnesium porphyrin composed of four pyrrole rings) with two alcohols (phytol and methanol) [109][113].
Carotenoids are polyene dyes, i.e., they have a conjugated double-bond system. Plant sources of carotenoids include Crocus sativus, from which the stigma, containing the yellow carotenoid pigment crocin, is used; Bixa orellana, whose fruits supply the yellow-orange carotenoid pigment bixin (annato, orlean); and Calendula officinalis, whose flowers contain α- and β-carotene, lutein, lycopene and violaxanthin [108][109][114].
Betalains are found in plants of the order Caryophyllales. Sources of betalain pigments include beet root (Beta vulgaris), the fruits of the prickly pear (Opuntia ficus-indica) or cacti of the Hylocereus genus and the flowers of numerous species of the Amaranthaceae family [108][109].
Natural colorants and dyes of plant origin have the important advantages of being nontoxic, safe, without side effects, non-carcinogenic, environmentally friendly (biodegradable and compatible with the environment) and economical. For these reasons, they are becoming an object of consumer interest with broad applications in the cosmetic industry. Plant dyes can be an alternative to synthetic dyes, which involve the use of petrochemical-based materials, and due to their allergic, toxic, mutagenic, genotoxic and carcinogenic effects, they are responsible for various health and skin problems [110][113][115].
References
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203.
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585.
- Menaa, F. Skin anti-aging benefits of phytoterapeutics-based emulsions. Pharm. Anal. Acta 2014, 5, 168.
- Jadoon, S.; Karim, S.; Bin Asad, M.H.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity. Oxid. Med. Cell Longev. 2015, 2015, 709628.
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537.
- Skarupova, D.; Vostalova, J.; Rajnochova Svobodova, A. Ultraviolet A protective potential of plant extracts and phytochemicals. Biomed. Pap. Med. Fac. Univ. Palacký Olomouc Czechoslov. 2020, 164, 1–22.
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, 11, 220.
- Park, K.; Choi, H.S.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Cactus cladodes (Opuntia humifusa) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm. Biol. 2017, 55, 1032–1040.
- Tobi, S.E.; Gilbert, M.; Paul, N.; McMillan, T.J. The green tea polyphenol, epi gallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int. J. Cancer. 2002, 102, 439–444.
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561.
- Napagoda, M.T.; Malkanthi, B.M.A.S.; Abayawardana, S.A.K.; Qader, M.M.; Jayasinghe, L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement. Altern. Med. 2016, 16, 479.
- Svobodová, A.; Zdařilová, A.; Walterová, D.; Vostálová, J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007, 48, 213–224.
- Alonso-Lebrero, J.L.; Domínguez-Jiménez, C.; Tejedor, R.; Brieva, A.; Pivel, J.P. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J. Photochem. Photobiol. B 2003, 70, 31–37.
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 2014, 132, 27–35.
- Svobodová, A.; Zdařilová, A.; Vostálová, J. Lonicera caerulea and Vaccinium myrtillus fruit polyphenols protect HaCaT keratinocytes against UVB-induced phototoxic stress and DNA damage. J. Dermatol. Sci. 2009, 56, 196–204.
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T.H. Thymus vul garis alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell Mol. Med. 2017, 21, 336–438.
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective effect of Opuntia ficus-indica L. cladodes against UVA-induced oxidative stress in normal human keratinocytes. Bioorg. Med. Chem. Lett. 2017, 27, 5485–5489.
- West, B.J.; Deng, S.; Palu, A.K.; Jensen, C.J. Morinda citrifolia Linn. (Rubiaceae) leaf extracts mitigate UVB-induced erythema. J. Nat. Med. 2009, 63, 351–354.
- Bazylko, A.; Borzym, J.; Parzonko, A. Determination of in vitro antioxidant and UV-protecting activity of aqueous and ethanolic extracts from Galinsoga parviflora and Galinsoga quadriradiata herb. J. Photochem. Photobiol. B 2015, 149, 189–195.
- Cho, Y.-H.; Bahuguna, A.; Kim, H.-H.; Kim, D.-I.; Kim, H.-J.; Yu, J.-M.; Jung, H.-G.; Jang, J.-Y.; Kwak, J.-H.; Park, G.-H.; et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B 2017, 174, 323–332.
- Bulla, M.K.; Hernandes, L.; Baesso, M.L.; Nogueira, A.C.; Bento, A.C.; Bortoluzzi, B.B.; Serra, L.Z.; Cortez, D.A.G. Evaluation of photoprotective potential and percutaneous penetration by photoacoustic spectroscopy of the Schinus terebinthifolius raddi extract. Photochem. Photobiol. 2015, 91, 558–566.
- de Lima-Saraiva, S.R.G.; da Silva Oliveira, F.G.; de Oliveira Junior, R.G.; de Souza Araújo, C.; de Oliveira, A.P.; Pacheco, A.G.M.; Rolim, L.A.; de Amorim, E.L.C.; César, F.C.S.; da Silva Almeida, J.R.G. Chemical analysis and evaluation of antioxidant, antimicrobial, and photoprotective activities of Schinopsis brasiliensis Engl. (Anacardiaceae). Sci. World J. 2017, 2017, 1713921.
- Ebrahimzadeh, M.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047.
- Jarzycka, A.; Lewińska, A.; Gancarz, R.; Wilk, K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B 2013, 128, 50–57.
- Lefahal, M.; Zaabat, N.; Ayad, R.; Makhloufi, E.H.; Djarri, L.; Benahmed, M.; Laouer, H.; Nieto, G.; Akkal, S. In vitro assessment of total phenolic and flavonoid contents, antioxidant and photoprotective activities of crude methanolic extract of aerial parts of Capnophyllum peregrinum (L.) Lange (Apiaceae) growing in Algeria. Medicines 2018, 5, 26.
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.F.; Barbosa, W.; Raposo, N.R.B. Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B 2016, 161, 34–39.
- Mejía-Giraldo, J.C.; Winkler, R.; Gallardo, C.; Sánchez-Zapata, A.M.; Puertas-Mejía, M.A. Photoprotective potential of Baccharis antioquensis (Asteraceae) as natural sunscreen. Photochem. Photobiol. 2016, 92, 742–752.
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111.
- Nunes, A.R.; Rodrigues, A.L.M.; de Queiróz, D.B.; Vieira, I.G.P.; Neto, J.F.C.; Junior, J.T.C.; Tintino, S.R.; de Morais, S.M.; Coutinho, H.D.M. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J. Photochem. Photobiol. B 2018, 189, 119–123.
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161.
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods 2021, 10, 1937.
- Quintero-Rincón, P.; Mesa-Arango, A.C.; Flórez-Acosta, O.A.; Zapata-Zapata, C.; Stashenko, E.E.; Pino-Benítez, N. Exploring the Potential of Extracts from Sloanea medusula and S. calva: Formulating Two Skincare Gels with Antioxidant, Sun Protective Factor, and Anti-Candida albicans Activities. Pharmaceuticals 2023, 16, 990.
- Vijayakumar, R.; Salwa Abd Gani, S.; Hasanah Zaidan, U.; Effendi Halmi, I.; Karunakaran, T.; Razak Hamdan, M. Exploring the Potential Use of Hylocereus polyrhizus Peels as a Source of Cosmeceutical Sunscreen Agent for Its Antioxidant and Photoprotective Properties. Evid.-Based Complement. Altern. Med. 2020, 2020, 7520736.
- Fuentes, J.L.; Pedraza Barrera, C.A.; Villamizar Mantilla, D.A.; Flórez González, S.J.; Sierra, L.J.; Ocazionez, R.E.; Stashenko, E.E. Flower Extracts from Ornamental Plants as Sources of Sunscreen Ingredients: Determination by In Vitro Methods of Photoprotective Efficacy, Antigenotoxicity and Safety. Molecules 2022, 27, 5525.
- Fernandes, A.S.; Mazzei, J.L.; Oliveira, C.G.; Evangelista, H.; Marques, M.R.C.; Ferraz, E.R.A.; Felzenszwalb, I. Protection against UV-induced toxicity and lack of mutagenicity of Antarctic Sanionia uncinata. Toxicology 2017, 376, 126–136.
- Fernandes Ada, S.; Santos Alencar, A.; Evangelista, H.; Luiz Mazzei, J.; Felzenszwalb, J. Photoprotective and toxicological activities of extract from the Antarctic moss Sanionia uncinata. Pharmacogn. Mag. 2015, 11, 38–43.
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.d.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The synergistic behavior of antioxidant phenolic compounds obtained from Winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants 2019, 8, 530.
- Mota, M.D.; da Boa Morte, A.N.; Silva, L.C.R.C.; Chinalia, F.A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L.) ethanolic extract. J. Photochem. Photobiol. B 2020, 205, 111837.
- Mota, M.D.; Costa, R.Y.S.; Guedes, A.A.S.; Silva, L.C.R.C.; Chinalia, F.A. Guava-fruit extract can improve the UV-protection efficiency of synthetic filters in sun cream formulations. J. Photochem. Photobiol. B 2019, 201, 111639.
- Catelan, T.B.S.; Gaiola, L.; Ferreira Duarte, B.; Lima Cardoso, C.A. Evaluation of the in vitro photoprotective potential of ethanolic extracts of four species of the genus Campomanesia. J. Photochem. Photobiol. B 2019, 197, 111500.
- Fernandes, A.S.; Mazzei, J.L.; Evangelista, H.; Marques, M.R.C.; Ferraz, E.R.A.; Felzenszwalb, I. Protection against UV-induced oxidative stress and DNA damage by Amazon moss extracts. J. Photochem. Photobiol. B 2018, 183, 331–341.
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620.
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108.
- Bass, S.; Chowdhury, M.; Raj, S.; Raj Chaudhary, N. Effects of Phytomedicines on Wound Healing. Eur. J. Exper. Biol. 2021, 11, 133–138.
- Bindschadler, M.; McGrath, J.L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 2007, 120, 876–884.
- Parent, C.A.; Devreotes, P.N. A cell’s sense of direction. Science 1999, 284, 765–770.
- Horwitz, R.; Webb, D. Cell migration. Curr. Biol. 2003, 13, 756–759.
- Sivamani, R.K.; Garcia, M.S.; Rivkah Isseroff, R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868.
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2015, 4, 213–224.
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607.
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722.
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, In Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022, 23, 142.
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, European Legislation and Folk Medicine-A Review. Int. J. Mol. Sci. 2021, 22, 10746.
- Nowak, A.; Zagórska-Dziok, M.; Perużyńska, M.; Cybulska, K.; Kucharska, E.; Ossowicz-Rupniewska, P.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; Sulikowski, T.; et al. Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts. Front. Pharmacol. 2022, 13, 896706.
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The Therapeutic Wound Healing Bioactivities of Various Medicinal Plants. Life 2023, 13, 317.
- Pain, S.; Altobelli, C.; Boher, A.; Cittadini, L.; Favre-Mercuret, M.; Gaillard, C.; Sohm, B.; Vogelgesang, B.; André-Frei, V. Surface rejuvenating effect of Achillea millefolium extract. Int. J. Cosmet. Sci. 2011, 33, 535–542.
- Sajithlal, G.B. Influence of aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J. Exp. Biol. 2000, 36, 896–901.
- Rezaei, M.; Dadgar, Z.; Noori-Zadeh, A.; Mesbah-Namin, S.A.; Pakzad, I.; Davodian, E. Evaluation of the antibacterial activity of the Althaea officinalis L. leaf extract and its wound healing potency in the rat model of excision wound creation. Avicenna J. Phytomed. 2015, 5, 105–112.
- Preethi, K.C.; Kuttan, R. Wound healing activity of flower extract of Calendula officinalis. J. Basic Clin. Physiol. Pharmacol. 2009, 20, 73–80.
- Yen, Y.H. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9,-SMA, and collagen. Int. Wound J. 2018, 15, 605–617.
- Mumtaz, R.; Zubair, M.; Khan, M.A.; Muzammil, S.; Siddique, M.H. Extracts of Eucalyptus alba Promote Diabetic Wound Healing by Inhibiting α-Glucosidase and Stimulating Cell Proliferation. Evid. Based Complement. Altern. Med. 2022, 2022, 4953105.
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011, 134, 443–449.
- Wang, C.; Shang, H.; Cui, W.; Zhou, F.; Zhang, S.; Wang, X.; Gao, P.; Wei, K.; Zhu, R. Pine pollen polysaccharides promote cel proliferation and accelerate wound healing by activating the JAK2-STAT3 signaling pathway. Int. J. Biol. Macromol. 2022, 210, 579–587.
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686.
- George, J.; Sneed, K.; Pathak, Y. The Skin Aging Process and Anti-Aging Strategies. Biomed. J. Sci. Tech. Res. 2022, 42, 33377–33386.
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195.
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27.
- Yoon, J.H.; Park, S.H.; Yoon, S.E.; Hong, S.Y.; Lee, J.B.; Lee, J.; Cho, J.Y. Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation. Nutrients 2023, 15, 2436.
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802.
- Poomanee, W.; Yaowiwat, N.; Pattarachaidaecharuch, T.; Leelapornpisid, P. Optimized multiherbal combination and in vivo anti-skin aging potential: A randomized double blind placebo controlled study. Sci. Rep. 2023, 13, 5633.
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Ki, Y.H.; Ryu, E.K.; Chang, M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396.
- Ito, S. A chemist’s view of melanogenesis. Pigment. Cell Res. 2003, 16, 230–236.
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276.
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment. Cell Melanoma Res. 2009, 22, 563–579.
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment. Cell Melanoma Res. 2015, 28, 520–544.
- Hearing, V.J. Determination of melanin synthetic pathway. J. Investig. Dermatol. 2011, 131, E8–E11.
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475.
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment. Cell Res. 2006, 19, 550–571.
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27.
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349.
- Agbai, O.N.; Taylor, S.C. Melasma and Depigmentation Agents. Cosmeceuticals and Active Cosmetics. In Cosmeceuticals and Active Cosmetics; Sivamani, R.K., Jagdeo, J., Elsner, P., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 343–356.
- Lee, B.D.; Eun, J.B. Optimum extraction conditions for arbutin from Asian peal peel by supercritical fluid extraction (SFE) using Box-Behnken design. J. Med. Plant Res. 2012, 6, 2348–2364.
- Lukas, B.; Schmiderer, C.; Mitteregger, U.; Novak, J. Arbutin in marjoram and oregano. Food Chem. 2010, 121, 185–190.
- Kenndler, I.E.; Schwer, C.; Fritsche, B.; Pöhm, M. Determination of arbutin in Uvae-ursi folium (bearberry leaves) by capillary zone electrophoresis. J. Chromatogr. A 1990, 514, 383–388.
- Pyka, A.; Bober, K.; Stolarczyk, A. Densitometric Determination of arbutin in cowberry leaves (Vaccinium vitis idaeae). Acta Pol. Pharmaceutic. Drug Res. 2007, 63, 395–400.
- Pop, C.; Vlase, L.; Tamas, M. Natural Resources Containing Arbutin. Determination of Arbutin in the Leaves of Bergenia crassifolia (L.) Fritsch. acclimated in Romania. Not. Bot. Hort. Agrobot. Cluj 2009, 37, 129–132.
- Nguyen, M.H.K.; Nguyen, H.X.; Nguyen, M.T.T.; Nguyen, N.T. Phenolic Constituents from the Heartwood of Artocapus altilis and their Tyrosinase Inhibitory Activity. Nat. Prod. Commun. 2012, 7, 185–186.
- Soifoini, T.; Donno, D.; Jeannoda, V.; Rakoto, D.D.; Msahazi, A.; Farhat, S.M.M.; Oulam, M.Z.; Beccaro, G.L. Phytochemical Composition, Antibacterial Activity, and Antioxidant Properties of the Artocarpus altilis Fruits to Promote Their Consumption in the Comoros Islands as Potential Health-Promoting Food or a Source of Bioactive Molecules for the Food Industry. Foods 2021, 10, 2136.
- Özer, Ö.; Mutlu, B.; Kıvçak, B. Antityrosinase Activity of Some Plant Extracts and Formulations Containing Ellagic Acid. Pharmaceut. Biol. 2007, 45, 519–524.
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment. Cell Res. 2002, 15, 335–340.
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927.
- Zheng, Y.; Zhou, S.; Zhang, H.; Lu, Z.; Deng, R.; Feng, Y.; Li, P. Comparative Study of the Flavonoid Content in Radix Scutellaria from Different Cultivation Areas in China. Int. J. Anal. Chem. 2023, 2023, 3754549.
- Eghlima, G.; Kheiry, A.; Sanikhani, M.; Hadian, J.; Aelaei, M.; Ebrahimi, S.N. Investigation of Phytochemical Variability, Antioxidant Activity and Ecological Conditions of Native Iranian Glycyrrhiza glabra L. Int. J. Hort. Sci. Technol. 2020, 7, 387–400.
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611.
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017, 2017, 9268468.
- Michalak, M. Aromatherapy and methods of applying essential oils. Arch. Physiother. Glob. Res. 2018, 22, 25–31.
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114.
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666.
- Śmigielski, K.; Raj, A.; Krosowiak, K.; Gruska, R. Chemical Composition of the Essential Oil of Lavandula angustifolia Cultivated in Poland. J. Essent. Oil Bear. Plants 2009, 12, 338–347.
- Shabnum, S.; Wagay, M.G. Essential Oil Composition of Thymus vulgaris L. and their Uses. J. Res. Develop. 2011, 11, 23617694.
- Golparvar, A.R.; Hadipanah, A. Chemical compositions of the essential oil from peppermint (Mentha piperita L.) cultivated in Isfahan conditions. J. Herb. Drugs 2013, 4, 75–80.
- Chakraborty, A.; Sankaran, V.; Ramar, M.; Chellappan, D.R. Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J. Chem. Pharm. Sci. 2015, 8, 476–479.
- Hatami, T.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Supercritical fluid extraction assisted by cold pressing from clove buds: Extraction performance, volatile oil composition, and economic evaluation. J. Supercrit. Fluids 2019, 144, 39–47.
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Chemical Composition of Essential Oils from Eucalyptus globulus and Eucalyptus maculata Grown in Tanzania. Sci. Afr. 2021, 12, e00758.
- Khalil, R.; Li, Z.G. Antimicrobial activity of essential oil of Salvia officinalis L. collected in Syria. Afr. J. Biotechnol. 2011, 10.
- Borotová, P.; Galovicová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kacániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558.
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145.
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289.
- Michalak, M.; Glinka, R. Sources of vegetable dyes and their use in cosmetology. Pol. J. Cosmetol. 2017, 20, 196–205.
- Bhalekar, O.S.; Waghmare, S.A.; Kamble, H.V.; Dhamal, K.S. Natural colourants and dyes from plant origin. Int. J. Sci. Res. Eng. Dev. 2022, 5, 537–549.
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-Derived Colorants for Food, Cosmetic and Textile Industries: A Review. Materials 2021, 14, 3484.
- Cui, H.; Xie, W.; Hua, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Yuan, Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules 2022, 27, 8062.
- Mohana Priya, M.; Chidambara Rajan, P.; Lavanya, M. Use of natural pigments as colorants in cosmetics—A review. J. Emerg. Technol. Innov. Res. 2020, 7, 907–917.
- Chandrasekar, R.; Sivagami, B.; Swapna, D. Herbal Cosmetics an Overview. Int. J. Pharm. Res. Rev. 2016, 5, 1–20.
- Prabhu, K.H.; Bhute, A.S. Plant based natural dyes and mordnats: A Review. J. Nat. Prod. Plant Resour. 2012, 2, 649–664.
More
Information
Subjects:
Dermatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
19 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




