Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irena Kostova | -- | 8034 | 2024-01-17 06:26:06 | | | |
| 2 | Camila Xu | Meta information modification | 8034 | 2024-01-17 06:29:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kostova, I. Main Group Metals and Metalloids in Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/53933 (accessed on 14 January 2026).
Kostova I. Main Group Metals and Metalloids in Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/53933. Accessed January 14, 2026.
Kostova, Irena. "Main Group Metals and Metalloids in Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/53933 (accessed January 14, 2026).
Kostova, I. (2024, January 17). Main Group Metals and Metalloids in Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/53933
Kostova, Irena. "Main Group Metals and Metalloids in Cancer Treatment." Encyclopedia. Web. 17 January, 2024.
Copy Citation
Cancer is one of the leading causes of human death among all major diseases. Metal-based complexes are considered as the most promising vital part in the existing arsenal of cytotoxic candidates used in cancer therapy and diagnostics. The efforts of many scientific groups resulted in the development of numerous metal-based compounds featuring different biologically active organic ligands in order to modulate their bioactivity.
metals
metalloids
complexes
organometallic compounds
anticancer activity
1. Introduction
Long ago, metal-based compounds were broadly used in the treatment of different diseases. Many of them have been reconsidered due to the absence of distinction between their therapeutic and toxic dosages. Nowadays, the research in the field of metal-based chemotherapeutic compounds is mostly directed to the rational design of metal complexes that can target specific biomolecules.
In recent decades, a number of classes of new metal-based coordination compounds have been intensively explored as potent antineoplastic agents based on a wide variety of metals, primarily from the d-block transition metals. The main representatives are complexes containing platinum [1], ruthenium [2], rhodium [3], palladium [4], iridium [5], gold [6], silver [7], titanocenes [8], vanadium [9] and lanthanide [10].
In general, non-transition metal cations are vital natural cell components involved in numerous bioprocesses. This is why, for the normal wellbeing maintenance of physiological processes, it is significant to find a balance between the cell requirement and the quantity existing in the living body. On the other hand, many metals and metalloids are not essential to the organism, but can hinder carcinogenesis. This has prompted an extensive demand for metal-and metalloid-based compounds of s- and p-block elements with low toxicity, high selectivity, a wide-ranging activity spectrum and mechanisms of action different from that of the classic metal-based chemotherapeutics, thus providing new alternatives.
Given the variety of metal centers available among the s- and p-block elements, different complexes can be designed and modified to give a wide variety of coordination sites, specific biochemical properties and structural diversity to be exploited for developing new anticancer therapeutics. The systematic study of the coordination chemistry of main group elements holds the potential to unveil new categories of cytotoxic compounds with targeted bioactivity and their associated pharmacological properties and modes of action. Because of their specific mechanisms of action and different pharmacological profiles, metal- and metalloid-based pharmaceuticals provide new perspectives for scientific research and for drug design development [11][12][13].
2. Properties of Metals and Their Significance for Biological Systems
Metals are s-, p-, d- and f-block elements of the periodic table. From a chemical point of view, metal cations possess characteristic features and unique biochemical properties that include the following:
-
Tendency to exhibit charge variations: Metal ions exist mainly as positively charged species, although they can form cationic, anionic and neutral species which depends on the coordination environment, which can modify their charges. The positively charged metal ions bind to negatively charged biomolecules in physiological conditions. In the s- and p-block elements of the periodic table, this tendency appears for the IVA (+2, +4), VA (+3, +5) and VIA (+2, +4, +6) groups.
-
Chemical bonds and structural geometries: Corresponding to organic biomolecules, metal complex compounds can aggregate to various coordination geometries, giving them unique polyhedral shapes. The bond lengths, bond angles and coordination states can vary; this is contingent on the metal ions and their oxidation states. The variation in the different geometries can also be observed for the s- and p-block elements; see below.
-
Metal–bioligand interactions: Different kinds of metal–ligand interactions may exist in the body and these bio-interactions generally initiate complex formations where the properties of obtained complexes are different from those of the free bioligands and metal cations. This characteristic feature is especially pronounced for the metals and metalloids from the s- and p-blocks of the periodic table.
-
Features of the acid–base properties: Characterized by high electron affinity and a large set of oxidation states, most metal cations can readily polarize the coordinated functional groups, therefore facilitating the hydrolysis processes. The oxidation states dramatically affect the acid–base properties. These alterations with the variations in the oxidation states are specifically pronounced in p-elements; for instance, selenium and tellurium occur in different chemical forms with varied bioactivity: selenates (SeO42−, HSeO4−, H2SeO4), selenides (H2Se, HSe−), selenites (SeO32−, HSeO3−, H2SeO3), parts of many enzymes and the tellurium oxyanions tellurite (TeO32−) and tellurate (TeO42−).
-
Partially filled d-shells: For transition metals, the variable number of electrons in the d- or f-shell (for lanthanides and actinides) impacts the magnetic and electronic properties of the resulting metal complexes. Due to the incompleteness of d- and f-shells and the presence of energetically similar unfilled shells, d- and f-elements are predisposed to complexation reactions in which the complex compounds formed are usually colored and paramagnetic. When moving along a large period, an increase in the ability to chelate in both directions toward the center of the period is clearly observed and maximal complexation capacity is typical for the VIIIB group metals (Fe, Co, Ni, Pt, etc.), elements with unfilled d-subshells.
-
Tendency to exhibit oxidation–reduction properties: Many transition metals are strong reducing agents with a strong predisposition to undergoing oxidation–reduction reactions. Their reducing capacity is determined by the electronic configuration structures and the size of the respective cations. Only heavy metals of the VIIIB and IB groups are termed noble because of their inertness. The oxidation states of metal cations are responsible for the modulation and the rational design of bio-coordination compounds. In biochemical redox catalytic reactions, metal cations are activators of the coordinated substrates. Compounds of p-block metals in the maximal oxidation state possess oxidative properties.
Ions of s-elements, including biogenic Na+, K+, Md2+ and Ca2+, form coordination compounds in aqueous solutions, but their stability is negligible, because the bonds of s-elements with the ligands are typically ionic. Moreover, cations of s-elements are resistant to the action of oxidizing and reducing agents; therefore, oxidation–reduction reactions are not typical for them.
Most of the known chemical bonds in nature are the bonds formed by p-elements of the second to third periods. When passing to the long periods, various chemical bond types of p-elements appear, which increases the capacity for the formation of coordination complexes with different coordination numbers.
There are around 40 d-elements in the periodic table, located between s- and p-elements. All d-elements are typical metals, although their metallic properties are less pronounced than those of s-elements. d-elements form the group of trace microelements with numerous stable oxidation states and a high affinity to various donor atoms. This is why d-block microelements are involved in most of the important life processes, including enzymatic catalysis of cellular synthetic and energy processes, electron and ion transfer in biomolecules, regulation of cellular mechanisms of biosystems, etc. In fact, transition metal ions cannot exist in a free state in the organism due to biochemical interactions, and most frequently these elements are involved in the formation of metal-based bio-complexes.
During the design of anticancer treatments based on metal complexes, it is necessary to take into account different aspects: first, the characteristics of the transition metal, like charge variation, Lewis acid properties and its role in biological processes; second, the three-dimensional configuration of the metal compounds that can be modified to suit specific molecular targets; third, the oxidation state of the metal due to the biological redox chemistry and its influence in bioavailability and the optimal dose for the treatment; and finally, metal–ligand interactions owing to its thermodynamic and kinetic properties, which influence ligand exchange reactions.
In the human body, many metal cations form complex compounds with various endogenous and exogenous ligands with substantial biological functions. Additionally, many coordination complexes are widely used as therapeutics. The unique features of metal ions, listed above (e.g., redox activity, flexible coordination modes, wide range of coordination numbers, geometries, ligand substitution kinetic properties and affinity to bioorganic substrates), determine their great therapeutic potential in cancer therapy. The attractive properties of metal complexes are the main reason for the success of various metal-based compounds which selectively bind to the biotargets, resulting in modifications of their cellular mechanism of proliferative action. Metal-based compounds afford therapeutic mechanisms that organic compounds cannot achieve. Metal complexes with great therapeutic indexes include coordination compounds with a variety of ligands, for instance, ionic organic molecules, inorganic anions and many organometallic agents that have been studied. The biological functions, medical applications related to their anticancer activity and toxic effects of s- and p-block metals and metalloids and their compounds are collected in Table 1.
Table 1. Biological functions, medical anticancer applications and toxic effects of metals and metalloids and their compounds.
| Element | Location and Biofunctions | Compounds with Anticancer Activity | Toxicity, Antidotes | References |
|---|---|---|---|---|
| Cesium | 134Cs and 137Cs—radioactive pollutants | 131Cs—in brachytherapy and oncology for prostate cancer | Low abundance, insignificant influence | [14] |
| Strontium | In bone tissue affecting bone formation; 90Sr disturbs bone marrow hematopoiesis | Strontium-89—β-emitter in therapy for breast and metastatic prostate cancer | Brittle bones, Sr rickets, extracting Sr from bones is impossible | [15][16][17][18][19] |
| Radium | 223Ra2+ is a Ca2+-mimetic | 223Ra—α-emitter for bone metastases and prostate cancer | Ultratrace amounts in human body | [20][21][22][23][24] |
| Gallium | Ga3+—metabolism stimulator; anticancer, antibacterial, anti-inflammation; Fe3+-mimetic | Ga(NO3)3 in treatment of cancer-related hypercalcemia, 68Ga-, 67Ga-scintigraphy of malignant tumors, incl. Hodgkin’s and non-Hodgkin’s lymphomas | Particularly nontoxic | [25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41] |
| Indium | In3+—metabolism stimulator; 111In—radiotracer for tagging red, white blood cells and proteins | 111In—γ-emitter in radiopharmaceuticals for neuroendocrine and prostate cancer |
Damages kidneys; low abundance, insignificant influence | [42][43][44][45][46] |
| Thallium | Tl causes mitochondrial and energy production damages; interferes with K+ pathways | 205Tl—in NMR detection, 201Tl—in SPECT for perfusion tests of myocardium in diagnostics of heart attacks and coronary artery disease | Toxic metal; antidotes—dialysis and Prussian Blue in colloid solutions | [47][48][49][50] |
| Germanium | Improves immunity, removes toxins, controls pain, antitumor | Tumor prevention; Ge-132—in cancer and cardiovascular therapy |
Ge compounds with low solubility are not toxic to human cells | [51][52][53][54][55][56][57] |
| Tin | Stimulating growth effect; easily enters the bloodstream | Photodynamic therapy agent Purlytin for cutaneous cancer, Kaposi’s sarcoma, AIDS, breast metastases | Low-abundance nontoxic metal; toxic organotins | [58][59][60][61][62][63][64][65] |
| Lead | In erythrocytes; contributes to other metals’ toxicity; disturbs nervous system | 212Pb (β−emission) in radioimmunotherapy, 212Pb-TCMC-trastuzumab—antitumor agent | Saturnism; Pb2+ binds SH-groups; Antidotes—CaNa2EDTA, dimercaprol |
[66][67][68][69][70][71][72][73][74] |
| Arsenic | In the brain and muscle tissues, contributes to hemoglobin synthesis | As2O3—for promyelocytic leukemia; Darinaparsin—anticancer therapeutic | Toxic metal—arsenolysis; antidotes—Na2S2O3, dimercaprol, DMPS, DMSA | [75][76][77][78][79][80][81][82][83][84] |
| Bismuth | In kidneys; inhibits enzymes amino- and carboxy-polypeptidaze | 213Bi (α-emitter); 213Bi-lintuzumab for α-targeted radiotherapy in patients with AML; Bi2O3—shielding γ-rays |
Nontoxic with reversible toxicity; antidotes— D-penicillamine |
[85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102] |
| Selenium | In small amounts is vital for normal growth and metabolism; in selenoproteins | Cancer-preventing; selenomethionine and Se-methyl-seleno-L-cysteine for treatment of prostate cancer | Selenosis; toxic selenites and selenates | [103][104][105][106] |
| Tellurium | Constantly in the human body; its biofunctions are not clear | Te(IV) nontoxic compound AS101—for AML chemotherapy—antiapoptotic | Toxic volatile compounds—in glutathione metabolism | [107][108][109] |
| Astatine | Short-lived At radioisotopes; in spleen, thyroid gland and lungs | 221At, 222At, 223At (α-emitters); 211At in therapy of CNS and malignant brain tumors | Analogous to iodine; thyrotoxicosis | [110][111][112][113][114][115] |
3. s-Elements and Their Compounds in Cancer Chemotherapy
Radioactive 24Na, which has a half-life of around 15 h, is used as a label for determination of blood flow velocity and for therapy for some forms of leukemia [1][2][3].
The radioactive nuclide 131Cs, with electron capture decay and a half-life of 9.7 days, is used in oncology in therapy for prostate cancer and brain tumors [14]. The optimal combination of its half-life and radioactivity makes it an attractive radionuclide for brachytherapy for malignant (lung, brain, prostate gland, mammary gland, etc.) tumors. Cesium-131 brachytherapy is a safe and convenient treatment opportunity showing reduced radiation to normal tissues.
The radionuclides of Ca, Sr, Ba and Ra have been studied extensively, but up to the present time, only strontium and radium radioisotopes have been found to be important for nuclear medicine applications, mainly for pain-reducing and palliative therapy for bone metastases. Radioactive treatment with strontium is connected with malignancies that have spread to the bones. The best-known radioisotopes of strontium are 85Sr, 87mSr, 89Sr and 90Sr. Strontium-89 is an allowed β-emitting radioisotope (T1/2 = 50.5 d), often applied as SrCl2 for the treatment of breast and metastatic prostate cancer [16][17][18][19]. Its accumulation into osteoblastic bone metastases is greater than into normal bones. Strontium-89 is active in the calming relief of pain from bone, prostate and breast cancer metastases. Radioactive 85Sr is applied to treat severe bone pain from bone cancer. Like the other radionuclides of strontium, the extremely radioactive 85Sr accumulates in the bones. Radiation, in particular, kills the surrounding nerves and causes extreme pain. Strontium-90 (T1/2 = 28.8 years) undergoes β− decay. Radioactive 90Sr has applications in medicine and industry. Strontium-90 exhibits biochemical behavior similar to that of Ca and concentrates in bones, replacing Ca. Like calcium, strontium-90 is deposited mainly in bone and bone marrow and can cause bone cancer and leukemia [15][16].
All of the known isotopes of radium have radioactivity. The radioactive α-emitting 223Ra dichloride is a radiopharmaceutical with a half-life of 11.4 days. It is acceptable for the treatment of symptomatic bone metastases and prostate cancer. The ion 223Ra2+ is a typical Ca2+ mimetic that can selectively target bone metastases with α-particles, which causes double-strand breaks in DNA, exhibiting great local cytotoxicity with low myelosuppression [20][21]. Its activity in vivo has led to the estimation of its effectiveness in clinical trials of patients with bone-metastatic and prostate cancer, as well as for palliation of bone pain [23][24]. None of the presented Ca, Sr or Ra radioactive isotopes are appropriate for positron emission tomography (PET) or single-photon emission computed tomography (SPECT) imaging purposes.
4. p-Elements and Their Compounds as Anticancer Chemotherapeutics
4.1. Gallium
The typical oxidation state of gallium in most of its inorganic and coordination compounds is +III. Its chemical properties (electron configuration, electrical charge, ionic radius, coordination numbers) are close to those of iron(III). Gallium compounds are widely used in therapy and in diagnosis [25][26]. It has been reported that the anions of Ga(III) salts do not influence cytotoxicity; for instance, chloride, nitrate and sulphate have shown identical toxicity.
Gallium nitrate Ga(NO3)3 has been described as inhibiting the growth of subcutaneously implanted tumors [27]. Intravenously administered Ga(III) nitrate is effective for the therapy of certain cancer types (brain tumors, neuroblastoma, rhabdomyosarcoma, non-Hodgkin’s lymphoma, refractory solid tumors and bone metastases), Paget’s bone disease and hypercalcemia. Studies of gallium nitrate antitumor activity have shown that blood Ca(II) levels decrease in many cases [27][28]. As the most common complication in cancer patients is hypercalcemia, this hypocalcemia effect may serve as one of the possible explanations of the antitumor activity of Ga(NO3)3. The antiproliferative effects of Ga(III) are usually related to its ionic mimicry and competition with Fe(III) because of the resemblance of these ions [28][29]. Similar to ruthenium(III) complexes, gallium(III) complexes can compete for Fe-occupied sites in biomolecules. For instance, Ga(NO3)3 is beneficial for the treatment of cystic fibrosis, where redox-inactive Ga(III) inhibits redox-active Fe(III)-dependent pathways [29].
Gallium(III) affects the cellular transport of Fe(III) binding transferrin as most of the Ga(III) ions in the blood are absorbed by transferrin and many forms of cancer show overexpression of transferrin receptors. Additionally, it interferes with ribonucleotide reductase action, thus resulting in the inhibition of DNA synthesis. The best-studied biological effects of Ga(III) compounds are closely associated with possible damage to DNA synthesis, disturbance of mitochondrial function, reactive oxygen species (ROS) formation, complete inhibition of Fe-dependent enzymes and apoptosis.
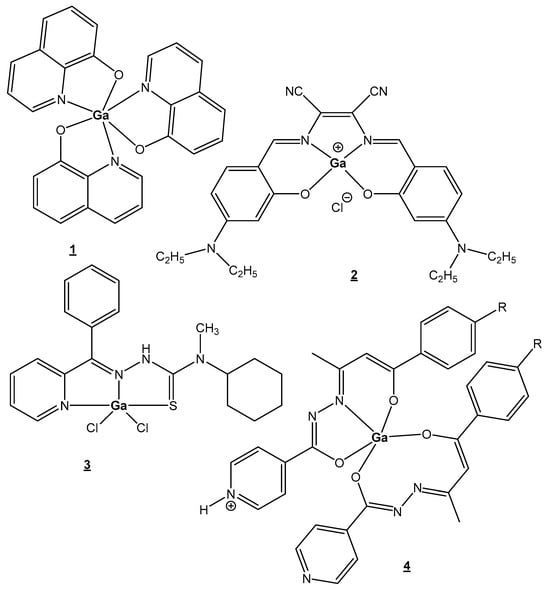
The recent studies are focused on bioligands that can stabilize Ga(III), prevent hydrolysis and facilitate membrane permeation. One of the promising new agents is the octahedral complex [Ga(III) (maltolate)3]. Oral plasma absorption of this compound is fast, followed by an almost complete transfer of Ga(III) to transferrin. The similar Ga(III) complex with the chelating anticancer ligand 8-hydroxyquinoline, tris(8-quinolinolato)gallium(III) (KP46/FFC11), Figure 1(1), has entered clinical trials as an oral anticancer drug [25][30]. This compound has shown a strong inhibitory in vitro effect on malignant cell growth, around 10 times higher than the effect of GaCl3. Tris(8-quinolinolato)gallium(III) makes it possible to overcome uni- and multicellular resistance.

Figure 1. Recently synthesized gallium(III) complexes.
New gallium compounds, with better bioavailability and stronger cytotoxic effects, are now under investigation and may improve the antitumor activity first demonstrated with inorganic gallium salts (Ga(NO3)3 and GaCl3). Recently, many Ga(III) complexes with different biologically active ligands have been synthesized and their cytotoxicity has been established; the best candidates are presented in Figure 1(2–4), [30][31][32][33][34][35][36][37][38]. Dissimilar from the most predominant octahedral complexes, those with planar tetra- or tridentate ligands have the propensity to block interactions between Ga(III) ions and bioligands to a lesser extent. In many cases, the vacant coordination sites may hold labile solvent fragments which would hypothetically allow improved interactions with bioactive ligands through solvent ligand exchange.
Various advantages of gallium radiopharmaceuticals have been discussed [40][41] in recent years. The isotopes of gallium 67Ga for SPECT and 68Ga for PET imaging are good alternatives to technetium-99m. The radioactive nuclide 68Ga with electron capture and a half-life of 1.1 h is predominant in clinical use as a tumor imaging agent in radiopharmaceuticals labeled with 68Ga [40]. 68Ga pasireotide tetraxetan (SOMscan) is used in PET imaging for gastroenteropancreatic neuroendocrine tumors. The synthetic pasireotide tetraxetan, analog of the natural hormone somatostatin, is attached to radioactive 68Ga in SOMscan. Another radioisotope of gallium 67Ga (half-life 3.3 days) decays via electron capture, emitting gamma radiation. It is widely used in standard nuclear medical imaging, being the longest-lived gallium radioisotope. 67Ga scintigraphy is applied in oncology for malignant tumor detection, including of Hodgkin’s and non-Hodgkin’s lymphomas [41].
4.2. Indium
Indium(III) exists entirely as complexes of In3+ and cannot undergo redox reactions under physiological conditions. As a result of the closed-shell electronic configurations, ions of the IIIA group elements are diamagnetic. In(III), like Ga(III), is categorized as a hard acid with high affinity for water. The In(III) ion prefers hard N and O donor ligands for coordination, but it can also form bonds with thiol groups; therefore, it can interact with many chelators. Indium(III) complexes present coordination numbers from 3 to 8 with a large variety of geometries. Although Ga(III) and In(III) are not considered vital ions, their resemblances to the biogenic ions Fe(III) or Ca(II) enable them to take part in bioprocesses in vivo.
Possible therapeutic applications of In(III) complexes have not been investigated sufficiently. Recently, In-based compounds have attracted great interest in view of their antitumor activity, useful photophysical properties and minor adverse effects. Between the pharmacological applications of In(III) complexes, antineoplastic activity deserves special consideration. The cytotoxic activities of many In(III) complexes against solid tumors and leukemia cells have been studied. InL3 complexes with curcumin, dimethoxycurcumin and diacetylcurcumin have been assayed [116][117] and demonstrated potent antineoplastic activity. Several studies have reported the interactions of In(III) complexes with DNA. An In(III) complex with N,N′-bis(5-sulfosalicyliden)-1,2-phenylendiamin (salsophen) was obtained and its interaction with DNA was investigated. It has been suggested that the complex binds to DNA through intercalation, which was confirmed with molecular docking studies [118]. The affinity of these complexes for transferrin and the fact that In(III)-transferrin binds transferrin receptors (TfR) make these compounds very attractive.
Indium compounds present a variety of clinical applications as diagnostic agents. Gallium-67, gallium-68 and indium-111 share the same group in the periodic table and can be harnessed for a range of applications in nuclear medicine, including scintigraphy, SPECT, PET and targeted radiotherapy. Main medical applications of In(III) complexes are presently related to the use of the isotope 111In. Indium-111 is a readily available γ-emitting radionuclide which is used in diagnostic radiopharmacy [44]. 111In was the first radionuclide to be employed in clinics for γ imaging that needs a long half-life, such as the labeling of monoclonal antibodies and cells. 111In can be used as a label for red cells, platelets and leukocytes. Furthermore, 111In is useful in cancer therapy, since it emits Auger electrons, causing DNA damage and subsequent cell death. Complexes with phthalocyanines are under study as photosensitizers in photodynamic cancer therapy. Commonly used chelators in radio medicine are polyamino acids, e.g., EDTA, DTPA and DOTA, with high coordination numbers and hard donor atoms, which can afford stabilization. Open-chain DTPA and macrocyclic DOTA are the best ligands for coordination to In(III) ions [45]. DTPA and its derivatives form very stable In(III) complexes with square planar geometries which have been exploited in numerous radioactive pharmaceuticals approved in clinical practice for treatment of prostate and neuroendocrine cancers. DOTA is very appropriate for 111In labeling of proteins. Due to its larger size, the In(III) cation fits the DOTA macrocycle and forms a very stable complex [46].
4.3. Thallium
The heaviest of the IIIA group elements, thallium, is a very toxic metal that concentrates in tissues with high numbers of K+ ions. Naturally occurring thallium consists of a mixture of two stable isotopes: 29.5% 203Tl and 70.5% 205Tl. The radioisotope thallium-205 is suitable for NMR detection. Thallium-201 is a potentially valuable radioisotope for various medical purposes including myocardial visualization, as a renal medullary imaging agent and for tumor detection. Thallium-201 (T1/2 = 73 h) decays via electron capture. It is used widely in radio diagnostic imaging (SPECT) and particularly for perfusion tests of the myocardium. Thallous chloride of 201Tl is applied for diagnostics of heart diseases, including coronary artery disease and heart attacks [50].
4.4. Germanium
Currently, Ge is widely recognized as an essential trace element, crucial for the immune system’s functions and cancer prevention. In the body, Ge binds to O-donor molecules, thus displaying efficiency at getting oxygen to the tissues. The enlarged O2 supply in the body improves the functions of the immune system and excretes harmful toxins. Additionally, Ge interacts with SH-groups of proteins, therefore showing bactericidal properties, so its compounds can be used as astringent, cauterizing and antiseptic agents in medicine. Germanium compounds are included in nutritional supplements which play a significant role in the prevention and treatment of some diseases, such as malignancies, arthritis, allergies, high blood pressure, high cholesterol levels and AIDS, stimulating the immune system [52][53]. It has been found that Ge levels in malignant tissues are significantly lower than in normal tissues, which elucidates its anticancer activity. Germanium compounds display low carcinogenicity, mutagenicity and teratogenicity [55].
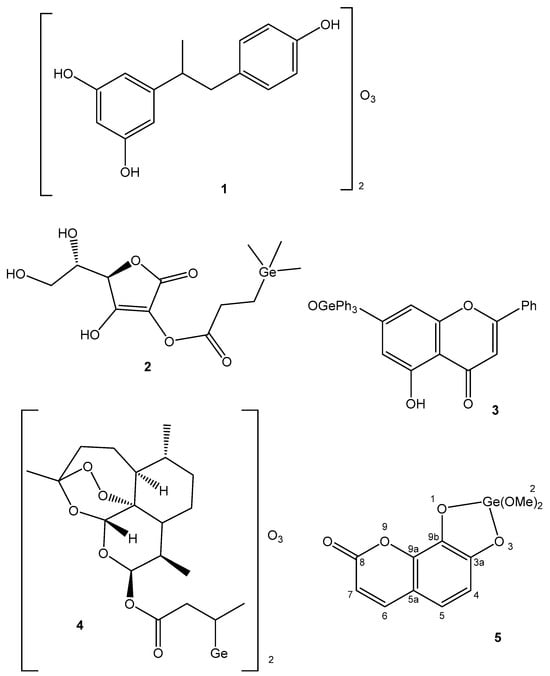
Organogermanium compounds garnered much scientific attention, among which bis(carboxyethylgermanium) sesquioxide (Ge-132) was the most famous. This compound prevents the appearance of malignant tumors and metastases, acts as an anesthetic, lowers blood pressure and protects against radioactivity [56][57]. Similar to organomercury compounds, organic germanium compounds are highly toxic. Only the high amounts of Ge-132 lead to toxic effects as a result of its hydrolysis and the formation of GeO2 in the body. Presently, attention is drawn to water-soluble germanium compounds to make them bioavailable and safe. When dissolved in H2O, bis(carboxyethylgermanium) sesquioxide turns into a hydrated form, 3-(trihydroxygermil)propanoic acid, which has been proven to inhibit melanoma cell proliferation. The molecule of 3-(trihydroxygermil)propanoic acid holds three OH-groups, which can react with the hydroxy groups of biomolecules (adrenaline, ATP). These interactions lead to their respective physiological functions. Numerous chemical modifications have been explored to enhance bioactivity and to extend the possibility of the application of Ge-132 [56][57]. The introduction of quinoline, anthraquinone and naphthalene into the Ge-132 structure has increased the anticancer action of the parent compound [57].
Along with the Ge-132 derivatives, a Ge sesquioxide with resveratrol has been synthesized (Figure 2(1)) with higher antioxidant activity than that of Ge-132 [119]. Germatranes are a class of bioactive Ge compounds, which are cyclic structures stabilized by the hypervalent Ge atom. Germatrane derivatives have shown strong antitumor and antioxidant activity. The bicyclic analogues of germatranes—germocanes and monocyclic analogues—and hypogermatranes have been found to possess the same activity [120].

Figure 2. Germanium complexes.
Germanium was introduced as a trimethylgermylpropionic acid substituent in the structure of ascorbic acid, shown in Figure 2(2), where a synergistic effect is demonstrated. The resulting stable and lipophilic derivative of ascorbic acid exhibited high antioxidant activity [121]. The natural flavone crysin (5,7-Dihydroxyflavone), which has numerous biological effects, has also been modified like this. The obtained Ge complex with crysin (Figure 2(3)) displayed a similar synergistic effect [122]. It has shown high anticancer activity with a substantial inhibition of the proliferation and growth of the MCF-7, HepG2 and Colo205 human cancer cell lines. High selectivity between malignant and normal cells has been observed. The mechanism of this inhibitory effect is supposed to be connected with the induction of apoptosis through the ROS-dependent mitochondrial pathway. Germanium has also been incorporated into dihydroartemisinin (artenimol) as an analogue of Ge-132 [123]. The obtained complex, shown in Figure 2(4), displayed synergistic effects and efficiently inhibited the proliferation of HepG2 cells inducing apoptosis.
Many Ge(IV) complex compounds with natural polyphenols have been synthesized and revealed to be potent anticancer agents. Among others, the Ge(IV) complex of the natural coumarin daphnetin (7,8-dihydroxychromen-2-one), shown in Figure 2(5), has exhibited high antioxidant activity, an intercalation of DNA and a strong antiproliferative effect on the HepG2 cancer cell line [124].
The study of the biological role of germanium is ongoing, and maybe novel, less harmful cancer treatments using this element can be developed [52][55].
4.5. Tin
Organotin(IV) compounds have attracted great attention as potential metal-based drugs since the detection of their anticancer activity, although the inorganic Sn compounds have been recognized as less toxic than organotin compounds [61]. Organotin(IV) compounds have been reported to possess antimicrobial, antiparasitic, antihypertensive, anti-hyperbilirubinemia, anticancer and antiviral activity, though none of them have reached clinical trials due to their high toxic effects, despite their multiple bioactivities.
Based on the varied range of organic donor molecules that are bound to Sn(IV), many diorganotin(IV) and triorganotin(IV) compounds with in vitro antineoplastic activity against various solid and hematologic cancers have been obtained [125]. They have low toxicity, are highly antiproliferative at low concentrations and have fewer adverse effects than the classic Pt-based drugs. Organotin(IV) compounds have demonstrated high selectivity toward cancer cells, regardless of the variety of ligands.
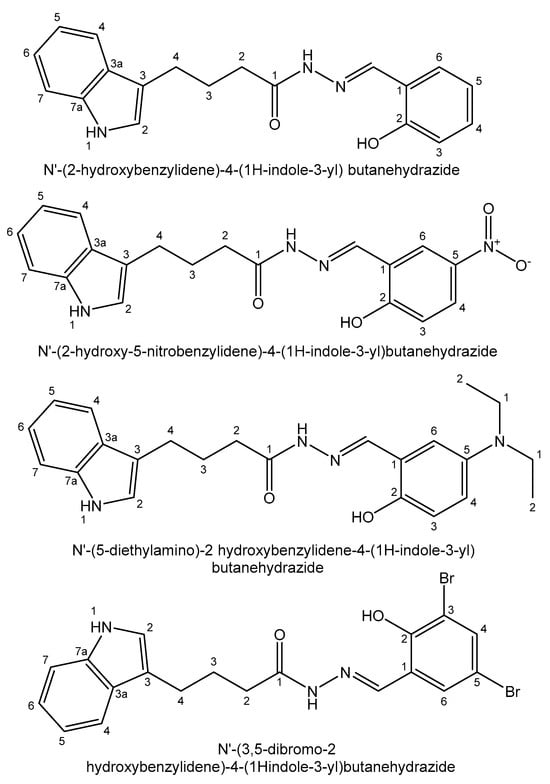
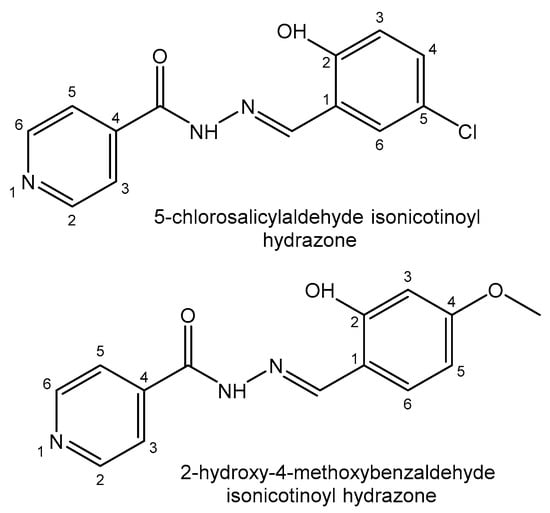
Recently, Devi et al. have reported a series of diorganotin(IV) compounds of different tridentate hydrazone Schiff bases, such as N′-(2-hydroxybenzylidene)-4-(1H-indole-3-yl) butanehydrazide, N′-(2-hydroxy-5 nitrobenzylidene)-4-(1H-indole-3-yl)butanehydrazide, N′-(5-diethylamino)-2 hydroxybenzylidene-4-(1H-indole-3-yl) butanehydrazide and N′-(3,5-dibromo-2 hydroxybenzylidene)-4-(1H-indole-3-yl)butanehydrazide (Figure 3). The cytotoxic activity of the compounds has been tested in the lung epithelial A549 and breast adenocarcinoma MCF7 human cell lines [126]. New diorganotin(IV) Me2SnL and Ph2SnL compounds of 5-chlorosalicylaldehyde isonicotinoyl hydrazone and 2-hydroxy-4-methoxybenzaldehyde isonicotinoyl hydrazone Schiff bases (Figure 4) have been synthesized and their in vitro cytotoxic effects have been screened against the lung cancer A549 and cervical carcinoma HeLa human cell lines. The diphenyltin(IV) compounds have exhibited stronger cytotoxicity than the dimethyltin(IV) compounds in the tested cell lines because of the better planarity of their phenyl groups, facilitating insertion into DNA [127].

Figure 3. Tridentate hydrazone Schiff bases.

Figure 4. Substituted hydrazone Schiff bases.
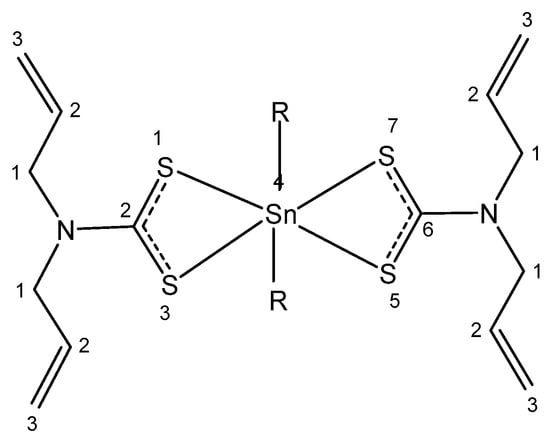
Four new organotin(IV) compounds holding N,N-diallyldithiocarbamate (Figure 5), where R = Cl, CH3, C4H9, C6H5, have been synthesized and characterized [128]. Their antiproliferative activity has been evaluated in HeLa human cervical carcinoma cells. The obtained results show that the compound with R= C6H5 had excellent cytotoxic effects (IC50 < 2 μM), which can be explained by the lipophilicity of this compound due to the presence of two phenyl groups. Dithiocarbamates can strongly bind and stabilize Sn(IV) ions. Chelation of Sn(IV with dithiocarbamate ligands decreases the polar character of the coordination center, thus increasing lipophilicity and enhancing permeability. It has been suggested that the individual properties of organotin(IV) and dithiocarbamate constituents induce synergistic effects and stronger biological activity.

Figure 5. Organotin(IV) complexes.
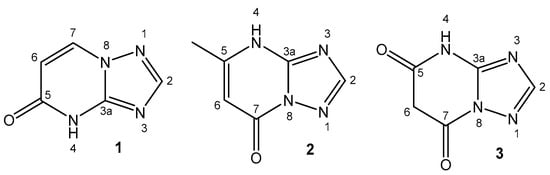
The in vitro antiproliferative activity of organotin(IV) compounds of 1,2,4-triazolo [1,5-a]pyrimidines in the HCT-116 colorectal carcinoma, HepG2 hepatocarcinoma and MCF-7 breast cancer human cell lines has been reported [129]. The structures of the used ligands 4,5-dihydro-5-oxo-[1,2,4] triazolo-[1,5-a]pyrimidine, 4,7-dihydro-5-methyl-7-oxo-[1,2,4]triazolo-[1,5-a]pyrimidine and 4,5,6,7-tetrahydro-5,7-dioxo-[1,2,4]triazolo-[1,5-a]-pyrimidine are presented in Figure 6. All of the organotin(IV) compounds possessed IC50 < 1 µM against the tested cell lines and high selectivity indexes, SI > 90. The participation of 1,2,4-triazolo[1,5-a] pyrimidine could be used as a model system to mimic the reactivity between purines and metal ions.

Figure 6. Triazolo-ligands for organotin(IV) compounds: 4,5-dihydro-5-oxo-[1,2,4]triazolo-[1,5-a]pyrimidine (1), 4,7-dihydro-5-methyl-7-oxo-[1,2,4]triazolo-[1,5-a]pyrimidine (2) and 4,5,6,7-tetrahydro-5,7-dioxo-[1,2,4]triazolo-[1,5-a]-pyrimidine (3).
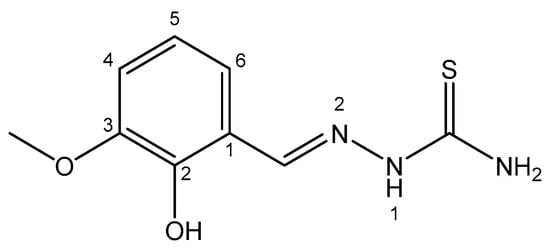
Khandani et al. have described diorganotin(IV) compounds of 3-methoxysalicylaldehyde thiosemicarbazone with antitumor activity against the Jurkat E6.1 acute lymphoblastic leukemia cell line [130] (Figure 7). The compositions of the diorganotin(IV) compounds were as follows: Ph2SnL, Me2SnL and Bu2SnL. The compounds Me2SnL and Bu2SnL adopted distorted square pyramid geometries, additionally stabilized by intermolecular hydrogen bonds. The activities of the compounds decreased in the order Bu2SnL > Ph2SnL > Me2SnL. The dibutyltin(IV) derivative showed higher antitumor activity than the other two compounds. Nevertheless, all the tested compounds showed high toxicity, with IC50 < 1 μM.

Figure 7. 3-methoxysalicylaldehyde thiosemicarbazone.
Many other organotin(IV) compounds with oxides, carboxylates, thiolates, halides and hydroxides as ligands have been reported. Their cytotoxic activity against numerous cancer cell lines has been evaluated and most of them have been classified as highly toxic with IC50 values in the micromolar range. Tri-n-butyltin(IV)lupinyl sulfide hydrogen fumarate displayed in vitro and in vivo activity against leukemia and melanoma [61][62][63]. In clinical practice, tin is used in the composition of the PDT agent purlytin (Sn(IV) ethyl-etiopurpurin). According to the clinical trials, purlytin is active against various cancers, like cutaneous basal cell cancer, Kaposi’s sarcoma in AIDS patients and breast metastases [64].
4.6. Lead
The unique coordination chemistry of this element is associated with its high atomic number and large radius, which make it suitable for adopting many different ligands, thus forming compounds with flexible coordination numbers and structures. In the body, Pb(II) ions can dislocate many biogenic metal ions, such as Ca(II), Zn(II), Fe(II) and Cu(II), which interrupts and inhibits the vital functions of enzymes [66][67][68]. Lead(II) ions show high attraction to sulfhydryl and amide groups of proteins and enzymes, affecting many vital biological processes.
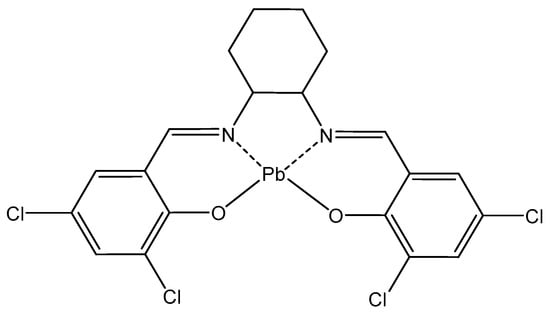
Schiff base-derived complexes are of particular interest in coordination chemistry and are highly suitable as antitumor agents. A novel Pb(II) complex (Figure 8) of a tetradentate ligand, obtained via condensation of 3,5-dichlorosalicylaldehyde and trans-1,2-diaminocyclohexane, has been synthesized and structurally characterized [131]. The cytotoxicity of the synthesized complex was evaluated against the MCF-7 cancer cell line. MTT results show that the Pb(II) complex delayed the development of uncontrolled growth cells.

Figure 8. Pb(II) complex.
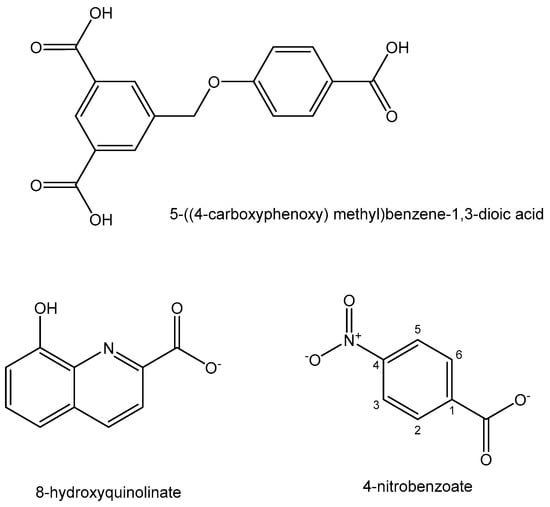
Coordination polymer compounds and nanoparticles have found a wide range of biomedical applications. A new Pb(II) coordination polymer [Pb(HL)(H2O)](H2O) with the tricarboxylate ligand 5-((4-carboxyphenoxy) methyl)benzene-1,3-dioic acid (Figure 9) has been synthesized [132]. The tumor cell growth inhibition activity of the nanosized Pb(II) coordination polymer has been investigated and proven against A549, H1299 and PC9 human lung cancer cells using the MTT assay. Polynuclear coordination compounds have attracted progressive attention in the past decades. A Pb(II) complex of the mixed ligands 8-hydroxyquinolinate and 4-nitrobenzoateligands (Figure 9) has been obtained and structurally characterized [133]. Its anticancer activity against the MCF-7, HepG-2 and A549 cell lines was determined using the MTT cell viability assay. The complex inhibited proliferation of the tested tumor cells.

Figure 9. Ligands for Pb(II) complexes.
Coordination compounds of radioactive metal ions are widely used in anticancer therapy to transport radiation to tumors, destroying malignancies, or to image the targets. The β− emitting radioisotope 212Pb (T1/2 = 10.6 h), which rapidly generates the α-emitting 212Bi isotope, is beneficial in targeted α-therapy and radioimmunotherapy [72]. Its decay leads to the emission of α-particles with a strong therapeutic action on cellular nuclei, proving its clinical feasibility [73]. The radiolabeled compound 212Pb-TCMC-trastuzumab, (TCMC = 2-(4-isothiocyanotobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamonyl methyl)-cyclododecane), a humanized monoclonal antibody carrying 212Pb, shows promising preclinical anticancer activity. IP administration of 212Pb-TCMC-trastuzumab has demonstrated activity against different human cancer xenografts. The antibody trastuzumab transports and delivers 212Pb to targeted malignant cells, leading to precise irradiation, which kills tumor cells and does not affect normal tissues [74]. 212Pb-TCMC-trastuzumab has shown promise against numerous HER-2-expressing malignancies.
4.7. Arsenic
In spite of the toxic character of this metal, its compounds do have beneficial efficiency. In small doses, arsenic compounds have therapeutic stimulant effects on Hb synthesis and erythrocyte development, inhibiting leukopoietic processes [75].
Arsenic trioxide As2O3 has been approved by the FDA for successful treatment of the rare and lethal acute promyelocytic leukemia (APL) [79], as well as for safe and effective therapy for other malignancies like unresectable hepatocellular, esophageal and renal cell carcinoma and small and non-small cell lung cancer [80] with almost complete remission in most of the cases.
The mechanism of the cytotoxic action of arsenic in cells is still not completely clear and cardiotoxicity has been reported as a side effect. Currently, there are several probable explanations for the anticarcinogenic effects of As2O3, which include induction of p53-dependent G1 or G2/M cell cycle arrest, induction of apoptosis and cancer cell differentiation, blocked by cAMP analogs. The apoptosis induction can be associated with an increase in ROS production and a reduction in antioxidation capability. In this context, As2O3 shows the anomaly of displaying antitumor and oncogenic properties simultaneously.
Arsenic is part of many organoarsenic compounds that are well-known drugs like Melarsoprol, 2-(4-amino)-(4,6-diamino 1,3,5-triazin-2-yl)-phenyl-1,2,3-dithiarsolan-4-methanol (Mel B, Arsobal), Salvarsan (3,3′-diamino-4,4′-dihydroxyarsenobenzene dihydrochloride) and Neosalvarsan (sodium 2-amino-4-[4-hydroxy-3-(sulfenatooxymethylamino)phenyl]arsanylidenearsanylphenol) [81].
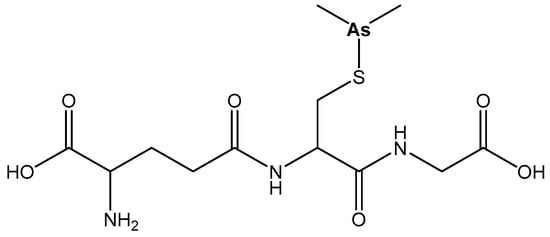
Darinaparsin, S-dimethylarsino-glutathione (DAR, ZIO101), Figure 10, which has potential anticancer activity against myeloma, induces greater accumulation of intracellular As and cellular death in vitro with lesser general toxic effects compared with As2O3 [82].

Figure 10. Darinaparsin, S-dimethylarsinoglutathione (DAR, ZIO101).
There are some radioisotopes of As, like 71As and 74As, which are appropriate for diagnostic imaging and radiotherapy emissions. Arsenic radioactive nuclides have been applied in blood diseases, as well as for diagnostics of brain tumors. Although As is popular as a radio diagnostic agent, the absence of suitable antidote chelators and problems with its toxicity have blocked its use in radiotherapy [84].
The biological role of antimony is comparable to that of As. The cations As3+, Sb3+ and to a lesser degree Bi3+ are synergistic [130]. This synergism, typical for As and Sb, is dependent on their affinity to sulfur-containing bioligands.
4.8. Antimony
The total amount of Sb and Bi in the human body is around 10−6%. The chemical properties and the biological role of antimony are analogous to those of As, as they are elements belonging to Group VA of the periodic table. Arsenic and antimony cations are synergistic in view of their accumulation in the thyroid gland [130]. This synergism is connected with their capability to form chemical bonds with thiol groups of biomolecules with a high specificity.
Antimony-containing compounds have been reported to display some medicinal functions and used as antiseptics and emetics. In recent years, substantial work has been devoted to the development of new antimony agents. Despite their known medicinal efficiency, the evaluation of the anticancer potential of antimony organometallic compounds has not been so well examined.
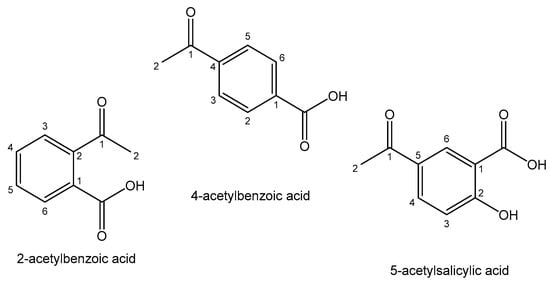
Inorganic Sb2O3 and antimony(III) complexes with polydentate carboxylic acids have shown notable therapeutic effects against acute promyelocytic leukemia without provoking side effects [134]. It has been proven that the tested trivalent antimonials (Sb2O3 and potassium antimonyl tartrat) can mimic the antileukemic effects of As2O3 on APL cells. The obtained Sb(III) coordination compounds have demonstrated higher potency than the free ligands, indicative of the role of antimony(III) in their cellular activity. The most considered Sb(III) and Sb(V) antitumor compounds are the complexes with Sb-C bonds with arylhydroxamates, thioamides and hydrazones as ligands, named organometallic compounds. Novel organobismuth(V) and organoantimony(V) complexes of the type Ph3ML2 with the heterocyclic ligands 2-acetylbenzoic acid, 4-acetylbenzoic acid and 5-acetylsalicylic acid (Figure 11) have been proven to have anticancer capacity against the human chronic myelogenous leukemia K562 cell line and metastatic murine melanoma B16F10 cells. They have been found to possess low toxic effects toward the noncancerous murine fibroblast L929 and murine melanocyte Melan-A cell lines [135]. Considering the selectivity indexes, the results indicate that the cell cycle arrest and induction of apoptosis contributed to the observed cytotoxicity.

Figure 11. Heterocyclic ligands.
4.9. Bismuth
Bismuth and its Bi(III) and Bi(V) compounds have remarkably low toxicity and are ecologically safe. Bismuth-based drugs are widely used in medicine to exert antimicrobial activity against digestive tract pathogenic microorganisms [86][87][88]. Bismuth affects gram-negative Helicobacter pylori, which contributes to gastrointestinal diseases, including gastric lymphoma [89]. The best candidates are Bi(III)-based compounds with hydroxy-carboxylate ligands such as salicylate and citrate.
Along with the recognized gastroprotective effects of bismuth, it also has a broad spectrum of bioactivity, including high potential for cancer treatment. Bi(III) complex compounds holding one or two phenyl groups and a tetrazole/triazolethiolate ligand have been shown to possess high cytotoxic activity, minimal side effects and a nonspecific mechanism of action [95]. A bismuth(III) complex of pentadentate ligand 2,6-pyridinedicarboxaldehyde bis(4N-methylthiosemicarbazone) with the composition [BiL(NO3)2]NO3 has been obtained [136]. The in vitro pharmacological study indicated that the complex significantly suppressed colony formation inducing apoptosis of A549 and H460 lung cancer cell lines without decreasing the cell viability of normal lung fibroblast HLF cells. The Bi(III) complex has shown higher antineoplastic activity than the ligand with IC50 values less than 5 μM. The antitumor activities of new binuclear Bi(III) complexes of thiosemicarbazone derivatives have been investigated against the human urinary bladder cancer T-24 cell line [137]. The complexes exhibited multifunctional mechanisms, including the activation of mitochondria-mediated apoptosis, MAPK-mediated apoptosis, LC3II-mediated autophagy and endoplasmic reticulum stress-mediated cell damage.
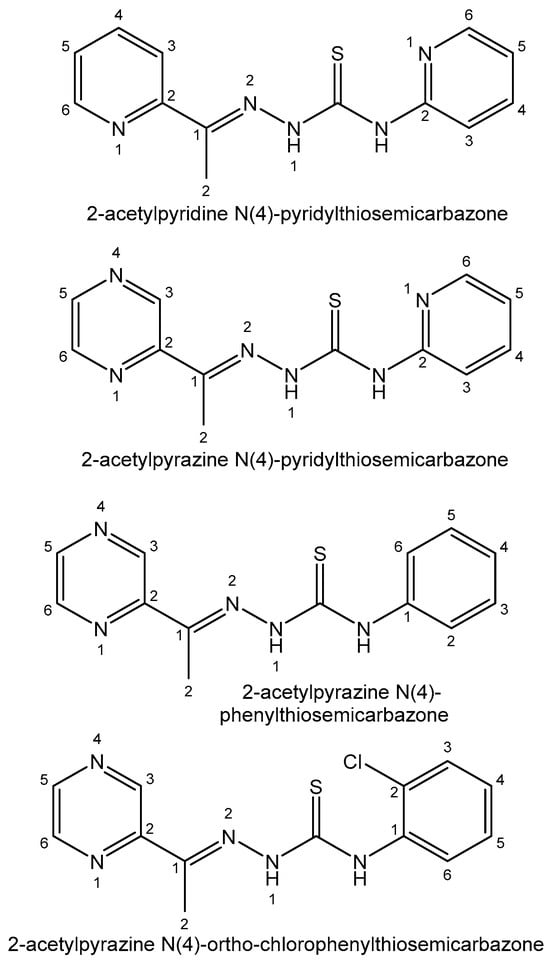
Thiosemicarbazone ligands and their bismuth(III) complexes are very selective toward different cell lines. In all the cases, the complexation reaction significantly increases the antiproliferative activity of thiosemicarbazone derivatives. Bismuth(III) complexes derived from 2-acetylpyridine N(4)-pyridylthiosemicarbazone [138] and 2-acetylpyrazine N(4)-pyridylthiosemicarbazone [139] have been tested against K562 cancer cells with IC50 values less than 2 μM. The cytotoxic activity of bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone [140] has been tested against the K562, HCT-116, HeLa and HepG2 cell lines. The Bi(III) complex of 2-acetylpyrazine N(4)-ortho-chlorophenylthiosemicarbazone [141] was obtained and its cytotoxicity against MCF-7, HT-29 and Vero cells was investigated. The thiosemicarbazone ligands, mentioned above, are presented in Figure 12.

Figure 12. Thiosemicarbazone ligands.
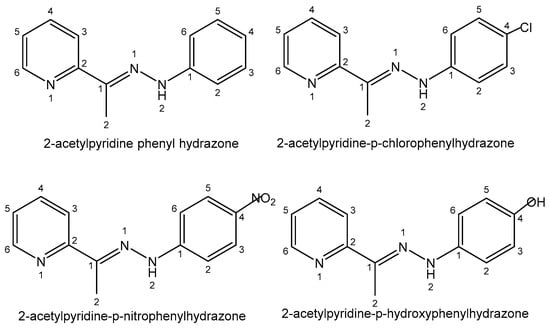
Another group of ligands widely used in the bismuth coordination complexes are hydrazone derivatives, very versatile and flexible ligands with various pharmacological activities. The cytotoxic activity of bismuth(III) complexes of several 2-acetylpyridine hydrazones para-substituted by -H, -Cl, -OH and -NO2 groups (2-acetylpyridine phenyl hydrazone, 2-acetylpyridine-p-chlorophenylhydrazone, 2-acetylpyridine-p-nitrophenylhydrazone and 2-acetylpyridine-p-hydroxyphenylhydrazone), shown in Figure 13, has been tested against the HL-60, Jurkat, THP-1, MCF-7, HCT-116 and Vero cancer cell lines [142]. It was found that the substitution of one H atom in a pyridine moiety by various groups generally caused an enhancement in their activity in the order -H < -NO2 < -Cl.

Figure 13. Hydrazone ligands.
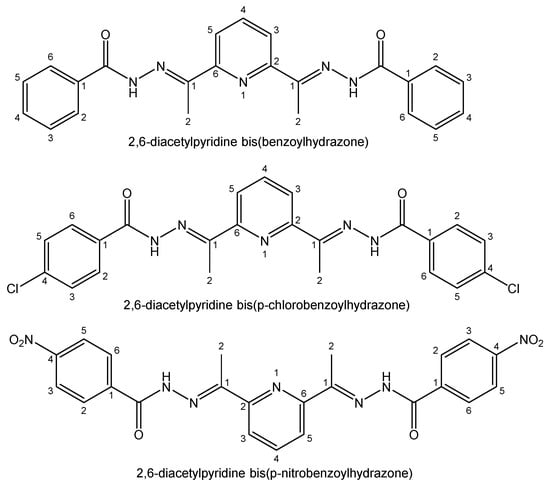
The bismuth(III) complexes of benzoyl substituted hydrazones (2-benzoylpyridinephenylhydrazone, 2-benzoylpyridine-p-chlorophenylhydrazone, 2-benzoylpyridine-p-nitrophenylhydrazone, 2-benzoylpyridine-p-hydroxyphenylhydrazone) have shown only slightly changed cytotoxic activity. The hydrophilic OH group significantly reduced the activity. A series of bismuth(III) complexes of three 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives (2,6-diacetylpyridine bis(benzoylhydrazone), 2,6-diacetylpyridine bis(p-chlorobenzoylhydrazone), 2,6-diacetylpyridine bis(p-nitrobenzoylhydrazone)), shown in Figure 14, has been reported [143].

Figure 14. Hydrazone ligands for bismuth(III) complexes.
It has been shown that none of the ligands were active against Jurkat and HCT-16 cells, but all of them were cytotoxic to HL-60 cells and H2L9. Coordination to Bi(III) ions increased the cytotoxicity of all the tested compounds. Among bismuth complexes, the most active was the Bi(III) complex of 2-benzoylpyridinephenylhydrazone, meaning that the p-substituents on the phenyl moiety diminished the activity of Bi(III) complexes. The therapeutic indexes and the values of IC50 proved that some hydrazine derivatives and their bismuth(III) complexes can be considered to be capable anticancer candidates, being more active and less toxic than some agents used in cancer therapy. Comparable results were obtained with Bi(III) coordination compounds of tiocarbono-derivative of hydrazone (bis(2-acetylpyrazine)thiocarbonohydrazone) [144].
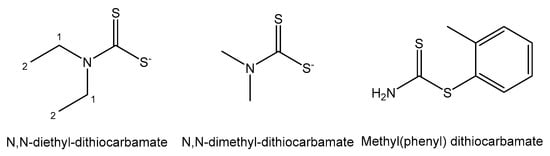
Derivatives of dithiocarbamate (1,1-dithiolate) have formed stable Bi(III) complexes, as shown in Figure 15 [145]. Their antiproliferative activity toward a panel of human tumor cell lines was investigated and proven for all the tested complexes. Bi(III) complexes of N,N-dimethyl-dithiocarbamate and N,N-diethyl-dithiocarbamate were screened on the HeLa, MCF-7, A498, EVSA-T, H226, IGROV, M19 MEL and WIDR cancer cell lines and demonstrated 10-fold stronger cytotoxicity than cisplatin, and MCF-7 cells of breast adenocarcinoma were the most sensitive. Methyl(phenyl) dithiocarbamate has shown lower antitumor activity against MCF-7.

Figure 15. Dithiocarbamate derivatives.
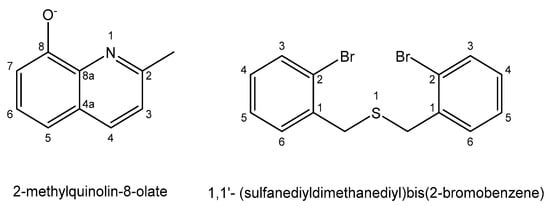
The antineoplastic activity of the organobismuth(III) 2-methylquinolin-8-olate complex, with the composition [BiPh(L)2], shown in Figure 16, has been tested against the L1210, L1210/DDP and SKOV-3 cell lines [146]. The bismuth(III) complex was found to be active against the cisplatin-resistant mouse leukemia cells L1210/DDP. Organobismuth(III) complexes derived from 1,1′-(sulfanediyldimethanediyl)bis(2-bromobenzene) with N-substituted aromatic benzyl or cyclohexyl, shown in Figure 16, were synthesized and evaluated in vitro against the MGC-803 cell line using the MTT method [147]. The complexes displayed substantial anticancer activity compared with cisplatin.

Figure 16. Ligands for organobismuth(III) complexes.
Along with thiosemicarbazone, hydrazone and dithiocarbamate ligands, many other pharmacologically active ligands have been used for the synthesis of bismuth(III) complexes that have been found to inhibit strong proliferation of colon, breast, lung, ovarian, etc. tumors, producing synergistic effects of Bi(III) ions and designated ligands [95].
Bismuth, the heaviest stable post-transition metal, shows weak radioactivity. The chemical properties of radioactive Bi are comparable to that of its analogs As and Sb. Bismuth-213, with a half-life of 45.6 min, is a clinically used α-emitter in radioimmunotherapy. Clinical trials of 213Bi-lintuzumab have shown that its administration against relapsed and refractory (R/R) acute myeloid leukemia (AML) was safe for healthy cells and led to remissions without significant extramedullary toxic effects [100][101][102]. 213Bi-lintuzumab has shown quick targeting of disease locations. Bi2O3 is lightweight and effective in protecting against ionizing radiations such as γ-rays, making it a promising agent for medical imaging and radiation therapy [102].
4.10. Selenium
Selenium compounds are used in medicine in various mineral–vitamin supplements because of their antioxidant activity. Se occurs in different chemical forms with varied bioactivity. Selenium inorganic selenates (SeO42−, HSeO4−, H2SeO4), selenides (H2Se, HSe−) and selenites (SeO32−, HSeO3−, H2SeO3) are part of many enzymes in the form of selenoamino acids (selenomethionine, seleno-L-methionine, selenocysteine, Se-methyl-seleno-L-cysteine), methylseleninic acid, selenoesters and selenophene-based compounds showing activating or inhibitory effects [148]. These inorganic and organic Se compounds have demonstrated a great potential for chemoprotective treatment of malignancies due to their great selectivity and efficacy, lower toxicity and reduced side effects in comparison with commonly used therapeutic agents [106].
Selenium possesses several oxidation states (−2, 0, +4, +6) and can be reduced or oxidized more easily than its analog sulfur, acting as a nucleophilic or electrophilic agent. Since Se behaves in this way, it is suitable for use in chemistry and biology. Se-containing compounds and Se-nanoparticles have been extensively studied for cancer treatment in recent years [149]. The main mechanism of anticancer activity of Se-based compounds is apoptosis, although additional nonapoptotic mechanisms, including necrosis, autophagy, cell cycle arrest, ferroptosis, necroptosis, etc. have been described as well.
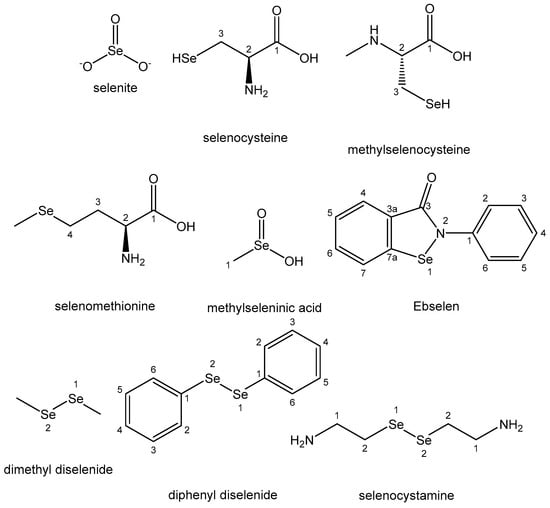
Inorganic salts, especially selenite ion and its sodium salt, have shown chemopreventive properties, in spite of their high toxicity. They displayed anticancer activity against prostate, pancreatic, lung, breast, bladder, hepatoma and osteosarcoma cell lines [106]. Selenites have synergistically improved the efficiency of anticancer agents, such as imatinib, 5-fluorouracil, cisplatin, oxaliplatin, ethaselen, irinotecan and auranofin, against different cancer cell lines. Selenoaminoacids (selenocysteine, methylselenocysteine and selenomethionine) are widely studied as broad-spectrum antineoplastic agents with an apoptotic mechanism of action. Methylseleninic acid, obtained via the decomposition of methylselenocysteine, has shown chemopreventive and antineoplastic activity against prostate, leukemia, lung, breast, head and neck, pancreatic and ovarian cancer cell lines with IC50 values between 1 and 40 µM. The induction of OS, mediated by higher ROS generation and GSH reduction, appears to be its key mechanism of action. The synthetic Se-based heterocyclic compound Ebselen is a chemopreventive agent possessing anti-inflammatory, antioxidant and GPx-mimicking properties [150]. It has been reported to increase the apoptosis in human hepatoma and multiple myeloma cell lines via oxidative stress (OS) induction. Selenocystamine and diselenides holding Se–Se groups (dimethyl diselenide and diphenyl diselenide) are antioxidants and have exhibited good antitumor activity mediated by apoptotic pathways. The above-mentioned Se-based compounds are listed in Figure 17.

Figure 17. Selenium complexes.
Most Se-containing organic compounds are low-toxicity and potent pleiotropic anticancer agents. Selenium has a narrow safety range. At low doses, its compounds induce antioxidant properties, whereas prooxidant and antineoplastic effects occur at high concentrations.
4.11. Tellurium
The tellurium oxyanions tellurite (TeO32−) and tellurate (TeO42−) are toxic to living organisms, leading to neurodegenerative, autoimmune and oncological diseases [107].
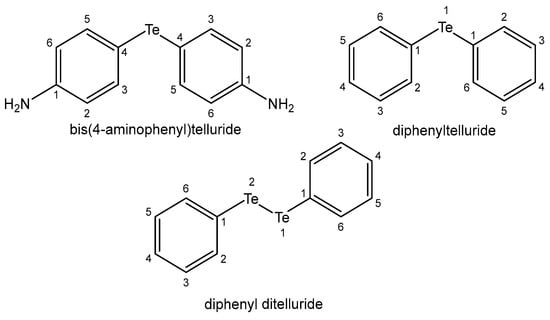
It has been found that tellurium compounds are potential active antioxidant, chemoprotective and antitumor agents, even more effective than their Se and S isosteres. Corresponding to selenium, numerous tellurium compounds have been found to possess antiproliferative properties and to interact synergistically with clinically used anticancer agents, overcoming tumor resistance. The compounds of Se and Te achieve this activity through various mechanisms including ROS generation, inducing cell cycle arrest, cell signaling and autophagy, apoptosis, etc. [151]. Additionally, they can inhibit tumor metastases and angiogenic processes. When Te compounds penetrate into cells, they change the structure of the cell membrane and glutathione metabolism, substitute essential biogenic metal ions in enzymes and cause OS. It has been found that Te compounds are less toxic than Se compounds, despite the apparent correspondence between their pathways. It has been reported that bis(4-aminophenyl)telluride and diphenyltelluride (Figure 18) were more cytotoxic in human lung fibroblasts than their selenium and sulfur analogs [152].

Figure 18. Tellurium complexes.
The synthetic and nontoxic organotellurium compound ammonium trichloro (dioxoethylene-O,O′) tellurate (AS101) exhibited significant immunomodulatory and neuroprotective activities [153]. AS101 is being used in phase II clinical trials with various possible clinical applications. It is also a potent agent in the reversal of VLA-4-mediated drug resistance in AML. The immunomodulator octa-O-bis-(R,R)tartarate ditellurane] exhibited promising activity in human multiple myeloma. Diphenyl ditelluride (Figure 18) and TeCl4 have been associated with a decreased GSH/GSSG ratio in the human colon cancer HT-29 cell line that finally resulted in apoptosis with diphenyl ditelluride and necrosis with TeCl4. 4,4′-dihydroxydiphenyl-telluride) has shown protective activity against ROS. Although the behavior of tellurium compounds is very similar to that of their selenium relatives, their biological profiles are somewhat different and they display stronger activity, which raises their toxicity. Several studies have reported that the toxicity of Na2TeO3 was associated with Te-induced OS, and furthermore with widespread damage to DNA.
Tellurium-based nanomaterials possess intrinsic biological potential for phototherapy and ROS-related applications [107].
4.12. Astatine
Astatine is rather less volatile than I2 and exhibits typical metallic properties. Its biological role is not clear. The chemical properties of At differ substantially from those of I2 because of its metallic character.
Astatine isotopes, except three (221At, 222At, 223At), have alpha emission of high-energy particles. α-induced radiolysis plays a significant role in the At behavior in many biosystems compared with that of the stable β-emitters of iodine. 211At (T1/2 = 7.2 h) is of great interest for usage in targeted α-oncotherapy. Only a few of the α-radioactive nuclides (227Th, 223Ra, 212Pb, 212Bi, 225Ac), along with 211At, meet the needed requirements for nuclear medical applications. Among them, 211At is one of the most promising candidates [110][111]. The radioactive astatine-211 has many advantages for targeted α-therapeutic purposes [112][113]. α-emitters have unique features for removal of focal locations of tumor cells which are close to the normal tissues of the central nervous system (CNS). For example, they may be useful for targeted treatment with no risks of the toxicity that occurs with β-emitters. The half-life of 211At is appropriately long and is well matched with the pharmacokinetic properties of other specific agents. Therefore, many 211At-labeled systems have been obtained and assessed as targeted radiotherapeutics, such as colloids, peptides, DNA precursors, antibodies, organic compounds like meta-[211At]astatobenzylguanidine, etc. [114]. The chimeric anti-tenascin mAb 81C6 labeled with astatine-211 has been assessed in malignant brain tumors in vivo. The radiopharmaceutical 211At-MX35 F(ab′)2 has been used in the remission of ovarian carcinoma for intraperitoneal administration. By radiolabeling the enzyme poly(ADP-ribose) polymerase 1 (PARP1) with 211At, the first α-emitting drug targeting cancer nuclei via PARP1, [211At]parthanatine ([211At]PTT), was obtained [115].
References
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137.
- Coverdale, J.P.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31.
- Sohrabi, M.; Saeedi, M.; Larijani, B.; Mahdavi, M. Recent advances in biological activities of rhodium complexes: Their applications in drug discovery research. Eur. J. Med. Chem. 2021, 216, 113308.
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A critical review of palladium organometallic anticancer agents. Cell Rep. Phys. Sci. 2021, 2, 100446.
- Ma, D.L.; Wu, C.; Wu, K.J.; Leung, C.H. Iridium (III) complexes targeting apoptotic cell death in cancer cells. Molecules 2019, 24, 2739.
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492.
- Soumya, R.S.; Hela, P.G. Nano silver based targeted drug delivery for treatment of cancer. Pharm. Lett. 2013, 5, 189–197.
- Skoupilova, H.; Hrstka, R.; Bartosik, M. Titanocenes as anticancer agents: Recent insights. Med. Chem. 2017, 13, 334–344.
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules 2020, 25, 1757.
- Chundawat, N.S.; Jadoun, S.; Zarrintaj, P.; Chauhan, N.P.S. Lanthanide complexes as anticancer agents: A review. Polyhedron 2021, 207, 115387.
- Kostova, I. Biological and Medical Significance of Chemical Elements; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023.
- Kostova, I. The Role of Complexes of Biogenic Metals in Living Organisms. Inorganics 2023, 11, 56.
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022.
- Palmisciano, P.; Haider, A.S.; Balasubramanian, K.; D’Amico, R.S.; Wernicke, A.G. The role of cesium-131 brachytherapy in brain tumors: A scoping review of the literature and ongoing clinical trials. J. Neuro-Oncol. 2022, 159, 117–133.
- Surdacka, A.; Stopa, J.; Torlinski, L. In situ effect of strontium toothpaste on artificially decalcified human enamel. Biol. Trace Elem. Res. 2007, 116, 147–153.
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588.
- Querido, W.; Campos, A.P.; Martins Ferreira, E.H.; San Gil, R.A.; Rossi, A.M.; Farina, M. Strontium ranelate changes the composition and crystal structure of the biological bone-like apatite produced in osteoblast cell cultures. Cell Tissue Res. 2014, 357, 793–801.
- Tomblyn, M. The role of bone-seeking radionuclides in the palliative treatment of patients with painful osteoblastic skeletal metastases. Cancer Control 2012, 19, 137–144.
- Gunawardana, D.H.; Lichtenstein, M.; Better, N.; Rosenthal, M. Results of strontium-89 therapy in patients with prostate cancer resistant to chemotherapy. Clin. Nucl. Med. 2004, 29, 81–85.
- Bruland, O.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s.
- Lewington, V.J. Bone-seeking radionuclides for therapy. J. Nucl. Med. 2005, 46, 38S–47S.
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with α-emitting 223Ra: Comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259.
- Larsen, R.H.; Saxtorph, H.; Skydsgaard, M.; Borrebaek, J.; Jonasdottir, T.J.; Bruland, O.S.; Klastrup, S.; Harling, R.; Ramdahl, T. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: Histology, clinical chemistry and hematology. In Vivo 2006, 20, 325–331.
- Nilsson, S.; Strang, P.; Aksnes, A.K.; Franzèn, L.; Olivier, P.; Pecking, A.; Bruland, O.S. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 678–686.
- Peng, X.X.; Gao, S.; Zhang, J.L. Gallium(III) complexes in cancer chemotherapy. Eur. J. Inorg. Chem. 2022, 2022, e202100953.
- Verron, E.; Bouler, J.M.; Scimeca, J.C. Gallium as a potential candidate for treatment of osteoporosis. Drug Discov. Today 2012, 17, 1127–1132.
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361.
- Leyland-Jones, B. Treating cancer-related hypercalcemia with gallium nitrate. J. Support. Oncol. 2004, 2, 509–516.
- Todorov, L.; Kostova, I.; Traykova, M. Lanthanum, Gallium and their Impact on Oxidative Stress. Curr. Med. Chem. 2019, 26, 4280–4295.
- Wilke, N.L.; Abodo, L.O.; Frias, C.; Frias, J.; Baas, J.; Jakupec, M.A.; Keppler, B.K.; Prokop, A. The gallium complex KP46 sensitizes resistant leukemia cells and overcomes Bcl-2-induced multidrug resistance in lymphoma cells via upregulation of Harakiri and downregulation of XIAP in vitro. Biomed. Pharmacother. 2022, 156, 113974.
- Hreusova, M.; Novohradsky, V.; Markova, L.; Kostrhunova, H.; Potočňák, I.; Brabec, V.; Kasparkova, J. Gallium (III) Complex with Cloxyquin Ligands Induces Ferroptosis in Cancer Cells and Is a Potent Agent against Both Differentiated and Tumorigenic Cancer Stem Rhabdomyosarcoma Cells. Bioinorg. Chem. Appl. 2022, 2022, 3095749.
- Chitambar, C.R.; Antholine, W.E. Iron-targeting antitumor activity of gallium compounds and novel insights into triapine®-metal complexes. Antiox. Redox Signal. 2013, 18, 956–972.
- Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium complexes as new promising metallodrug candidates. Inorg. Chim. Acta 2012, 393, 53–63.
- Chitambar, C.R. Gallium-containing anticancer compounds. Fut. Med. Chem. 2012, 4, 1257–1272.
- Qi, J.; Liu, T.; Zhao, W.; Zheng, X.; Wang, Y. Synthesis, crystal structure and antiproliferative mechanisms of gallium (III) complexes with benzoylpyridine thiosemicarbazones. RSC Adv. 2020, 10, 18553–18559.
- Firmino, G.d.S.S.; Andre, S.C.; Hastenreiter, Z.; Campos, V.K.; Abdel-Salam, M.A.; de Souza-Fagundes, E.M.; Lessa, J.A. In vitro assessment of the cytotoxicity of Gallium (III) complexes with Isoniazid-Derived Hydrazones: Effects on clonogenic survival of HCT-116 cells. Inorg. Chim. Acta 2019, 497, 119079.
- Robin, P.; Singh, K.; Suntharalingam, K. Gallium(III)-polypyridyl complexes as anti-osteosarcoma stem cell agents. Chem. Commun. 2020, 56, 1509–1512.
- Litecká, M.; Hreusova, M.; Kašpárková, J.; Gyepes, R.; Smolková, R.; Obuch, J.; David, T.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part XIV: High selective antiproliferative activity of tris (5-chloro-8-quinolinolato) gallium(III) complex against human cancer cell lines. Bioorg. Med. Chem. Lett. 2020, 30, 127206.
- Banerjee, S.R.; Pomper, M.G. Clinical applications of gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13.
- Khan, M.U.; Khan, S.; El-Refaie, S.; Win, Z.; Rubello, D.; Al-Nahhas, A. Clinical indications for gallium-68 positron emission tomography imaging. Eur. J. Surg. Oncol. 2009, 35, 561–567.
- Nishiyama, Y.; Yamamoto, Y.; Toyama, Y.; Satoh, K.; Nagai, M.; Ohkawa, M. Usefulness of 67Ga scintigraphy in extranodal malignant lymphoma patients. Ann. Nucl. Med. 2003, 17, 657–662.
- Beraldo, H. Pharmacological applications of non-radioactive indium(III) complexes: A field yet to be explored. Coord. Chem. Rev. 2020, 419, 213375.
- Xu, X.; Liu, J.H.; Yuan, C.X.; Xing, Y.M.; Wang, M.; Zhang, Y.H.; Xu, F.R. Masses of ground and isomeric states of In 101 and configuration-dependent shell evolution in odd-A indium isotopes. Phys. Rev. C 2019, 100, 051303.
- Goldsmith, S.J. Targeted radionuclide therapy: A historical and personal review. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2020; Volume 50, pp. 87–97.
- Kurdziel, K.A.; Mena, E.; McKinney, Y.; Wong, K.; Adler, S.; Sissung, T.; Lee, J.; Lipkowitz, S.; Lindenberg, L.; Turkbey, B.; et al. First-in-human phase 0 study of 111In-CHX-A"-DTPA trastuzumab for HER2 tumor imaging. J. Transl. Sci. 2019, 5, 10.
- Banerjee, S.R.; Kumar, V.; Lisok, A.; Plyku, D.; Nováková, Z.; Brummet, M.; Pomper, M.G. Evaluation of 111In-DOTA-5D3, a surrogate SPECT imaging agent for radioimmunotherapy of prostate-specific membrane antigen. J. Nucl. Med. 2019, 60, 400–406.
- Eghtesadi, R.; Safavi, S.; Shahmirzayi, F.; Banafshe, H.R.; Omidi, S.; Ghaderi, A. A narrative review of thallium toxicity; preventive measures. Int. J. Pharm. Res. 2019, 11, 322–330.
- Campanella, B.; Colombaioni, L.; Benedetti, E.; Di Ciaula, A.; Ghezzi, L.; Onor, M.; Bramanti, E. Toxicity of thallium at low doses: A review. Int. J. Environ. Res. Public Health 2019, 16, 4732.
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium use, toxicity, and detoxification therapy: An overview. Appl. Sci. 2021, 11, 8322.
- Al Badarin, F.J.; Malhotra, S. Diagnosis and prognosis of coronary artery disease with SPECT and PET. Curr. Card. Rep. 2019, 21, 1–11.
- Azam, F.; Volcani, B.E. Germanium-silicon interactions in biological systems. In Silicon and Siliceous Structures in Biological Systems; Springer: New York, NY, USA, 1981; pp. 43–67.
- Tao, S.H.; Bolger, P.M. Hazard assessment of germanium supplements. Regul. Toxicol. Pharmacol. 1997, 25, 211–219.
- Sabbioni, E.; Fortaner, S.; Bosisio, S.; Farina, M.; Del Torchio, R.; Edel, J.; Fischbach, M. Metabolic fate of ultratrace levels of GeCl4 in the rat and in vitro studies on its basal cytotoxicity and carcinogenic potential in Balb/3T3 and HaCaT cell lines. J. Appl. Toxicol. 2010, 30, 34–41.
- Schauss, A.G. Nephrotoxicity in humans by the ultratrace element germanium. Ren. Fail. 1991, 13, 1–4.
- Gerber, G.B.; Léonard, A. Mutagenicity, carcinogenicity and teratogenicity of germanium compounds. Mutat. Res. 1997, 387, 141–146.
- Gerik, S.; Maypole, J. Overview of biologically based therapies in rehabilitation. In Complementary Therapies for Physical Therapy; Deutsch, J.E., Anderson, E.Z., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2008; pp. 156–175.
- Menchikov, L.G.; Ignatenko, M.A. Biological activity of organogermanium compounds (a review). Pharm. Chem. J. 2013, 46, 635–638.
- Aldridge, W.N. The toxicology and biological properties of organotin compounds. In Tin as a Vital Nutrient; CRC Press: Boca Raton, FL, USA, 2019; pp. 245–262.
- Kucklick, J.R.; Ellisor, M.D. A review of organotin contamination in arctic and subarctic regions. Emerg. Contamin. 2019, 5, 150–156.
- Dodokhova, M.A.; Safronenko, A.V.; Kotieva, I.M.; Alkhuseyn-Kulyaginova, M.S.; Shpakovsky, D.B.; Milaeva, E.R. Impact of organotin compounds on the growth of epidermoid Lewis carcinoma. Res. Res. Pharmacol. 2021, 7, 81–88.
- Alama, A.; Tasso, B.; Novelli, F.; Sparatore, F. Organometallic compounds in oncology: Implications of novel organotins as antitumor agents. Drug Discov. Today 2009, 14, 500–508.
- Nath, M. Toxicity and the cardiovascular activity of organotin compounds: A review. Appl. Organomet. Chem. 2008, 22, 598–612.
- Graisa, A.M.; Zainulabdeen, K.; Salman, I.; Al-Ani, A.; Mohammed, R.; Hairunisa, N.; Yousif, E. Toxicity and anti-tumour activity of organotin (IV) compounds. Baghdad J. Biochem. Appl. Biol. Sci. 2022, 3, 99–108.
- Iornumbe, E.N.; Yiase, S.G.; Sha’Ato, R.; Tor-Anyiin, T.A. Synthesis, characterization and antifungal activity of some organotin(IV) derivatives of octanedioic acid. Int. J. Sci. Res. 2015, 4, 2095–2101.
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75.
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58.
- Kumar, A.; Kumar, A.; Cabral Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Yadav, K.K. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179.
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140.
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Proc. Engin. 2022, 45, 102518.
- Bjørklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Buha Djordjevic, A.; Aaseth, J. A review on coordination properties of thiol-containing chelating agents towards mercury, cadmium, and lead. Molecules 2019, 24, 3247.
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22.
- Tan, Z.; Chen, P.; Schneider, N.; Glover, S.; Cui, L.; Torgue, J.; Rixe, O.; Spitz, H.B.; Dong, Z. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int. J. Oncol. 2012, 40, 1881–1888.
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17.
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Baidoo, K.E.; Albert, P.S.; Wong, K.J.; Flynn, J.; Brechbiel, M.W. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2008, 14, 5108–5115.
- Prakash, S.; Verma, A.K. Arsenic: It’s Toxicity and Impact on Human health. Int. J. Biol. Innov. 2021, 3, 38–47.
- Flora, S.J.S. Preventive and therapeutic strategies for acute and chronic human arsenic exposure. In Arsenic in Drinking Water and Food; Springer: Dordrecht, The Netherlands, 2020.
- Rajavardhan, R.; Mamadapur, A.; Shyamala, N. Acute Arsenic Suicidal Poisoning—A Rare Case. Int. J. Med. Dent. Sci. 2021, 10, 1961–1965.
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302.
- Fang, J.; Chen, S.-J.; Tong, J.-H.; Wang, Z.-G.; Chen, G.-Q.; Chen, Z. Treatment of acute promyelocytic leukemia with ATRA and As2O3. Cancer Biol. Ther. 2002, 1, 614–620.
- Blower, P.J. 30 Inorganic pharmaceuticals. Annu. Rep. Prog. Chem. Sect. A 2004, 100, 633–658.
- Bisser, S.; N’Siesi, F.-X.; Lejon, V.; Preux, P.-M.; Van Nieuwenhove, S.; Miaka Mia Bilenge, C.; Büscher, P. Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness. J. Infect. Dis. 2007, 195, 322–329.
- Garnier, N.; Redstone, G.G.; Dahabieh, M.S.; Nichol, J.N.; del Rincon, S.V.; Gu, Y.; Miller, W.H. The novel arsenical darinaparsin is transported by cystine importing systems. Mol. Pharmacol. 2014, 85, 576–585.
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The composition of Ehrlich’s salvarsan: Resolution of a century-old debate. Angew. Chem. Int. Ed. Engl. 2005, 44, 941–944.
- Sanders, V.A.; Cutler, C.S. Radioarsenic: A promising theragnostic candidate for nuclear medicine. Nucl. Med. Biol. 2021, 92, 184–201.
- Liu, Y.; Shen, C.; Zhang, X.; Yu, H.; Wang, F.; Wang, Y.; Zhang, L.W. Exposure and nephrotoxicity concern of bismuth with the occurrence of autophagy. Toxicol. Ind. Health 2018, 34, 188–199.
- Li, H.; Sun, H. Recent advances in bioinorganic chemistry of bismuth. Curr. Opin. Chem. Biol. 2012, 16, 74–83.
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of bismuth in the eradication of Helicobacter pylori. Am. J. Therap. 2017, 24, e751–e757.
- Thomas, F.; Bialek, B.; Hensel, R. Medical use of bismuth: The two sides of the coin. J. Clin. Toxicol. 2011, 3, 004.
- Shiotani, A.; Roy, P.; Lu, H.; Graham, D.Y. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap. Adv. Gastroenterol. 2021, 14, 17562848211064080.
- Wang, R.; Li, H.; Ip, T.K.Y.; Sun, H. Bismuth drugs as antimicrobial agents. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 183–205.
- Halani, S.; Wu, P.E. Salicylate toxicity from chronic bismuth subsalicylate use. BMJ Case Rep. 2020, 13, e236929.
- Sadler, P.J.; Li, H.; Sun, H. Coordination chemistry of metals in medicine: Target sites for bismuth. Coord. Chem. Rev. 1999, 185–186, 689–709.
- Xie, Y.; Pan, X.; Li, Y.; Wang, H.; Du, Y.; Xu, J.; Wang, J.; Zeng, Z.; Chen, Y.; Zhang, G.; et al. New single capsule of bismuth, metronidazole and tetracycline given with omeprazole versus quadruple therapy consisting of bismuth, omeprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: A Chinese prospective, randomized, multicentre trial. J. Antimicrob. Chemother. 2018, 73, 1681–1687.
- Shetu, S.A.; Sanchez-Palestino, L.M.; Rivera, G.; Bandyopadhyay, D. Medicinal bismuth: Bismuth-organic frameworks as pharmaceutically privileged compounds. Tetrahedron 2022, 129, 133117.
- Kowalik, M.; Masternak, J.; Barszcz, B. Recent research trends on bismuth compounds in cancer chemo-and radiotherapy. Curr. Med. Chem. 2019, 26, 729–759.
- Yang, N.; Sun, H. Biocoordination chemistry of bismuth: Recent advances. Coord. Chem. Rev. 2007, 251, 2354–2366.
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A short review on biomedical applications of nanostructured bismuth oxide and related Nanomaterials. Materials 2020, 13, 5234.
- Peng, Z.; Tang, J. Intestinal Infection of Candida albicans: Preventing the Formation of Biofilm by C. albicans and Protecting the Intestinal Epithelial Barrier. Front. Microbiol. 2022, 12, 4413.
- Prakash, M.; Kavitha, H.P.; Abinaya, S.; Vennila, J.P.; Lohita, D. Green synthesis of bismuth-based nanoparticles and its applications—A review. Sust. Chem. Pharm. 2022, 25, 100547.
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Jurcic, J.G. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311.
- Jurcic, J. Alpha-Particle Therapy for Acute Myeloid Leukemia. J. Med. Imag. Rad. Sci. 2019, 50, S86–S87.
- Zare, O.; Afzalipour, R.; Yazdi, M.S.G. Review of Alloy Containing Bismuth Oxide Nanoparticles on X-Ray Absorption in Radiology Shields. Dis. Diagn. 2022, 11, 31–35.
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food sources of selenium and its relationship with chronic diseases. Nutrients 2021, 13, 1739.
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Veter. Sci. 2022, 9, 1868.
- Parish, L.; Parish, J.; Routh, H. Selenium sulfide in the 21st century. J. Am. Acad. Dermatol. 2008, 58, AB72.
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium compounds as novel potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 1009.
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Webster, T. Tellurium, the forgotten element: A review of the properties, processes, and biomedical applications of the bulk and nanoscale metalloid. In Racing for the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020.
- Chiaverini, L.; Marzo, T.; La Mendola, D. AS101: An Overview on a Leading Tellurium-Based Prodrug. Inorg. Chim. Acta 2022, 540, 121048.
- Halpert, G.; Eitan, T.; Voronov, E.; Apte, R.N.; Rath-Wolfson, L.; Albeck, M.; Kalechman, Y.; Sredni, B. Multifunctional activity of a small tellurium redox immunomodulator compound, AS101, on dextran sodium sulfate-induced murine colitis. J. Biol. Chem. 2014, 289, 17215–17227.
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Norenberg, J.P. Alpha-emitters and targeted alpha therapy in oncology: From basic science to clinical investigations. Targ. Oncol. 2018, 13, 189–203.
- Guerra Liberal, F.D.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417.
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing clinical trials with astatine-211: The chemistry infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436.
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and availability. Curr. Radiopharm. 2011, 4, 177–185.
- Vaidyanathan, G.; Zalutsky, M.R. Astatine radiopharmaceuticals: Prospects and problems. Curr. Radiopharm. 2008, 1, 177–196.
- Reilly, S.W.; Makvandi, M.; Xu, K.; Mach, R.H. Rapid Cu-Catalyzed Astatination and Iodination of Boronic Esters at Room Temperature. Org. Lett. 2018, 20, 1752–1755.
- Mohammadi, K.; Thompson, K.H.; Patrick, B.O.; Storr, T.; Martins, C.; Polishchuk, E.; Yuen, V.G.; McNeill, J.H.; Orvig, C. Synthesis and Characterization of Dual Function Vanadyl, Gallium and Indium Curcumin Complexes for Medicinal Applications. J. Inorg. Biochem. 2005, 99, 2217–2225.
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Pérez-González, L.L.; Tavera-Hernández, R.; Ramírez-Apan, M.T.; Toscano, R.A.; Sánchez-Obregón, R.; Obregón-Mendoza, M.A.; Enríquez, R.G. First Gallium and Indium Crystal Structures of Curcuminoid Homoleptic Complexes: All-Different Ligand Stereochemistry and Cytotoxic Potential. Int. J. Mol. Sci. 2023, 24, 16324.
- Shorkaei, M.R.; Asadi, Z.; Asadi, M. Synthesis, characterization, molecular docking and DNA binding studies of Al (III), Ga (III) and In (III) water-soluble complexes. J. Mol. Struct. 2016, 1109, 22–30.
- Yao, S.; Jiang, J.; Yang, P.; Cai, J.-Y. Synthesis, characterization and antioxidant activity of a novel organogermanium sesquioxide with resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122.
- Menchikov, L.G.; Popov, A.V. Physiological Activity of Trace Element Germanium Including Anticancer Properties. Biomedicines 2023, 11, 1535.
- Oh, C.; Li, M.; Kim, E.H.; Park, J.S.; Lee, J.C.; Ham, S.W. Antioxidant and Radical Scavenging Activities of Ascorbic Acid Derivatives Conjugated with Organogermanium. Bull. Korean Chem. Soc. 2010, 31, 3513–3514.
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium (IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732.
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-H. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisininorganogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297.
- Pi, J.; Zeng, J.; Luo, J.-J.; Yang, P.-H.; Cai, J.-Y. Synthesis and biological evaluation of Germanium(IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908.
- Syed Annuar, S.N.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular Basis of Organotin(IV) Derivatives as Anticancer Metallodrugs: A Review. Front. Chem. 2021, 9, 657599.
- Devi, J.; Yadav, J.; Kumar, D.; Jindal, D.K.; Basu, B. Synthesis, Spectral Analysis and In Vitro Cytotoxicity of Diorganotin (IV) Complexes Derived from Indole-3-Butyric Hydrazide. Appl. Organomet. Chem. 2020, 34, e5815.
- Liu, K.; Yan, H.; Chang, G.; Li, Z.; Niu, M.; Hong, M. Organotin(IV) Complexes Derived from Hydrazone Schiff Base: Synthesis, crystal Structure, In Vitro Cytotoxicity and DNA/BSA Interactions. Inorg. Chim. Acta 2017, 464, 137–146.
- Adeyemi, J.O.; Onwudiwe, D.C.; Ekennia, A.C.; Anokwuru, C.P.; Nundkumar, N.; Singh, M. Synthesis, Characterization and Biological Activities of Organotin(IV) Diallyldithiocarbamate Complexes. Inorg. Chim. Acta 2019, 485, 64–72.
- Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859.
- Khandani, M.; Sedaghat, T.; Erfani, N.; Haghshenas, M.R.; Khavasi, H.R. Synthesis, Spectroscopic Characterization, Structural Studies and Antibacterial and Antitumor Activities of Diorganotin Complexes with 3- methoxysalicylaldehyde Thiosemicarbazone. J. Mol. Struct. 2013, 1037, 136–143.
- Priya, J.; Madheswari, D. Biomolecular docking interactions, cytotoxicity and antioxidant property evaluations with novel Mn(II), Ni(II), Cd(II) and Pb(II) Schiff base ligand complexes: Synthesis and characterization. J. Biosci. 2022, 47, 29.
- Yang, K.N.; Yuan, K.D.; Jiang, L.L.; Zhang, Y. A new Pb (II)-based coordination polymer constructed from a semirigid tricarboxylate ligand: Crystal structure and anti-lung cancer activity. Main Group Met. Chem. 2019, 42, 8–12.
- Lv, L.L.; Xia, W.M.; Cheng, Y.Z.; Zhang, L.P.; Wang, X.D. Synthesis, structure, DNA binding and anticancer activity of a new tetranuclear Pb (II) complex constructed by 8-hydroxyquinolinate and 4-nitrobenzoate ligands. Main Group Met. Chem. 2019, 42, 60–66.
- Müller, S.; Miller, W.H., Jr.; Dejean, A. Trivalent Antimonials Induce Degradation of the PML-RARα Oncoprotein and Reorganization of the Promyelocytic Leukemia Nuclear Bodies in Acute Promyelocytic Leukemia NB4 Cells. Blood J. Am. Soc. Hematol. 1998, 92, 4308–4316.
- Islam, A.; Rodrigues, B.L.; Marzano, I.M.; Perreira-Maia, E.C.; Dittz, D.; Lopes, M.T.P.; Demicheli, C. Cytotoxicity and apoptotic activity of novel organobismuth (V) and organoantimony (V) complexes in different cancer cell lines. Eur. J. Med. Chem. 2016, 109, 254–267.
- Ouyang, R.; Yang, Y.; Tong, X.; Feng, K.; Yang, Y.; Tao, H.; Zhou, S. Potent anticancer activity of a new bismuth (III) complex against human lung cancer cells. J. Inorg. Biochem. 2017, 168, 18–26.
- Khan, M.H.; Cai, M.; Li, S.; Zhang, Z.; Zhang, J.; Wen, X.; Yang, F. Developing a binuclear multi-target Bi(III) complex by optimizing 2-acetyl-3-ethylpyrazine thiosemicarbazides. Eur. J. Med. Chem. 2019, 182, 111616.
- Li, M.; Lu, Y.; Yang, M.; Li, Y.; Zhang, L.; Xie, S. One dodecahedral bismuth(III) complex derived from 2- acetylpyridine N(4)-pyridylthiosemicarbazone: Synthesis, crystal structure and biological evaluation. Dalton Trans. 2012, 41, 12882–12887.
- Zhang, L.-Z.; An, G.-Y.; Yang, M.; Li, M.-X.; Zhu, X.-F. Synthesis, characterization, crystal structure and biological activities of the unusual main group 8-coordinate bismuth(III) complex derived from 2-acetylpyrazine N4- pyridylthiosemicarbazone. Inorg. Chem. Commun. 2012, 20, 37–40.
- Li, Y.-K.; Yang, M.; Li, M.-X.; Yu, H.; Wu, H.-C.; Xie, S.-Q. Synthesis, crystal structure and biological evaluation of a main group seven-coordinated bismuth(III) complex with 2- acetylpyridine N4-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2013, 23, 2288–2292.
- Parrilha, G.L.; Ferraz, K.S.O.; Lessa, J.A.; de Oliveira, K.N.; Rodrigues, B.L.; Ramos, J.P.; Souza-Fagundes, E.M.; Ott, I.; Beraldo, H. Metal complexes with 2-acetylpyridineN(4)-orthochlorophenylthiosemicarbazone: Cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur. J. Med. Chem. 2014, 84, 537–544.
- Ferreira, I.P.; Piló, E.D.L.; Recio-Despaigne, A.A.; Da Silva, J.G.; Ramos, J.P.; Marques, L.B.; Prazeres, P.H.D.M.; Takahashi, J.A.; Souza-Fagundes, E.M.; Rocha, W.; et al. Bismuth(III) complexes with 2- acetylpyridine- and 2-benzoylpyridine-derived hydrazones: Antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg. Med. Chem. 2016, 24, 2988–2998.
- Ferraz, K.S.O.; Silva, N.F.; da Silva, J.G.; de Miranda, L.F.; Romeiro, C.F.D.; Souza-Fagundes, E.M.; Mendes, I.C.; Beraldo, H. Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Eur. J. Med. Chem. 2012, 53, 98–106.
- Zhang, N.; Tai, Y.; Li, M.; Ma, P.; Zhao, J.; Niu, J. Main group bismuth(III), gallium(III) and diorganotin(IV) complexes derived from bis(2- acetylpyrazine)thiocarbonohydrazone: Synthesis, crystal structures and biological evaluation. Dalton Trans. 2014, 43, 5182–5189.
- Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Manos, M.J.; Tasiopoulos, A.J.; Owczarzak, A.M.; Kubicki, M.; Hadjikakou, S.K. Synthesis, characterization and biological activity of antimony(III) or bismuth(III) chloride complexes with dithiocarbamate ligands derived from thiuram degradation. Polyhedron 2014, 67, 89–103.
- Tiekink, E.R.T. Antimony and bismuth compounds in oncology. Crit. Rev. Oncol. Hematol. 2002, 42, 217–224.
- Zhang, X.W.; Xia, J.; Yan, H.W.; Luo, S.L.; Yin, S.F.; Au, C.T.; Wong, W.Y. Synthesis, structure, and in vitro antiproliferative activity of cyclic hypervalent organobismuth(III) chlorides and their triphenylgermylpropionate derivatives. J. Organomet. Chem. 2009, 694, 3019–3026.
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695.
- Garbo, S.; Di Giacomo, S.; Łazewska, D.; Honkisz-Orzechowska, E.; Di Sotto, A.; Fioravanti, R.; Zwergel, C.; Battistelli, C. Selenium-Containing Agents Acting on Cancer—A New Hope? Pharmaceutics 2023, 15, 104.
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263.
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Spengler, G. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updates 2022, 63, 100844.
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol. 1998, 55, 573–584.
- Gross, M.; Stanciu, E.; Kenigsbuch-Sredni, D.; Sredni, B.; Pinhasov, A. The immunomodulatory tellurium compound ammonium trichloro (dioxoethylene-O,O’) tellurate reduces anxiety-like behavior and corticosterone levels of submissive mice. Behav. Pharmacol. 2017, 28, 458–465.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
859
Revisions:
2 times
(View History)
Update Date:
17 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




