
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | RamaRao Malla | -- | 3755 | 2024-01-17 02:23:55 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 3758 | 2024-01-17 03:02:20 | | |
Video Upload Options
The hippo/yes-associated protein (YAP) protein is a critical oncogenic mediator within the Hippo signaling pathway and has been implicated in various cancer types. In breast cancer, it frequently becomes activated, thereby contributing to developing drug-resistance mechanisms. Studies have underscored the intricate interplay between YAP and ferroptosis within the breast tumor microenvironment. YAP exerts a negative regulatory effect on ferroptosis, promoting cancer cell survival and drug resistance.
1. Introduction
2. Hippo Signaling Pathway
3. YAP Protein and Its Impact on Breast Cancer
3.1. YAP Mediates Drug Resistance by Inducing Stemness in Breast Cancer Cells
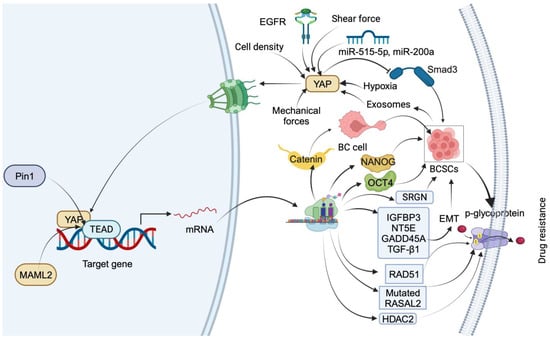
3.2. Ferroptosis Factors and Regulation Mechanisms in Breast Cancer
3.3. YAP Mediates Resistance to Ferroptosis in Breast Cancer
3.4. YAP in the Control of Metabolic and Oxidative Stress in Breast Cancer
4. Regulation of YAP
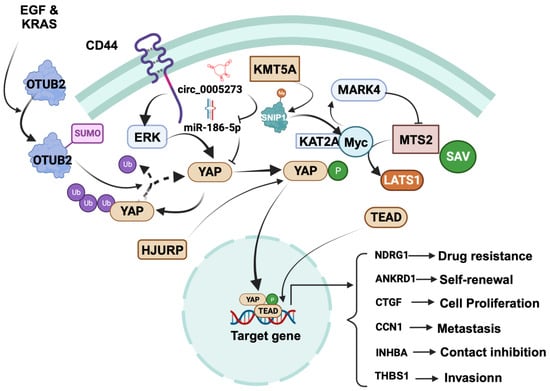
4.1. Tumor-Promoting Role of YAP in Breast Cancer
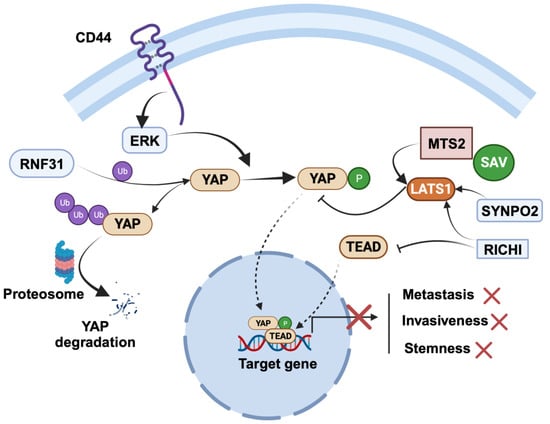
4.2. Tumor-Suppressor Role of YAP in Breast Cancer
5. Targeting YAP Signaling and Drug Resistance in BC
Over the past two decades, a multitude of potential natural compounds and synthetic derivatives have emerged as promising inhibitors of YAP (Table 1). These compounds demonstrate the ability to independently restrain growth and metastasis or sensitize TNBC cells to chemotherapeutic agents through either the inhibition of YAP signaling or the promotion of its degradation. Furthermore, select compounds exhibit the capacity to induce ferroptosis in TNBC cells by modulating YAP expression.
| Natural Compound | Cellular Mechanism | Molecular Mechanism | Ref. |
|---|---|---|---|
| Apigenin |
|
|
[54] |
|
|
[55] | |
| Luteolin |
|
|
[56] |
| Parthenolide derivative |
|
|
[57] |
| Alantolactone |
|
|
[58] |
| Curcumin |
|
|
[59] |
|
|
[60] | |
| Curcumin derivative |
|
|
[61] |
|
|
[61] | |
| Resveratrol |
|
|
[62] |
| Caudatin |
|
|
[63] |
| Rosmarinic acid |
|
|
[64] |
| Hydnocarpin |
|
|
[65] |
6. Conclusions
References
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. S2), S26–S35.
- Kobayashi, S. Basal-like subtype of breast cancer: A review of its unique characteristics and their clinical significance. Breast Cancer 2008, 15, 153–158.
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287.
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 2021, 497, 100–111.
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957.
- Yang, D.; Zhang, N.; Li, M.; Hong, T.; Meng, W.; Ouyang, T. The Hippo Signaling Pathway: The Trader of Tumor Microenvironment. Front. Oncol. 2021, 11, 772134.
- Wang, Z.; Wang, F.; Ding, X.Y.; Li, T.E.; Wang, H.Y.; Gao, Y.H.; Wang, W.J.; Liu, Y.F.; Chen, X.S.; Shen, K.W. Hippo/YAP signaling choreographs the tumor immune microenvironment to promote triple negative breast cancer progression via TAZ/IL-34 axis. Cancer Lett. 2022, 527, 174–190.
- Xu, N.; Li, B.; Liu, Y.; Yang, C.; Tang, S.; Cho, W.C.; Huang, Z. Ferroptosis and triple-negative breast cancer: Potential therapeutic targets. Front. Oncol. 2022, 12, 1017041.
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604.
- Calses, P.C.; Crawford, J.J.; Lill, J.R.; Dey, A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer 2019, 5, 297–307.
- Luo, J.; Yu, F.X. GPCR-Hippo Signaling in Cancer. Cells 2019, 8, 426.
- Samji, P.; Rajendran, M.K.; Warrier, V.P.; Ganesh, A.; Devarajan, K. Regulation of Hippo signaling pathway in cancer: A MicroRNA perspective. Cell Signal. 2021, 78, 109858.
- Ouyang, T.; Meng, W.; Li, M.; Hong, T.; Zhang, N. Recent Advances of the Hippo/YAP Signaling Pathway in Brain Development and Glioma. Cell Mol. Neurobiol. 2020, 40, 495–510.
- Cao, L.; Sun, P.L.; Yao, M.; Jia, M.; Gao, H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum. Pathol. 2017, 68, 166–174.
- Kim, S.K.; Jung, W.H.; Koo, J.S. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 3224–3234.
- Abylkassov, R.; Xie, Y. Role of Yes-associated protein in cancer: An update (Review). Oncol. Lett. 2016, 12, 2277–2282.
- Yu, Y.; Su, X.; Qin, Q.; Hou, Y.; Zhang, X.; Zhang, H.; Jia, M.; Chen, Y. Yes-associated protein and transcriptional coactivator with PDZ-binding motif as new targets in cardiovascular diseases. Pharmacol. Res. 2020, 159, 105009.
- Zhao, W.; Wang, M.; Cai, M.; Zhang, C.; Qiu, Y.; Wang, X.; Zhang, T.; Zhou, H.; Wang, J.; Zhao, W. Transcriptional co-activators YAP/TAZ: Potential therapeutic targets for metastatic breast cancer. Biomed. Pharmacother. 2021, 133, 110956.
- Morciano, G.; Vezzani, B.; Missiroli, S.; Boncompagni, C.; Pinton, P.; Giorgi, C. An Updated Understanding of the Role of YAP in Driving Oncogenic Responses. Cancers 2021, 13, 3100.
- Yang, C.E.; Lee, W.Y.; Cheng, H.W.; Chung, C.H.; Mi, F.L.; Lin, C.W. The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem. Biol. Interact. 2019, 302, 28–35.
- Maugeri-Saccà, M.; De Maria, R. Hippo pathway and breast cancer stem cells. Crit. Rev. Oncol. Hematol. 2016, 99, 115–122.
- Rong, X.; Liang, Y.; Han, Q.; Zhao, Y.; Jiang, G.; Zhang, X.; Lin, X.; Liu, Y.; Zhang, Y.; Han, X.; et al. Molecular Mechanisms of Tyrosine Kinase Inhibitor Resistance Induced by Membranous/Cytoplasmic/Nuclear Translocation of Epidermal Growth Factor Receptor. J. Thorac. Oncol. 2019, 14, 1766–1783.
- Sadri, F.; Hosseini, S.F.; Rezaei, Z.; Fereidouni, M. Hippo-YAP/TAZ signaling in breast cancer: Reciprocal regulation of microRNAs and implications in precision medicine. Genes Dis. 2024, 11, 760–771.
- Quinn, H.M.; Vogel, R.; Popp, O.; Mertins, P.; Lan, L.; Messerschmidt, C.; Landshammer, A.; Lisek, K.; Château-Joubert, S.; Marangoni, E.; et al. YAP and β-Catenin Cooperate to Drive Oncogenesis in Basal Breast Cancer. Cancer Res. 2021, 81, 2116–2127.
- Sun, H.L.; Men, J.R.; Liu, H.Y.; Liu, M.Y.; Zhang, H.S. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch. Biochem. Biophys. 2020, 685, 108349.
- Guo, Z.; Guo, A.; Zhou, C. Breast Cancer Stem Cell-Derived ANXA6-Containing Exosomes Sustain Paclitaxel Resistance and Cancer Aggressiveness in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 718721.
- Khanal, P.; Yeung, B.; Zhao, Y.; Yang, X. Identification of Prolyl isomerase Pin1 as a novel positive regulator of YAP/TAZ in breast cancer cells. Sci. Rep. 2019, 9, 6394.
- Kim, J.; Jang, G.; Sim, S.H.; Park, I.H.; Kim, K.; Park, C. SMARCA4 Depletion Induces Cisplatin Resistance by Activating YAP1-Mediated Epithelial-to-Mesenchymal Transition in Triple-Negative Breast Cancer. Cancers 2021, 13, 5474.
- Jiang, Y.; Ji, F.; Liu, Y.; He, M.; Zhang, Z.; Yang, J.; Wang, N.; Zhong, C.; Jin, Q.; Ye, X.; et al. Cisplatin-induced autophagy protects breast cancer cells from apoptosis by regulating yes-associated protein. Oncol. Rep. 2017, 38, 3668–3676.
- Elaimy, A.L.; Amante, J.J.; Zhu, L.J.; Wang, M.; Walmsley, C.S.; FitzGerald, T.J.; Goel, H.L.; Mercurio, A.M. The VEGF receptor neuropilin 2 promotes homologous recombination by stimulating YAP/TAZ-mediated Rad51 expression. Proc. Natl. Acad. Sci. USA 2019, 116, 14174–14180.
- Koh, S.B.; Ross, K.; Isakoff, S.J.; Melkonjan, N.; He, L.; Matissek, K.J.; Schultz, A.; Mayer, E.L.; Traina, T.A.; Carey, L.A.; et al. RASAL2 Confers Collateral MEK/EGFR Dependency in Chemoresistant Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 4883–4897.
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165625.
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414.
- Yun, T.; Liu, Z.; Wang, J.; Wang, R.; Zhu, L.; Zhu, Z.; Wang, X. Microenvironment immune response induced by tumor ferroptosis-the application of nanomedicine. Front. Oncol. 2022, 12, 1019654.
- Jiang, L.; Gao, X.M.; Cao, J. The Achilles heel of TNBCs: Ferroptosis heterogeneity. Cell Metab. 2023, 35, 1–2.
- Yang, F.; Xiao, Y.; Ding, J.H.; Jin, X.; Ma, D.; Li, D.Q.; Shi, J.X.; Huang, W.; Wang, Y.P.; Jiang, Y.Z.; et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023, 35, 84–100.e8.
- Brown, C.W.; Amante, J.J.; Chhoy, P.; Elaimy, A.L.; Liu, H.; Zhu, L.J.; Baer, C.E.; Dixon, S.J.; Mercurio, A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell 2019, 51, 575–586.e4.
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406.
- Morrow, K.A.; Das, S.; Metge, B.J.; Ye, K.; Mulekar, M.S.; Tucker, J.A.; Samant, R.S.; Shevde, L.A. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J. Biol. Chem. 2011, 286, 40376–40385.
- White, S.M.; Avantaggiati, M.L.; Nemazanyy, I.; Di Poto, C.; Yang, Y.; Pende, M.; Gibney, G.T.; Ressom, H.W.; Field, J.; Atkins, M.B.; et al. YAP/TAZ Inhibition Induces Metabolic and Signaling Rewiring Resulting in Targetable Vulnerabilities in NF2-Deficient Tumor Cells. Dev. Cell 2019, 49, 425–443.e9.
- Petrilli, A.M.; Fernández-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35, 537–548.
- Mota, M.; Metge, B.J.; Hinshaw, D.C.; Alsheikh, H.A.; Chen, D.; Samant, R.S.; Shevde, L.A. Merlin deficiency alters the redox management program in breast cancer. Mol. Oncol. 2021, 15, 942–956.
- Wu, M.; Zhang, X.; Zhang, W.; Chiou, Y.S.; Qian, W.; Liu, X.; Zhang, M.; Yan, H.; Li, S.; Li, T.; et al. Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat. Commun. 2022, 13, 1371.
- Koo, J.H.; Guan, K.-L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206.
- Dai, J.Z.; Wang, Y.J.; Chen, C.H.; Tsai, I.L.; Chao, Y.C.; Lin, C.W. YAP Dictates Mitochondrial Redox Homeostasis to Facilitate Obesity-Associated Breast Cancer Progression. Adv. Sci. 2022, 9, e2103687.
- Sorrentino, G.; Ruggeri, N.; Zannini, A.; Ingallina, E.; Bertolio, R.; Marotta, C.; Neri, C.; Cappuzzello, E.; Forcato, M.; Rosato, A.; et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017, 8, 14073.
- You, M.; Xie, Z.; Zhang, N.; Zhang, Y.; Xiao, D.; Liu, S.; Zhuang, W.; Li, L.; Tao, Y. Signaling pathways in cancer metabolism: Mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 196.
- Shen, J.; Cao, B.; Wang, Y.; Ma, C.; Zeng, Z.; Liu, L.; Li, X.; Tao, D.; Gong, J.; Xie, D. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 175.
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971.
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell Biol. 2008, 28, 2426–2436.
- Yu, S.; Cai, X.; Wu, C.; Wu, L.; Wang, Y.; Liu, Y.; Yu, Z.; Qin, S.; Ma, F.; Thiery, J.P.; et al. Adhesion glycoprotein CD44 functions as an upstream regulator of a network connecting ERK, AKT and Hippo-YAP pathways in cancer progression. Oncotarget 2015, 6, 2951–2965.
- Liu, J.; Ye, L.; Li, Q.; Wu, X.; Wang, B.; Ouyang, Y.; Yuan, Z.; Li, J.; Lin, C. Synaptopodin-2 suppresses metastasis of triple-negative breast cancer via inhibition of YAP/TAZ activity. J. Pathol. 2018, 244, 71–83.
- Tian, Q.; Gao, H.; Zhou, Y.; Zhu, L.; Yang, J.; Wang, B.; Liu, P.; Yang, J. RICH1 inhibits breast cancer stem cell traits through activating kinases cascade of Hippo signaling by competing with Merlin for binding to Amot-p80. Cell Death Dis. 2022, 13, 71.
- Malla, R.R.; Deepak, K.G.K.; Merchant, N.; Dasari, V.R. Breast Tumor Microenvironment: Emerging target of therapeutic phytochemicals. Phytomedicine 2020, 70, 153227.
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105.
- Cao, D.; Zhu, G.-Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.-J.; Peng, S.-X.; Chen, L.-W.; Li, Y.-W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462.
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19.
- Nakatani, K.; Maehama, T.; Nishio, M.; Otani, J.; Yamaguchi, K.; Fukumoto, M.; Hikasa, H.; Hagiwara, S.; Nishina, H.; Mak, T.W.; et al. Alantolactone is a natural product that potently inhibits YAP1/TAZ through promotion of reactive oxygen species accumulation. Cancer Sci. 2021, 112, 4303–4316.
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE 2022, 17, e0261370.
- Li, R.; Zhang, J.; Zhou, Y.; Gao, Q.; Wang, R.; Fu, Y.; Zheng, L.; Yu, H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid. Med. Cell Longev. 2020, 2020, 3469840.
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X.; et al. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 460.
- Kim, Y.N.; Choe, S.R.; Cho, K.H.; Cho, D.Y.; Kang, J.; Park, C.G.; Lee, H.Y. Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Exp. Mol. Med. 2017, 49, e296.
- Zhen, X.; Choi, H.S.; Kim, J.H.; Kim, S.L.; Liu, R.; Ko, Y.C.; Yun, B.S.; Lee, D.S. Caudatin Isolated from Cynanchum auriculatum Inhibits Breast Cancer Stem Cell Formation via a GR/YAP Signaling. Biomolecules 2020, 10, 925.
- Kim, C.-L.; Shin, Y.-S.; Choi, S.-H.; Oh, S.; Kim, K.; Jeong, H.-S.; Mo, J.-S. Extracts of Perilla frutescens var. Acuta (Odash.) Kudo Leaves Have Antitumor Effects on Breast Cancer Cells by Suppressing YAP Activity. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5619761.
- Ou, H.L.; Wu, H.; Ren, Y.L.; Si, Y.; Duan, Z.Q.; Liu, X.W. Hydnocarpin inhibits malignant progression of triple negative breast cancer via CNOT4-mediated ubiquitination and degradation of YAP. Zhongguo Zhong Yao Za Zhi 2023, 48, 4483–4492.




