In recent years, with the development of science and technology and the enhancement of social awareness of environmental protection, the development and utilization of new energy sources and new materials have received attention from various countries
[1]. Graphene, a new two-dimensional carbon material with sp
2 hybridization, is the thinnest two-dimensional material ever discovered. Due to its properties of being very small and difficult to peel off the monolayer structure, even though scientists have carried out a lot of research, little has been achieved. In 2004, British researchers Andrei Geim and Kostia Novoselov succeeded in obtaining graphene from graphite by repeatedly folding special tapes
[2], which won them the Nobel Prize in Physics in 2010. Since then, more and more research has been conducted on graphene, and many advantages of graphene have been gradually discovered, such as the fact that its strength is very large
[3] and that it has a high transmittance
[4]. Because of its many excellent optical, mechanical, and electrical properties, it is a revolutionary material with very important application potential in many fields.
2. Structure of Graphene Quantum Dots
Carbon nanomaterials can be categorized into zero-dimensional, one-dimensional, and two-dimensional carbon nanomaterials according to their dimensionality. Zero-dimensional carbon nanomaterials refer to carbon materials that are all nanoscale in three dimensions. There are mainly fullerene and fullerene-like Cn-structured carbon nanomaterials, nanodiamonds, carbon nanodots, and graphene quantum dots, which have different structures. In fullerenes, C atoms are hybridized into bonds with sp
2, a single carbon atom has a coordination number of 3, forming 3 σ bonds with the surrounding three carbon atoms, and the remaining orbital forms a cloud of π electrons, thus forming aromatic carbon molecules
[5]. Different from the sp
2 hybrid bonding of fullerene and Cn structures, nanodiamonds is generally sp
3 bonded inside, maintaining the integrity of the crystal structure as a whole, and there are graphite shells or carbon suspension bonds outside. Similar to nanodiamonds, carbon quantum dots are roughly spherical particles with internal carbon nuclei that maintain crystalline integrity, but the particle size of carbon quantum dots is smaller compared to nanodiamonds, typically around 10 nm.
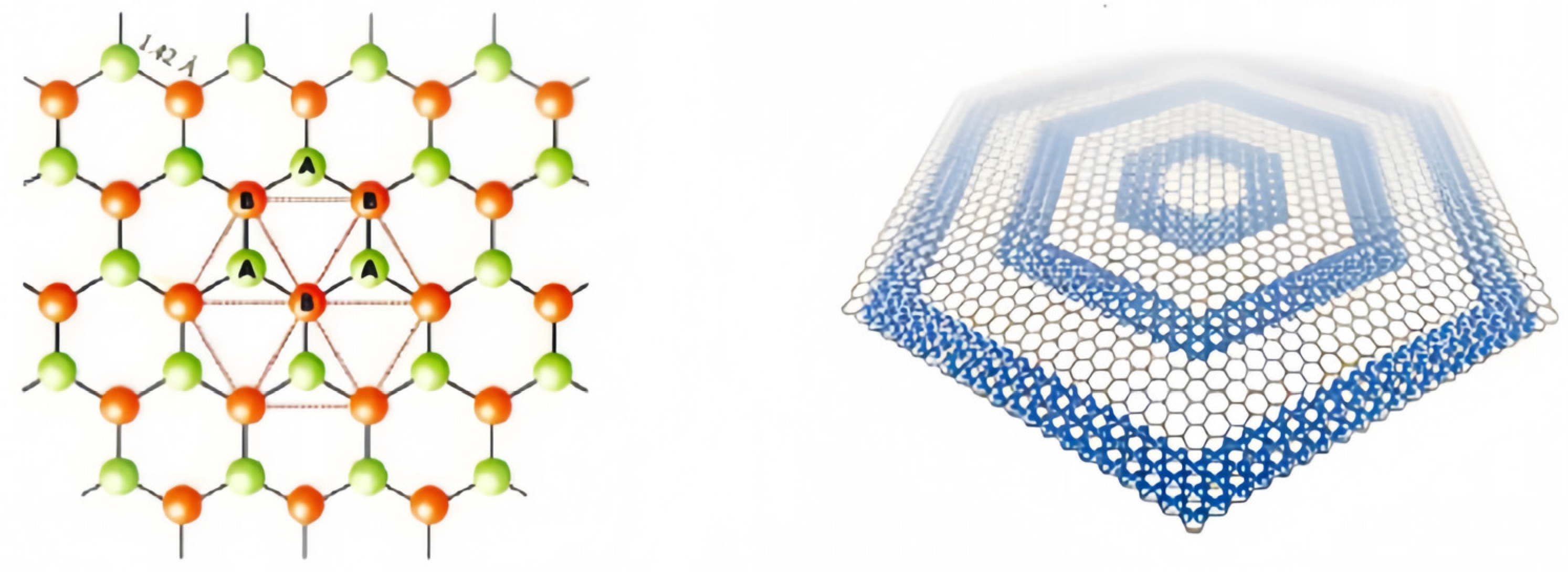
Graphene quantum dots (GQDs) are a quasi-zero-dimensional form of graphene with the same crystal structure as graphene. The ideal infinite graphene crystal consists of two sets of nested sublattices (lattices A and B) with a distance of 0.142 nm between neighboring cells. and they belong to different sublattices, with three x-bonds inside each lattice and carbon atoms C occupying lattice points to form a periodic six-membered ring structure in the graphene plane
[6]. C atoms are closely stacked, each carbon atom is connected to 3 carbon atoms by sp
2, 3 of the 4 valence electrons are paired with the valence electrons of the surrounding 3 carbon atoms to form δ-bonds, and the unpaired π-electrons work together to form large π-bonds, and this unique electronic structure confers an allotropic nature that is different from that of other carbons. The structure of the graphene quantum dot is shown in
Figure 1.
Figure 1. The structure of the GQDs.
3. Nature of Graphene Quantum Dots
3.1. Optical Performance
Graphene quantum dots have excellent photostability and photoluminescence properties, and their light-absorbing capacity is generally closely related to the structure of graphene oxygen-containing groups and quantum dots. They are usually reflective in the ultraviolet region, which decreases with increasing wavelengths
[7][8]. The causes of light mainly include two aspects: the surface edge state and the conjugate π bond. The effects of surface edge states on the photoluminescence of graphene quantum dots include their jagged edge types, oxygen-containing groups, amino groups, and quantum dot nuclei. When graphene quantum dots only have fewer surface chemical groups, the band gap of conjugated π bonds is considered to be the main cause of photoluminescence, and the photoluminescence of graphene quantum dots can be adjusted by adjusting the size of the conjugated π bonds. Studies have found that GQDS has a long luminous wavelength and low energy. Compared with carbon quantum dots, there is a higher PL quantum yield. Zhu et al.
[9] synthesized GQD with different fluorescences, which can range from the UV to the red light region and have better photostability than conventional fluorescent moieties. Li et al.
[10] found that carbon quantum dots obtained by the alkali-assisted electrochemical method have good transition luminescence properties, excitation with a long wavelet length, and low energy. It has an important potential for applications in bioimaging and medicine.
3.2. Water Solubility
The surface of GQDs is rich in oxygen-containing functional groups, such as hydroxyl and carboxyl groups. Therefore, it is particularly water-soluble and can form a stable aqueous solution. In addition, some groups can be introduced on the surface of GQDs to change the types of hydrophilic and hydrophobic groups and synthesize many different graphene quantum dots. Cho et al.
[11] coupled the synthesized hydrophilic GQD with N,N′-dicyclohexyl carbon diimide and reacted with epoxy ring-opening simultaneously, realizing the change of hydrophilic to hydrophobic GQDs.
3.3. Electrical Properties
(ECL) electrochemiluminescence is a luminescent phenomenon produced by electrochemical reactions in solution. As an analytical technique, ECL combines the advantages of chemiluminescence analysis without background light signals and reaction electrochemical analysis utilizing electrode potentials that are easy to control with the advantages of high sensitivity, good selectivity, and no acoustic noise. It should have outstanding advantages, such as a wide range. In recent years, many researchers have found that semiconductor quantum dots have ECL properties, while GQDs have a higher specific surface area and richer surface states and therefore have better potential as ECL luminophores
[12]. Typically, GQD nanosheets, especially monolayer GQDs, have a higher specific surface area than CQD nanoparticles, which means that GQDs may have richer surface states. In addition, oxidized GQDs increase the emission sites of GQDs due to the increase of oxygen-containing functional groups, resulting in stronger electrochemiluminescence effects. For example, the hydrothermal onset potential of GQDs prepared from graphene oxide sheets can be as low as 0.4 V, and a stable ECL signal with a relative standard deviation of only 1.0% was measured in a continuous cyclic scan. Jung et al. assembled GO nanoplates into a porous network using an ion-mediated assembly (IMA) method. The metal substrate was immersed in GO solution, and GO nanosheets were attracted to the anode and adhered to the surface with the assistance of an applied voltage. The GO was then converted to reduced graphene oxide (rGO). Conductive rGO filters with a porous structure and a high specific surface area can be connected to voltage applications for novel electrostatic PM filtration. Ionic liquids (ILs) are liquids composed entirely of ions and have been widely studied in the field of electrochemistry due to their high conductivity and electrochemical stability
[13].
3.4. Toxicity and Cytocompatibility
Carbon is a vital element of human tissue and is non-toxic. In addition, a series of carbon-based compounds are the basis of human tissues. And the carbon material is chemically inert, so GQDs have excellent biocompatibility and environmentally friendly properties. Chong
[14] et al. studied the toxicity of graphene quantum dots in organisms and in vitro in detail. In vitro experiments found that graphene quantum dots have extremely low toxicity due to their ultra-small size and high oxygen content, while in vivo experiments on the distribution of graphene quantum dots in mice showed that graphene quantum dots do not accumulate in the major organs of the mice but are rapidly excreted through the kidneys and do not show significant toxicity at high doses. Some researchers have demonstrated the very low cytotoxicity of graphene quantum dots by co-culturing graphene quantum dots with MC
3T
3 cells and conducting MTT assay experiments. In conclusion, graphene quantum dots have very low cytotoxicity and good biocompatibility and can be used for high-concentration bioimaging and other biomedical applications. The effect of functional groups on the cytotoxicity of graphene quantum dots was determined by comparing their ability to produce reactive oxygen species. It has been shown that the keto carbonyl group has a significant effect on the reactive oxygen species formation ability of graphene quantum dots, so the removal of oxygen functional groups from graphene quantum dots improves the photostability and reduces the cytotoxicity of graphene quantum dots, which provides a molecular-level indication for better design of biocompatible graphene quantum dots. Graphene quantum dots with a large number of oxygen-containing groups are effectively used as surgical nanoradiation sensitizers for radiation therapy due to their good biocompatibility
[15]. The results show that GQDs have very high biocompatibility and low in vivo cytotoxicity compared to quantum dots
[16].