Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adonis Sfera | -- | 1346 | 2024-01-16 12:04:39 | | | |
| 2 | Jessie Wu | Meta information modification | 1346 | 2024-01-17 02:16:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sfera, A. Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs. Encyclopedia. Available online: https://encyclopedia.pub/entry/53892 (accessed on 07 February 2026).
Sfera A. Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs. Encyclopedia. Available at: https://encyclopedia.pub/entry/53892. Accessed February 07, 2026.
Sfera, Adonis. "Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs" Encyclopedia, https://encyclopedia.pub/entry/53892 (accessed February 07, 2026).
Sfera, A. (2024, January 16). Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs. In Encyclopedia. https://encyclopedia.pub/entry/53892
Sfera, Adonis. "Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs." Encyclopedia. Web. 16 January, 2024.
Copy Citation
In 1957, Arvid Carlsson discovered that dopamine, at the time believed to be nothing more than a norepinephrine precursor, was a brain neurotransmitter in and of itself. By 1963, postsynaptic dopamine blockade had become the cornerstone of psychiatric treatment as it appeared to have deciphered the “chlorpromazine enigma”, a 1950s term, denoting the action mechanism of antipsychotic drugs.
microbial translocation
antipsychotic drugs
autophagy
1. Introduction
The dopamine hypothesis (DH) of schizophrenia (SCZ), launched by Carlsson and Lindqvist in 1963, surmises that the postsynaptic blockade of dopamine (DA) receptors is responsible for the beneficial effect of antipsychotic drugs in SCZ and SCZ-like disorders [1]. Arvid Carlson’s experiments with DA depletion by reserpine, followed by chlorpromazine inhibition of dopaminergic transmission, led to a better understanding of the pathology at work in Parkinson’s disease (PD) and acute psychosis [2]. Carlsson and Lindqvist surmised that excessive DA activation of the postsynaptic DA type 2 receptors (D2R) in the central nervous system (CNS) triggered psychotic symptoms, while dopaminergic blockade of these receptors comprised the remedy [3]. This model was further substantiated by the 1970s observation that amphetamine enhanced dopaminergic signaling, often exacerbating psychotic symptoms [4]. However, the realization that antipsychotic drugs exert properties, seemingly unrelated to DA, such as antimicrobial, antiviral, antiproliferative, cell cycle arrest, autophagy activation, alteration of iron metabolism, DNA methylation, and telomere elongation, led to the third DH revision, which emphasized genetic and epigenetic input in dopaminergic transmission [5][6][7][8][9][10].
Aside from the antipsychotic drugs, several characteristics of SCZ itself may be incongruous with the DH, including increased prevalence in industrialized countries and urban areas, variance with latitude, autoantibodies, and high comorbidity with inflammatory bowel disease (IBD) and human immunodeficiency virus 1 (HIV-1) (Table 1). Moreover, excessive DA in the CNS would likely promote euphoria, motivation, and heightened alertness instead of hallucinations or delusions. Furthermore, DH equates acute psychosis with SCZ, an assumption not always shared by clinicians, many of whom conceptualize positive symptoms of SCZ as epiphenomena of this pathology [11].
Table 1. DA-unrelated SCZ characteristics explained by translocated microbes/AhR activation.
| DH-Discordant SCZ Features | Non-DA Mechanisms | References |
|---|---|---|
| Negative symptoms | Translocation of Hafnei alvei, Pseudomonas aeruginosa, Morganella morganii, Pseudomonas putida, and Klebsiella pneumoniae | [12][13][14] |
| Comorbidity with IBD | AhR/STAT3/IL-22-regulated intestinal permeability and microbiota translocation | [11][15][16] |
| Comorbidity with HIV | AhR/STAT3/IL22-regulateted gut barrier permeability |
[12][17][18] |
| Poor insight (anosognosia) | IC activation by gut Prevotella and Bacteroides abundance | [19][20][21][22][23][24][25] |
| Higher prevalence in urban areas | Pollutants are AhR ligands associated with SCZ and are more prevalent in industrialized countries and urban areas | [26][27][28][29][30][31][32][33][34] |
| Increasing prevalence with the distance from the equator | Sunlight-driven vitamin D derivatives and tryptophan light metabolites are AhR ligands | [35][36][37][38][39] |
| Autoantibodies | Gut microbes express molecules, including GABA and NMDA, which can elicit formation of antibodies upon translocation | [40][41][42] |
Aryl hydrocarbon receptor (AhR), a cytosolic transcription factor, initially described as the dioxin receptor, responds to numerous exogenous and endogenous ligands, inducing both immune tolerance of gut microbes as well as their prompt elimination upon translocation into host tissues [43][44]. In the cytosol, AhR is bound by two heat shock protein 90 (HSP90) chaperones, molecules recently identified as both SCZ and Parkinson’s disease (PD) targets [45][46][47][48][49][50][51][52]. Other AhR ligands significant for SCZ include DA, carbidopa, clozapine, melatonin, serotonin, microbial phenazines, and various pollutants, linking endogenous and exogenous molecules to this pathology [53][54][55][56].
Several properties of antipsychotic agents appear difficult to reconcile with the DH, even when taking into consideration environmental and genetic factors [57]. For example, the antimicrobial properties of these drugs could alleviate psychosis by eliminating translocated microbes and subsequent inflammation [58]. The non-dopaminergic, therapeutic properties of antipsychotic drugs are likely driven by AhR, a protein involved in the regulation of antimicrobial, antiviral, and antiproliferative host defenses as well as pathogen clearance via reactive oxygen species (ROS), or autophagy [59][60][61]. For example, AhR-induced ROS can ameliorate psychotic symptoms by clearing intracellular pathogens, including Toxoplasma gondii, a SCZ-associated parasite [62]. In addition, neuroleptic drug-activated autophagy and clearance of molecular debris and damaged cells lower the organismal inflammatory burden, likely generating antipsychotic effects [63][64]. Conversely, dysfunctional autophagy and accumulation of cellular remains at the gut barrier and BBB may promote inflammation and microbial translocation into the host systemic circulation [65]. This may explain elevated translocation markers and the more diverse blood microbiome documented in SCZ patients [65][66][67].
Gut commensals enjoy immunological tolerance in the GI tract; however, this protection ceases upon migration outside the intestinal barrier, where they can be vehemently attacked by the host immune defenses [68]. Bactericidal antipsychotic drugs likely facilitate the clearance of both translocated microbes and damaged cells (by autophagy activation), lowering the odds of neuroinflammation and psychosis [69][70]. Other non-dopaminergic beneficial effects of antipsychotic drugs, such as telomere elongation, microglial de-escalation, and inhibition of ferroptosis, may likely be explained by the agonist/antagonist AhR binding [6][71][72][73]. For example, AhR antagonists and some partial agonists, including resveratrol, quercetin, or the synthetic compound HBU651, were demonstrated to elongate telomeres, reverse microglial activation, and avert ferroptosis, placing AhR at the epicenter of SCZ pathology [74][75][76][77] (Table 2).
Taken together, the clinical efficacy of antipsychotic drugs may be mediated by both dopaminergic and non-dopaminergic pathways, the latter including lowering neuroinflammation, ferroptosis inhibition, telomere lengthening, and deactivation of microglia [72][78][79][80].
2. Antipsychotics as Antibacterials
First- and second-generation antipsychotic drugs possess antibacterial properties, suggesting that the elimination of translocated microbes may drive symptomatic relief in psychosis [81]. This is further substantiated by the current efforts to repurpose several antipsychotic drugs as antibiotics [82]. Conversely, antibiotics such as doxycycline and minocycline exert antipsychotic properties, indicating that postsynaptic DA blockade may not be the only mechanism for alleviating psychotic symptoms [83][84].
Other antipsychotic drugs with antimicrobial properties include phenothiazines, compounds capable of eliminating E. coli, a bacterium previously associated with SCZ [85][86]. Moreover, haloperidol exerts fungicidal action against Candida albicans (C. albicans), a BBB-crossing fungus, linked by previous studies to SCZ [87][88][89]. Furthermore, both antipsychotic drugs and IL-22, including the recombinant form, inhibit IFN-γ, a cytokine with established antifungal properties [90][91]. Interestingly, IL-22 exhibits fungicidal action against C. albicans as well as antipsychotic-like properties [88][92][93][94][95] (Table 2).
Table 2. Antipsychotic-like properties of IL22 (or recombinant IL22).
| Antipsychotic Drugs | Recombinant IL-22 | References |
|---|---|---|
| JAK-STAT activation | JAK-STAT activation | [96][97] |
| Neuroprotective | Neuroprotective | [98][99] |
| IFN-γ inhibitor | IFN-γ inhibitor | [100][101] |
| Activate autophagy | Activate autophagy | [102][103][104] |
| Antibacterial/antiviral | Antibacterial/antiviral | [87][105][106][107] |
3. Antipsychotics as Antivirals
Many antipsychotic drugs possess antiviral properties inherited from their parent compound and phenothiazine dye, methylene blue (MB) [108]. For example, chlorpromazine exerts antiviral properties against SARS-CoV-2, the etiologic agent of the COVID-19 pandemic, and is currently in clinical trials (NCT04366739) for this viral infection [109]. Other viruses enter host cells via clathrin-dependent endocytosis (CDE) and are inhibited by several neuroleptics, including chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine, emphasizing an alternative, DA-independent mechanisms of action, likely mediated by AhR (functioning as an E3 ubiquitin ligase) [110][111][112].
Several antipsychotic drugs alter the biophysical properties of cell membranes by intercalating themselves into the lipid bilayer, blocking viral fusion with host cells. At the same time, this process may comprise an antipsychotic mechanism by depolarizing neuronal membranes, lowering pathological connectivity and likely calcium entry [113][114]. Moreover, several antipsychotics, including haloperidol, are cationic, amphiphilic compounds that accumulate in lysosomes, likely sabotaging viral replication as well as SCZ-associated lysosomal dysfunction [115][116].
4. Antipsychotics as Anticancer Drugs
Several first- and second-generation antipsychotic drugs can arrest the cell cycle at the G2/M point, explaining their beneficial effects against some cancers [117][118]. In patients with SCZ, antipsychotic drugs were found to block the paradoxical attempt of mature neurons to re-enter the cell cycle, emphasizing another possible DA-independent mechanism of alleviating psychosis [119][120][121][122][123].
5. Microbial Phenazines vs. Antipsychotic Phenothiazines
Phenazines are ubiquitous nitrogen-based AhR ligands, released by a wide variety of bacteria, including gut commensals Pseudomonas spp. [124][125]. Like phenothiazine antipsychotics, phenazines exhibit anti-inflammatory, anticancer, antimicrobial, and neuroprotective properties, suggesting that they likely bind AhR [126]. Microbial phenazines are natural phenothiazines, generated by the gut commensals to eliminate pathogenic bacteria and malignant cells by ROS production [127][128][129] (Figure 1).
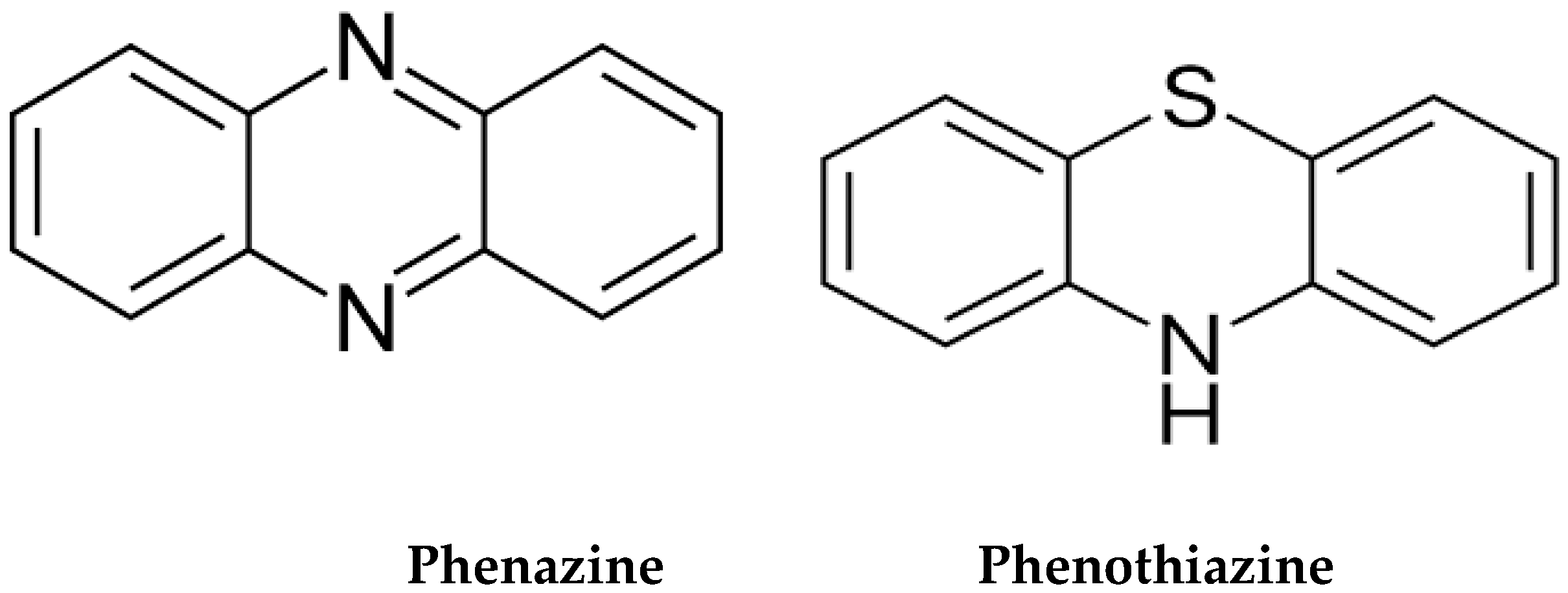
Figure 1. Structural similarity between microbial phenazines and phenothiazines. In the gut, Pseudomonas aeruginosa is the main producer of phenazines. Phenazines upregulate ACh by inhibiting its degrading enzymes, acetylcholinesterase (AChE) and butyryl acetylcholinesterase (BChE), enhancing cholinergic transmission.
Phenothiazines are MB derivatives which led to the development of the first marketed antipsychotic drug, chlorpromazine, which in 1954 ushered in the era of psychopharmacology [130][131]. Since microbial phenazines are AhR ligands, phenothiazines very likely attach to AhR, suggesting an alternative antipsychotic mechanism [132][133].
References
- Baumeister, A.A. The Chlorpromazine Enigma. J. Hist. Neurosci. 2013, 22, 14–29.
- Carlsson, A. Basic concepts underlying recent developments in the field of Parkinson’s disease. Contemp. Neurol. Ser. 1971, 8, 1–31.
- McKenna, P.J. Pathology, Phenomenology and the Dopamine Hypothesis of Schizophrenia. Br. J. Psychiatry 1987, 151, 288–301.
- Snyder, S.H. The dopamine hypothesis of schizophrenia: Focus on the dopamine receptor. Am. J. Psychiatry 1976, 133, 197–202.
- Nehme, H.; Saulnier, P.; Ramadan, A.A.; Cassisa, V.; Guillet, C.; Eveillard, M.; Umerska, A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS ONE 2018, 13, e0189950.
- Hirata, Y.; Oka, K.; Yamamoto, S.; Watanabe, H.; Oh-Hashi, K.; Hirayama, T.; Nagasawa, H.; Takemori, H.; Furuta, K. Haloperidol Prevents Oxytosis/Ferroptosis by Targeting Lysosomal Ferrous Ions in a Manner Independent of Dopamine D2 and Sigma-1 Receptors. ACS Chem. Neurosci. 2022, 13, 2719–2727.
- Iasevoli, F.; Avagliano, C.; D’ambrosio, L.; Barone, A.; Ciccarelli, M.; De Simone, G.; Mazza, B.; Vellucci, L.; de Bartolomeis, A. Dopamine Dynamics and Neurobiology of Non-Response to Antipsychotics, Relevance for Treatment Resistant Schizophrenia: A Systematic Review and Critical Appraisal. Biomedicines 2023, 11, 895.
- Yang, A.C.; Tsai, S.-J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689.
- Woldman, I.; Reither, H.; Kattinger, A.; Hornykiewicz, O.; Pifl, C. Dopamine inhibits cell growth and cell cycle by blocking ribonucleotide reductase. Neuropharmacology 2005, 48, 525–537.
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines 2020, 8, 595.
- Bernstein, C.N.; A Hitchon, C.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; A Graff, L.; Patten, S.B.; Singer, A.; Lix, L.M.; et al. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 25, 360–368.
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox. Res. 2018, 35, 684–698.
- Secher, T.; Samba-Louaka, A.; Oswald, E.; Nougayrède, J.-P. Escherichia coli Producing Colibactin Triggers Premature and Transmissible Senescence in Mammalian Cells. PLoS ONE 2013, 8, e77157.
- Ma, Q.; Gao, F.; Zhou, L.; Fan, Y.; Zhao, B.; Xi, W.; Wang, C.; Zhu, F.; Ma, X.; Wang, W.; et al. Characterizing serum amino acids in schizophrenic patients: Correlations with gut microbes. J. Psychiatr. Res. 2022, 153, 125–133.
- Sung, K.; Zhang, B.; Wang, H.E.; Bai, Y.; Tsai, S.; Su, T.; Chen, T.; Hou, M.; Lu, C.; Wang, Y.; et al. Schizophrenia and risk of new-onset inflammatory bowel disease: A nationwide longitudinal study. Aliment. Pharmacol. Ther. 2022, 55, 1192–1201.
- Bartocci, B.; Buono, A.D.; Gabbiadini, R.; Busacca, A.; Quadarella, A.; Repici, A.; Mencaglia, E.; Gasparini, L.; Armuzzi, A. Mental Illnesses in Inflammatory Bowel Diseases: Mens sana in corpore sano. Medicina 2023, 59, 682.
- Helm, E.Y.; Zhou, L. Transcriptional regulation of innate lymphoid cells and T cells by aryl hydrocarbon receptor. Front Immunol. 2023, 14, 1056267.
- Sewell, D.D. Schizophrenia and HIV. Schizophr. Bull. 1996, 22, 465–473.
- Lehrer, D.S.; Lorenz, J. Anosognosia in schizophrenia: Hidden in plain sight. Innov. Clin. Neurosci. 2014, 11, 10–17.
- Torregrossa, L.J.; Amedy, A.; Roig, J.; Prada, A.; Park, S. Interoceptive functioning in schizophrenia and schizotypy. Schizophr. Res. 2021, 239, 151–159.
- Ardizzi, M.; Ambrosecchia, M.; Buratta, L.; Ferri, F.; Peciccia, M.; Donnari, S.; Mazzeschi, C.; Gallese, V. Interoception and Positive Symptoms in Schizophrenia. Front. Hum. Neurosci. 2016, 10, 379.
- Klein, T.A.; Ullsperger, M.; Danielmeier, C. Error awareness and the insula: Links to neurological and psychiatric diseases. Front. Hum. Neurosci. 2013, 7, 14.
- Gil-Lievana, E.; Ramírez-Mejía, G.; Urrego-Morales, O.; Luis-Islas, J.; Gutierrez, R.; Bermúdez-Rattoni, F. Photostimulation of Ventral Tegmental Area-Insular Cortex Dopaminergic Inputs Enhances the Salience to Consolidate Aversive Taste Recognition Memory via D1-Like Receptors. Front. Cell. Neurosci. 2022, 16, 823220.
- Karnath, H.-O.; Baier, B.; Nägele, T. Awareness of the Functioning of One’s Own Limbs Mediated by the Insular Cortex? J. Neurosci. 2005, 25, 7134–7138.
- Ullsperger, M.; Harsay, H.A.; Wessel, J.R.; Ridderinkhof, K.R. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 2010, 214, 629–643.
- Sánchez-Martín, F.J.; Fernández-Salguero, P.M.; Merino, J.M. Aryl hydrocarbon receptor-dependent induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells from mouse. J. Neurochem. 2011, 118, 153–162.
- Attademo, L.; Bernardini, F. Air Pollution as Risk Factor for Mental Disorders: In Search for a Possible Link with Alzheimer’s Disease and Schizophrenia. J. Alzheimer Dis. 2020, 76, 825–830.
- Antonsen, S.; Mok, P.L.H.; Webb, R.T.; Mortensen, P.B.; McGrath, J.J.; Agerbo, E.; Brandt, J.; Geels, C.; Christensen, J.H.; Pedersen, C.B. Exposure to air pollution during childhood and risk of developing schizophrenia: A national cohort study. Lancet Planet. Health 2020, 4, e64–e73.
- Dagher, J.B.; Al Mansi, M.; Jacob, E.; Kaimal, A.; Chuang, Y.; Mohankumar, P.S.; MohanKumar, S.M.J. Prenatal Exposure to Bisphenol A and Diethylhexyl Phthalate Induces Apoptosis in the Thymus of Male and Female Offspring. FASEB J. 2019, 33, 812.5.
- Maurya, P.K.; Rizzo, L.B.; Xavier, G.; Tempaku, P.F.; Ota, V.K.; Santoro, M.L.; Spíndola, L.M.; Moretti, P.S.; Mazzotti, D.R.; Gadelha, A.; et al. Leukocyte telomere length variation in different stages of schizophrenia. J. Psychiatr. Res. 2018, 96, 218–223.
- Holahan, M.R.; Smith, C.A.; Luu, B.E.; Storey, K.B. Preadolescent Phthalate (DEHP) Exposure Is Associated With Elevated Locomotor Activity and Reward-Related Behavior and a Reduced Number of Tyrosine Hydroxylase Positive Neurons in Post-Adolescent Male and Female Rats. Toxicol. Sci. 2018, 165, 512–530.
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. Msystems 2019, 4, e00724-19.
- Kim, H.; Kim, W.-H.; Kim, Y.-Y.; Park, H.-Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public Health 2020, 8, 575330.
- Dey, S.K.; Sugur, K.; Venkatareddy, V.G.; Rajeev, P.; Gupta, T.; Thimmulappa, R.K. Lipid peroxidation index of particulate matter: Novel metric for quantifying intrinsic oxidative potential and predicting toxic responses. Redox Biol. 2021, 48, 102189.
- Li, X.; Luck, M.E.; Hammer, A.M.; Cannon, A.R.; Choudhry, M.A. 6-Formylindolo (3, 2-b) Carbazole (FICZ)–mediated protection of gut barrier is dependent on T cells in a mouse model of alcohol combined with burn injury. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165901.
- Memari, B.; Nguyen-Yamamoto, L.; Salehi-Tabar, R.; Zago, M.; Fritz, J.H.; Baglole, C.J.; Goltzman, D.; White, J.H. Endocrine aryl hydrocarbon receptor signaling is induced by moderate cutaneous exposure to ultraviolet light. Sci. Rep. 2019, 9, 8486.
- Rannug, A.; Fritsche, E. The aryl hydrocarbon receptor and light. Biol. Chem. 2006, 387, 1149–1157.
- Saatci, D.; Johnson, T.; Smee, M.; van Nieuwenhuizen, A.; Handunnetthi, L. The role of latitude and infections in the month-of-birth effect linked to schizophrenia. Brain Behav. Immun. Health 2022, 24, 100486.
- Dikongué, E.; Ségurel, L. Latitude as a co-driver of human gut microbial diversity? Bioessays 2017, 39, 1600145.
- Krøll, J. E. coli antibodies in schizophrenia. Psychol. Med. 1986, 16, 209–211.
- Al-Diwani, A.A.J.; Pollak, T.A.; Irani, S.R.; Lennox, B.R. Psychosis: An autoimmune disease? Immunology 2017, 152, 388–401.
- Dagorn, A.; Chapalain, A.; Mijouin, L.; Hillion, M.; Duclairoir-Poc, C.; Chevalier, S.; Taupin, L.; Orange, N.; Feuilloley, M.G.J. Effect of GABA, a Bacterial Metabolite, on Pseudomonas fluorescens Surface Properties and Cytotoxicity. Int. J. Mol. Sci. 2013, 14, 12186–12204.
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2013, 26, 209–220.
- Postal, B.G.; Ghezzal, S.; Aguanno, D.; André, S.; Garbin, K.; Genser, L.; Brot-Laroche, E.; Poitou, C.; Soula, H.; Leturque, A.; et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 2020, 39, 101007.
- Ishima, T.; Iyo, M.; Hashimoto, K. Neurite outgrowth mediated by the heat shock protein Hsp90α: A novel target for the antipsychotic drug aripiprazole. Transl. Psychiatry 2012, 2, e170.
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W.; et al. Chronic Treatment with Novel Small Molecule Hsp90 Inhibitors Rescues Striatal Dopamine Levels but Not α-Synuclein-Induced Neuronal Cell Loss. PLoS ONE 2014, 9, e86048.
- Carver, L.; Jackiw, V.; Bradfield, C. The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system. J. Biol. Chem. 1994, 269, 30109–30112.
- Whitelaw, M.L.; McGuire, J.; Picard, D.; A Gustafsson, J.; Poellinger, L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 4437–4441.
- Kaneta, H.; Ukai, W.; Tsujino, H.; Furuse, K.; Kigawa, Y.; Tayama, M.; Ishii, T.; Hashimoto, E.; Kawanishi, C. Antipsychotics promote GABAergic interneuron genesis in the adult rat brain: Role of heat-shock protein production. J. Psychiatr. Res. 2017, 92, 108–118.
- Uemura, S.; Nakajima, Y.; Yoshida, Y.; Furuya, M.; Matsutani, S.; Kawate, S.; Ikeda, S.-I.; Tsuji, N.; Grave, E.; Wakui, H.; et al. Biochemical properties of human full-length aryl hydrocarbon receptor (AhR). J. Biochem. 2020, 168, 285–294.
- Kim, J.J.; Lee, S.J.; Toh, K.Y.; Lee, C.U.; Lee, C.; Paik, I.H. Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr. Res. 2001, 52, 127–135.
- Zhong, W.; Chen, W.; Liu, Y.; Zhang, J.; Lu, Y.; Wan, X.; Qiao, Y.; Huang, H.; Zeng, Z.; Li, W.; et al. Extracellular HSP90α promotes cellular senescence by modulating TGF -β signaling in pulmonary fibrosis. FASEB J. 2022, 36, e22475.
- Park, H.; Jin, U.-H.; Karki, K.; Jayaraman, A.; Allred, C.H.; Michelhaugh, S.K.; Mittal, S.; Chapkin, R.S.; Safe, S.H. Dopamine is an aryl hydrocarbon receptor agonist. Biochem. J. 2020, 477, 3899–3910.
- Fehsel, K.; Schwanke, K.; Kappel, B.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201.
- Eisenhofer, G.; Åneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial Production of Dopamine in the Human Gastrointestinal Tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871.
- Gopinath, A.; Mackie, P.M.; Phan, L.T.; Mirabel, R.; Smith, A.R.; Miller, E.; Franks, S.; Syed, O.; Riaz, T.; Law, B.K.; et al. Who Knew? Dopamine Transporter Activity Is Critical in Innate and Adaptive Immune Responses. Cells 2023, 12, 269.
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2022, 48, 151–167.
- Jia, L.; Chen, H.; Yang, J.; Fang, X.; Niu, W.; Zhang, M.; Li, J.; Pan, X.; Ren, Z.; Sun, J.; et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. J. Endotoxin Res. 2019, 26, 48–61.
- Vasilev, A.; Sofi, R.; Tong, L.; Teschemacher, A.G.; Kasparov, S. In Search of a Breakthrough Therapy for Glioblastoma Multiforme. Neuroglia 2018, 1, 292–310.
- Mori, M.; Hitora, T.; Nakamura, O.; Yamagami, Y.; Horie, R.; Nishimura, H.; Yamamoto, T. Hsp90 inhibitor induces autophagy and apoptosis in osteosarcoma cells. Int. J. Oncol. 2014, 46, 47–54.
- Boule, L.A.; Burke, C.G.; Jin, G.-B.; Lawrence, B.P. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: Ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci. Rep. 2018, 8, 1826.
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int. J. Mol. Sci. 2021, 22, 5705.
- Congdon, E.E.; Wu, J.W.; Myeku, N.; Figueroa, Y.H.; Herman, M.; Marinec, P.S.; Gestwicki, J.E.; Dickey, C.A.; Yu, W.H.; Duff, K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 2012, 8, 609–622.
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 347.
- Loohuis, L.M.O.; Mangul, S.; Ori, A.P.S.; Jospin, G.; Koslicki, D.; Yang, H.T.; Wu, T.; Boks, M.P.; Lomen-Hoerth, C.; Wiedau-Pazos, M.; et al. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 2018, 8, 96.
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Origoni, A.E.; Khushalani, S.; Leweke, F.M.; Dickerson, F.B.; Yolken, R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013, 148, 130–137.
- Talukdar, P.M.; Abdul, F.; Maes, M.; Binu, V.; Venkatasubramanian, G.; Kutty, B.M.; Debnath, M. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol. Neurobiol. 2020, 57, 4345–4361.
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240.
- Al-Amin, M.; Uddin, M.M.N.; Reza, H.M. Effects of Antipsychotics on the Inflammatory Response System of Patients with Schizophrenia in Peripheral Blood Mononuclear Cell Cultures. Clin. Psychopharmacol. Neurosci. 2013, 11, 144–151.
- Marcinowicz, P.; Więdłocha, M.; Zborowska, N.; Dębowska, W.; Podwalski, P.; Misiak, B.; Tyburski, E.; Szulc, A. A Meta-Analysis of the Influence of Antipsychotics on Cytokines Levels in First Episode Psychosis. J. Clin. Med. 2021, 10, 2488.
- Russo, P.; Prinzi, G.; Proietti, S.; Lamonaca, P.; Frustaci, A.; Boccia, S.; Amore, R.; Lorenzi, M.; Onder, G.; Marzetti, E.; et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of most recent literature. Schizophr. Res. 2018, 202, 37–45.
- De-Paula, V.J.; Polho, G.B.; Cardillo, G.M.; Kerr, D.S.; Chile, T.; Gattaz, W.F.; Forlenza, O.V.; Brentani, H.P. Antipsychotics preserve telomere length in peripheral blood mononuclear cells after acute oxidative stress injury. Neural Regen. Res. 2022, 17, 1156–1160.
- Lauterbach, E.C. Repurposing psychiatric medicines to target activated microglia in anxious mild cognitive impairment and early Parkinson’s disease. Am. J. Neurodegener. Dis. 2016, 5, 29–51.
- Ling, X.; Yang, W.; Zou, P.; Zhang, G.; Wang, Z.; Zhang, X.; Chen, H.; Peng, K.; Han, F.; Liu, J.; et al. TERT regulates telomere-related senescence and apoptosis through DNA damage response in male germ cells exposed to BPDE in vitro and to BP in vivo. Environ. Pollut. 2018, 235, 836–849.
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718.
- Panda, S.K.; Peng, V.; Sudan, R.; Antonova, A.U.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812.e4.
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 2023, 938, 175407.
- Mahapatra, S.; Marques, T.R. Antipsychotics, versatility in action. Proc. Natl. Acad. Sci. USA 2021, 118, e2108946118.
- Ben-Shachar, D.; Livne, E.; Spanier, I.; Zuk, R.; Youdim, M.B. Iron modulates neuroleptic-induced effects related to the dopaminergic system. Isr. J. Med. Sci. 1993, 29, 587–592.
- Kato, T.; Monji, A.; Hashioka, S.; Kanba, S. Risperidone significantly inhibits interferon-γ-induced microglial activation in vitro. Schizophr. Res. 2007, 92, 108–115.
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics 2023, 12, 137.
- Liu, Y.; She, P.; Xu, L.; Chen, L.; Li, Y.; Liu, S.; Li, Z.; Hussain, Z.; Wu, Y. Antimicrobial, Antibiofilm, and Anti-persister Activities of Penfluridol Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 727692.
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A Double-Blind, Randomized Study of Minocycline for the Treatment of Negative and Cognitive Symptoms in Early-Phase Schizophrenia. J. Clin. Psychiatry 2009, 71, 138–149.
- De Witte, L.D.; Laursen, T.M.; Corcoran, C.M.; Kahn, R.S.; Birnbaum, R.; Munk-Olsen, T.; Bergink, V. A Sex-Dependent Association Between Doxycycline Use and Development of Schizophrenia. Schizophr. Bull. 2023, 49, 953–961.
- Fatemi, S.H. Potential microbial origins of schizophrenia and their treatments. Drugs Today 2009, 45, 305–318.
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 880568.
- Chattopadhyay, D.; Dastidar, S.G.; Chakrabarty, A.N. Antimicrobial properties of methdilazine and its synergism with antibiotics and some chemotherapeutic agents. Arzneimittelforschung 1988, 38, 869–872.
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2017, 62, 41–45.
- Ji, C.; Liu, N.; Tu, J.; Li, Z.; Han, G.; Li, J.; Sheng, C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect. Dis. 2019, 6, 768–786.
- Villar, C.C.; Kashleva, H.; Nobile, C.J.; Mitchell, A.P.; Dongari-Bagtzoglou, A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect. Immun. 2007, 75, 2126–2135.
- Cataldi, S.; Codini, M.; Hunot, S.; Légeron, F.-P.; Ferri, I.; Siccu, P.; Sidoni, A.; Ambesi-Impiombato, F.S.; Beccari, T.; Curcio, F.; et al. e-Cadherin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson Disease. Mediat. Inflamm. 2016, 2016, 3937057.
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.-C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373.
- Hawi, Z.; Tong, J.; Dark, C.; Yates, H.; Johnson, B.; Bellgrove, M.A. The role of cadherin genes in five major psychiatric disorders: A literature update. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 177, 168–180.
- Jong, A.Y.; Stins, M.F.; Huang, S.-H.; Chen, S.H.M.; Kim, K.S. Traversal of Candida albicans across Human Blood-Brain Barrier In Vitro. Infect. Immun. 2001, 69, 4536–4544.
- Ezeonwumelu, I.J.; Garcia-Vidal, E.; Ballana, E. JAK-STAT Pathway: A Novel Target to Tackle Viral Infections. Viruses 2021, 13, 2379.
- Singh, R.K.; Dai, Y.; Staudinger, J.L.; Muma, N.A. Activation of the JAK-STAT pathway is necessary for desensitization of 5-HT2A receptor-stimulated phospholipase C signalling by olanzapine, clozapine and MDL. Int. J. Neuropsychopharmacol. 2008, 12, 651–665.
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.-C. Interleukin-22 (IL-22) Activates the JAK/STAT, ERK, JNK, and p38 MAP Kinase Pathways in a Rat Hepatoma Cell Line. J. Biol. Chem. 2002, 277, 33676–33682.
- He, J.; Kong, J.; Tan, Q.-R.; Li, X.-M. Neuroprotective effect of atypical antipsychotics in cognitive and non-cognitive behavioral impairment in animal models. Cell Adhes. Migr. 2009, 3, 129–137.
- Mattapallil, M.J.; Kielczewski, J.L.; Zárate-Bladés, C.; Leger, A.J.S.; Raychaudhuri, K.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.-C.; Caspi, R.R. Interleukin 22 ameliorates neuropathology and protects from central nervous system autoimmunity. J. Autoimmun. 2019, 102, 65–76.
- Cazzullo, C.L.; Sacchetti, E.; Galluzzo, A.; Panariello, A.; Adorni, A.; Pegoraro, M.; Bosis, S.; Colombo, F.; Trabattoni, D.; Zagliani, A.; et al. Cytokine profiles in schizophrenic patients treated with risperidone: A 3-month follow-up study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 33–39.
- Kato, T.; Mizoguchi, Y.; Monji, A.; Horikawa, H.; Suzuki, S.O.; Seki, Y.; Iwaki, T.; Hashioka, S.; Kanba, S. Inhibitory effects of aripiprazole on interferon-induced microglial activation via intracellular Ca2+ regulation in vitro. J. Neurochem. 2008, 106, 815–825.
- Merenlender-Wagner, A.; Malishkevich, A.; Shemer, Z.; Udawela, M.; Gibbons, A.; Scarr, E.; Dean, B.; Levine, J.; Agam, G.; Gozes, I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 2013, 20, 126–132.
- Mo, R.; Lai, R.; Lu, J.; Zhuang, Y.; Zhou, T.; Jiang, S.; Ren, P.; Li, Z.; Cao, Z.; Liu, Y.; et al. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics 2018, 8, 4170–4180.
- Vucicevic, L.; Misirkic-Marjanovic, M.; Harhaji-Trajkovic, L.; Maric, N.; Trajkovic, V. Mechanisms and therapeutic significance of autophagy modulation by antipsychotic drugs. Cell Stress 2018, 2, 282–291.
- Dempsey, L. Antimicrobial IL-22. Nat. Immunol. 2017, 18, 373.
- Das, S.; Croix, C.S.; Good, M.; Chen, J.; Zhao, J.; Hu, S.; Ross, M.; Myerburg, M.M.; Pilewski, J.M.; Williams, J.; et al. Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience 2020, 23, 101256.
- Girgis, R.R.; Lieberman, J.A. Anti-viral properties of antipsychotic medications in the time of COVID-19. Psychiatry Res. 2020, 295, 113626.
- Karwaciak, I.; Karaś, K.; Sałkowska, A.; Pastwińska, J.; Ratajewski, M. Chlorpromazine, a Clinically Approved Drug, Inhibits SARS-CoV-2 Nucleocapsid-Mediated Induction of IL-6 in Human Monocytes. Molecules 2022, 27, 3651.
- Plaze, M.; Attali, D.; Petit, A.-C.; Blatzer, M.; Simon-Loriere, E.; Vinckier, F.; Cachia, A.; Chrétien, F.; Gaillard, R. Repositionnement de la chlorpromazine dans le traitement du COVID-19: Étude reCoVery. Repurposing of chlorpromazine in COVID-19 treatment: The reCoVery study. 2020, 46, S35–S39.
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 2020, 887, 173553.
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148.
- Hu, J.; Ding, Y.; Liu, W.; Liu, S. When AHR signaling pathways meet viral infections. Cell Commun. Signal. 2023, 21, 42.
- Özkucur, N.; Quinn, K.P.; Pang, J.C.; Du, C.; Georgakoudi, I.; Miller, E.; Levin, M.; Kaplan, D.L. Membrane potential depolarization causes alterations in neuron arrangement and connectivity in cocultures. Brain Behav. 2014, 5, 24–38.
- Skrede, S.; Holmsen, H. Har antipsykotika en rolle i membranhypotesen ved schizofreni? A role for antipsychotic agents in the membrane hypothesis of schizophrenia? Tidsskr. Nor. Laegeforen. 2003, 123, 2568–2570.
- Canfrán-Duque, A.; Barrio, L.C.; Lerma, M.; De la Peña, G.; Serna, J.; Pastor, O.; Lasunción, M.A.; Busto, R. First-Generation Antipsychotic Haloperidol Alters the Functionality of the Late Endosomal/Lysosomal Compartment in Vitro. Int. J. Mol. Sci. 2016, 17, 404.
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044.
- Shin, S.Y.; Lee, K.S.; Choi, Y.-K.; Lim, H.J.; Lee, H.G.; Lim, Y.; Lee, Y.H. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis 2013, 34, 2080–2089.
- Katsel, P.; Davis, K.L.; Li, C.; Tan, W.; Greenstein, E.; Hoffman, L.B.K.; Haroutunian, V. Abnormal Indices of Cell Cycle Activity in Schizophrenia and their Potential Association with Oligodendrocytes. Neuropsychopharmacology 2008, 33, 2993–3009.
- E Grant, C.; Flis, A.L.; Ryan, B.M. Understanding the role of dopamine in cancer: Past, present and future. Carcinogenesis 2022, 43, 517–527.
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun. 2020, 40, 694–710.
- Okazaki, S.; Boku, S.; Otsuka, I.; Mouri, K.; Aoyama, S.; Shiroiwa, K.; Sora, I.; Fujita, A.; Shirai, Y.; Shirakawa, O.; et al. The cell cycle-related genes as biomarkers for schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 70, 85–91.
- Fan, Y.; Abrahamsen, G.; McGrath, J.J.; Mackay-Sim, A. Altered Cell Cycle Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 129–135.
- Barrio-Alonso, E.; Hernández-Vivanco, A.; Walton, C.C.; Perea, G.; Frade, J.M. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci. Rep. 2018, 8, 14316.
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas Spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445.
- Valliappan, K.; Sun, W.; Li, Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377.
- Lavaggi, M.L.; Aguirre, G.; Boiani, L.; Orelli, L.; García, B.; Cerecetto, H.; González, M. Pyrimidoquinoxaline 6-oxide and phenazine 5,10-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1737–1741.
- Anthérieu, S.; Azzi, P.B.-E.; Dumont, J.; Abdel-Razzak, Z.; Guguen-Guillouzo, C.; Fromenty, B.; Robin, M.-A.; Guillouzo, A. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2012, 57, 1518–1529.
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670.
- Bock, K.W. Aryl hydrocarbon receptor (AHR) functions in infectious and sterile inflammation and NAD+-dependent metabolic adaptation. Arch. Toxicol. 2021, 95, 3449–3458.
- Bernthsen, A. Ueber das Methylenblau. Berichte Dtsch Chem Ges. 1883, 16, 1025–1028.
- Elkes, J.; Elkes, C. Effect of chlorpromazine on the behavior of chronically overactive psychotic patients. Br. Med. J. 1954, 2, 560–565.
- Pinto, C.J.; Ávila-Gálvez, M.; Lian, Y.; Moura-Alves, P.; dos Santos, C.N. Targeting the aryl hydrocarbon receptor by gut phenolic metabolites: A strategy towards gut inflammation. Redox Biol. 2023, 61, 102622.
- Moura-Alves, P.; Faé, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
473
Revisions:
2 times
(View History)
Update Date:
23 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




