
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ulvi Bayraktutan | -- | 4228 | 2024-01-15 16:28:18 | | | |
| 2 | Lindsay Dong | -7 word(s) | 4221 | 2024-01-16 01:14:23 | | |
Video Upload Options
Stroke remains one of the leading causes of death and disability worldwide. Current reperfusion treatments for ischaemic stroke are limited due to their narrow therapeutic window in rescuing ischaemic penumbra. Stem cell therapy offers a promising alternative. As a regenerative medicine, stem cells offer a wider range of treatment strategies, including long-term intervention for chronic patients, through the reparation and replacement of injured cells via mechanisms of differentiation and proliferation.
1. Introduction
2. Pathology of Ischaemic Stroke
2.1. Excitotoxic Cell Death
2.2. Apoptosis, Necrosis, and Necroptosis Pathways
3. Blood–Brain Barrier
4. Stem Cell as Therapeutics
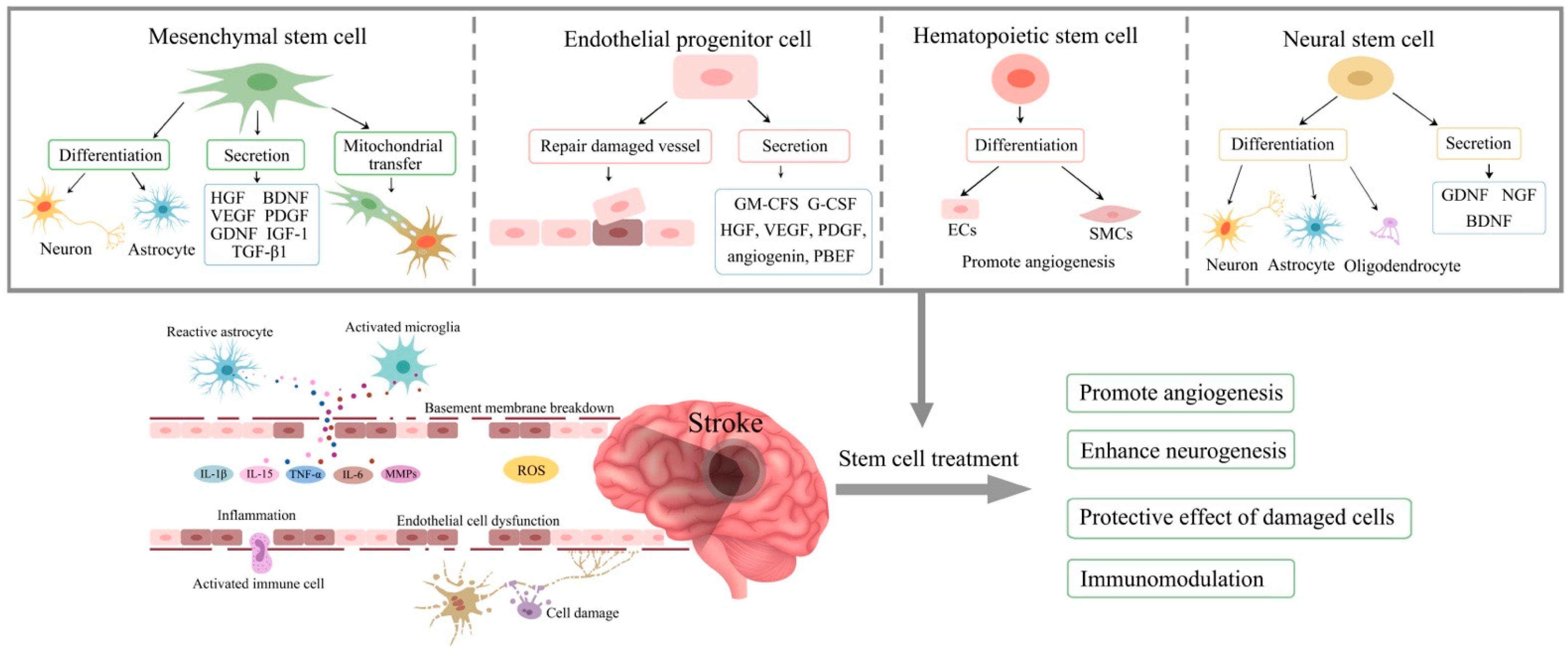
5. Mesenchymal Stem Cells
6. Endothelial Progenitor Cells (EPCs)
7. Haematopoietic Stem Cells
8. Neural Stem Cells
9. Route, Dose, and Timing of Treatment
9.1. Route
9.2. Dose
9.3. Timing
9.4. Comparison of Treatments with Different Types of Stem Cells
10. Conclusions
In conclusion, stem cell treatment presents possibilities for patients with all types of ischaemic stroke. With evidence of safety and efficacy measured in patients with acute, subacute, and chronic disease, therapeutic interventions appear to be promising for patients at every stage of the disease. However, further clinical research is necessary to standardise the treatment regimens.
References
- Brine, S. New Figures Show Larger Proportion of Strokes in the Middle Aged. 2018. Available online: https://www.gov.uk/government/news/new-figures-show-larger-proportion-of-strokes-in-the-middle-aged (accessed on 22 October 2023).
- Clark, W.M.; Albers, G.W.; Madden, K.P.; Hamilton, S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276 g): Results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000, 31, 811–816.
- Del Zoppo, G.J.; Saver, J.L.; Jauch, E.C.; Adams, H.P., Jr.; American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the American Heart Association/American Stroke Association. Stroke 2009, 40, 2945–2948.
- Broocks, G.; Kniep, H.; Kemmling, A.; Flottmann, F.; Nawabi, J.; Elsayed, S.; Schön, G.; Thomalla, G.; Fiehler, J.; Hanning, U. Effect of intravenous alteplase on ischaemic lesion water homeostasis. Eur. J. Neurol. 2020, 27, 376–383.
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21.
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110.
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2011, 300, C385–C393.
- Losenkova, K.; Zuccarini, M.; Helenius, M.; Jacquemet, G.; Gerasimovskaya, E.; Tallgren, C.; Jalkanen, S.; Yegutkin, G.G. Endothelial cells cope with hypoxia-induced depletion of ATP via activation of cellular purine turnover and phosphotransfer networks. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1804–1815.
- Annunziato, L.; Cataldi, M.; Pignataro, G.; Secondo, A.; Molinaro, P. Glutamate-independent calcium toxicity: Introduction. Stroke 2007, 38, 661–664.
- Bano, D.; Nicotera, P. Ca2+ Signals and Neuronal Death in Brain Ischemia. Stroke 2007, 38, 674–676.
- Garcia, J.H.; Liu, K.F.; Yoshida, Y.; Lian, J.; Chen, S.; del Zoppo, G.J. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am. J. Pathol. 1994, 144, 188–199.
- Gursoy-Ozdemir, Y.; Can, A.; Dalkara, T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 2004, 35, 1449–1453.
- Gibson, C.L.; Srivastava, K.; Sprigg, N.; Bath, P.M.; Bayraktutan, U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J. Neurochem. 2014, 129, 816–826.
- Rakkar, K.; Bayraktutan, U. Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-α and apoptosis. Biochim. Biophys. Acta 2016, 1862, 56–71.
- Kim, K.-A.; Shin, D.; Kim, J.-H.; Shin, Y.-J.; Rajanikant, G.K.; Majid, A.; Baek, S.-H.; Bae, O.-N. Role of Autophagy in Endothelial Damage and Blood–Brain Barrier Disruption in Ischemic Stroke. Stroke 2018, 49, 1571–1579.
- Shi, S.X.; Li, Y.J.; Shi, K.; Wood, K.; Ducruet, A.F.; Liu, Q. IL (Interleukin)-15 Bridges Astrocyte-Microglia Crosstalk and Exacerbates Brain Injury Following Intracerebral Hemorrhage. Stroke 2020, 51, 967–974.
- He, T.; Yang, G.Y.; Zhang, Z. Crosstalk of Astrocytes and Other Cells during Ischemic Stroke. Life 2022, 12, 910.
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339.
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516.
- Skulachev, V.P. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 2006, 11, 473–485.
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48.
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front. Physiol. 2020, 11, 605398.
- Winkler, L.; Blasig, R.; Breitkreuz-Korff, O.; Berndt, P.; Dithmer, S.; Helms, H.C.; Puchkov, D.; Devraj, K.; Kaya, M.; Qin, Z.; et al. Tight junctions in the blood–brain barrier promote edema formation and infarct size in stroke—Ambivalent effects of sealing proteins. J. Cereb. Blood Flow Metab. 2021, 41, 132–145.
- Nakano-Doi, A.; Sakuma, R.; Matsuyama, T.; Nakagomi, T. Ischemic stroke activates the VE-cadherin promoter and increases VE-cadherin expression in adult mice. Histol. Histopathol. 2018, 33, 507–521.
- Kalka, C.; Masuda, H.; Takahashi, T.; Gordon, R.; Tepper, O.; Gravereaux, E.; Pieczek, A.; Iwaguro, H.; Hayashi, S.-I.; Isner, J.M.; et al. Vascular Endothelial Growth Factor165 Gene Transfer Augments Circulating Endothelial Progenitor Cells in Human Subjects. Circ. Res. 2000, 86, 1198–1202.
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864.
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356.
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow–Derived ALD-401 Cells in Patients with Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205.
- Abdullah, Z.; Bayraktutan, U. Suppression of PKC-α attenuates TNF-α-evoked cerebral barrier breakdown via regulations of MMP-2 and plasminogen-plasmin system. Biochim. Biophys. Acta 2016, 1862, 1354–1366.
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732.
- Fujimoto, M.; Takagi, Y.; Aoki, T.; Hayase, M.; Marumo, T.; Gomi, M.; Nishimura, M.; Kataoka, H.; Hashimoto, N.; Nozaki, K. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 1674–1685.
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82.
- Yao, Y. Basement membrane and stroke. J. Cereb. Blood Flow Metab. 2019, 39, 3–19.
- Kang, M.; Yao, Y. Basement Membrane Changes in Ischemic Stroke. Stroke 2020, 51, 1344–1352.
- Krabbe, C.; Zimmer, J.; Meyer, M. Neural transdifferentiation of mesenchymal stem cells—A critical review. APMIS 2005, 113, 831–844.
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825–1834.
- Croft, A.P.; Przyborski, S.A. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp. Neurol. 2009, 216, 329–341.
- Lian, Q.; Zhang, Y.; Liang, X.; Gao, F.; Tse, H.-F. Directed Differentiation of Human-Induced Pluripotent Stem Cells to Mesenchymal Stem Cells. In Mesenchymal Stem Cells: Methods and Protocols; Gnecchi, M., Ed.; Springer: New York, NY, USA, 2016; pp. 289–298.
- Khan, A.A.; Huat, T.J.; Al Mutery, A.; El-Serafi, A.T.; Kacem, H.H.; Abdallah, S.H.; Reza, M.F.; Abdullah, J.M.; Jaafar, H. Significant transcriptomic changes are associated with differentiation of bone marrow-derived mesenchymal stem cells into neural progenitor-like cells in the presence of bFGF and EGF. Cell Biosci. 2020, 10, 126.
- Kruminis-Kaszkiel, E.; Osowski, A.; Bejer-Oleńska, E.; Dziekoński, M.; Wojtkiewicz, J. Differentiation of Human Mesenchymal Stem Cells from Wharton’s Jelly Towards Neural Stem Cells Using a Feasible and Repeatable Protocol. Cells 2020, 9, 739.
- Venkat, P.; Shen, Y.; Chopp, M.; Chen, J. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology 2018, 134, 310–322.
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784.
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin Promotes Mesenchymal Stem Cell Migration and Lessens Cell Stiffness via Integrin β1, FAK, and ERK Pathways. Cell Biochem. Biophys. 2013, 65, 455–462.
- Huang, P.; Gebhart, N.; Richelson, E.; Brott, T.G.; Meschia, J.F.; Zubair, A.C. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy 2014, 16, 1336–1344.
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359.
- Tai, L.; Saffery, N.S.; Chin, S.P.; Cheong, S.K. Secretome profile of TNF-α-induced human umbilical cord mesenchymal stem cells unveils biological processes relevant to skin wound healing. Regen. Med. 2023, 18, 839–856.
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1.
- Wang, J.; Fu, X.; Jiang, C.; Yu, L.; Wang, M.; Han, W.; Liu, L.; Wang, J. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF–VEGFR2 signaling pathway in a rat model of vascular dementia. Behav. Brain Res. 2014, 265, 171–180.
- Mu, J.; Bakreen, A.; Juntunen, M.; Korhonen, P.; Oinonen, E.; Cui, L.; Myllyniemi, M.; Zhao, S.; Miettinen, S.; Jolkkonen, J. Combined Adipose Tissue-Derived Mesenchymal Stem Cell Therapy and Rehabilitation in Experimental Stroke. Front. Neurol. 2019, 10, 235.
- Tsai, M.J.; Tsai, S.K.; Hu, B.R.; Liou, D.Y.; Huang, S.L.; Huang, M.C.; Huang, W.C.; Cheng, H.; Huang, S.S. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J. Biomed. Sci. 2014, 21, 5.
- Zang, J.; Sha, M.; Zhang, C.; Ye, J.; Zhang, K.; Gao, J. Senescent hepatocyte secretion of matrix metalloproteinases is regulated by nuclear factor-κB signaling. Life Sci. 2017, 191, 205–210.
- Bhasin, A.; Padma Srivastava, M.V.; Mohanty, S.; Bhatia, R.; Kumaran, S.S.; Bose, S. Stem cell therapy: A clinical trial of stroke. Clin. Neurol. Neurosurg. 2013, 115, 1003–1008.
- Honmou, O.; Houkin, K.; Matsunaga, T.; Niitsu, Y.; Ishiai, S.; Onodera, R.; Waxman, S.G.; Kocsis, J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134, 1790–1807.
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y.; STARTING Collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106.
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824.
- Bhatia, V.; Gupta, V.; Khurana, D.; Sharma, R.R.; Khandelwal, N. Randomized Assessment of the Safety and Efficacy of Intra-Arterial Infusion of Autologous Stem Cells in Subacute Ischemic Stroke. Am. J. Neuroradiol. 2018, 39, 899–904.
- Han, H.; Hu, J.; Yan, Q.; Zhu, J.; Zhu, Z.; Chen, Y.; Sun, J.; Zhang, R. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 2016, 13, 1517–1524.
- Tseng, N.; Lambie, S.C.; Huynh, C.Q.; Sanford, B.; Patel, M.; Herson, P.S.; Ormond, D.R. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow Metab. 2021, 41, 761–770.
- Yang, Y.; Ye, G.; Zhang, Y.-L.; He, H.-W.; Yu, B.-Q.; Hong, Y.-M.; You, W.; Li, X. Transfer of mitochondria from mesenchymal stem cells derived from induced pluripotent stem cells attenuates hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells. Neural Regen. Res. 2020, 15, 464–472.
- Noronha, N.d.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131.
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Treatment with outgrowth endothelial cells protects cerebral barrier against ischemic injury. Cytotherapy 2022, 24, 489–499.
- Bayraktutan, U. Endothelium, endothelial progenitor cells and stroke. J. Neurol. Clin. Neurosci. 2017, 1, 21–22.
- Williamson, K.; Stringer, S.E.; Alexander, M.Y. Endothelial progenitor cells enter the aging arena. Front. Physiol. 2012, 3, 30.
- Ya, J.; Bayraktutan, U. Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11538.
- Rakkar, K.; Othman, O.; Sprigg, N.; Bath, P.; Bayraktutan, U. Endothelial progenitor cells, potential biomarkers for diagnosis and prognosis of ischemic stroke: Protocol for an observational case-control study. Neural Regen. Res. 2020, 15, 1300–1307.
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ. Res. 2004, 95, 343–353.
- Yuan, J.-J.; Yang, J.; Sun, S.-L.; Zhang, R.; Xu, Y.-M. Endothelial Progenitor Cells’ Classification and Application in Neurological Diseases. Tissue Eng. Regen. Med. 2017, 14, 327–332.
- Bayraktutan, U. Endothelial progenitor cells: Potential novel therapeutics for ischaemic stroke. Pharmacol. Res. 2019, 144, 181–191.
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Protein kinase C-beta distinctly regulates blood-brain barrier-forming capacity of Brain Microvascular endothelial cells and outgrowth endothelial cells. Metab. Brain Dis. 2022, 37, 1815–1827.
- Alwjwaj, M.; Kadir, R.R.A.; Bayraktutan, U. Outgrowth endothelial progenitor cells restore cerebral barrier function following ischaemic damage: The impact of NOX2 inhibition. Eur. J. Neurosci. 2022, 55, 1658–1670.
- Shmelkov, S.V.; Butler, J.M.; Hooper, A.T.; Hormigo, A.; Kushner, J.; Milde, T.; St Clair, R.; Baljevic, M.; White, I.; Jin, D.K.; et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Investig. 2008, 118, 2111–2120.
- Alwjwaj, M.; Kadir, R.R.A.; Bayraktutan, U. The secretome of endothelial progenitor cells: A potential therapeutic strategy for ischemic stroke. Neural Regen. Res. 2021, 16, 1483–1489.
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial Progenitor Cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189.
- Liao, S.; Luo, C.; Cao, B.; Hu, H.; Wang, S.; Yue, H.; Chen, L.; Zhou, Z. Endothelial Progenitor Cells for Ischemic Stroke: Update on Basic Research and Application. Stem Cells Int. 2017, 2017, 2193432.
- Niu, G.; Chen, X. Vascular Endothelial Growth Factor as an Anti-Angiogenic Target for Cancer Therapy. Curr. Drug Targets 2010, 11, 1000–1017.
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105.
- Li, L.; Liu, H.; Xu, C.; Deng, M.; Song, M.; Yu, X.; Xu, S.; Zhao, X. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res. Ther. 2017, 8, 237.
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation 2006, 113, 1708–1714.
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 2013, 35, 175–185.
- Feliers, D.; Chen, X.; Akis, N.; Choudhury, G.G.; Madaio, M.; Kasinath, B.S. VEGF regulation of endothelial nitric oxide synthase in glomerular endothelial cells. Kidney Int. 2005, 68, 1648–1659.
- Ren, C.; Yao, Y.; Han, R.; Huang, Q.; Li, H.; Wang, B.; Li, S.; Li, M.; Mao, Y.; Mao, X.; et al. Cerebral ischemia induces angiogenesis in the peri-infarct regions via Notch1 signaling activation. Exp. Neurol. 2018, 304, 30–40.
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 642352.
- Mack, J.J.; Iruela-Arispe, M.L. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018, 25, 212–218.
- Smith, B.R. Regulation of hematopoiesis. Yale J. Biol. Med. 1990, 63, 371–380.
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389.
- Ngo, N.; Patel, K.; Isaacson, P.G.; Naresh, K.N. Leucocyte common antigen (CD45) and CD5 positivity in an “undifferentiated” carcinoma: A potential diagnostic pitfall. J. Clin. Pathol. 2007, 60, 936–938.
- Britannica, T.; Editors of Encyclopaedia. organ. Encyclopedia Britannica. 22 August 2023. Available online: https://www.britannica.com/science/organ-biology (accessed on 22 October 2023).
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An Evolving Paradigm for Stem Cell Biology. Cell 2008, 132, 631–644.
- Mayani, H. The regulation of hematopoietic stem cell populations . F1000Research 2016, 5, 1524.
- Rodrigues, N.P.; Tipping, A.J.; Wang, Z.; Enver, T. GATA-2 mediated regulation of normal hematopoietic stem/progenitor cell function, myelodysplasia and myeloid leukemia. Int. J. Biochem. Cell Biol. 2012, 44, 457–460.
- McIver, S.C.; Kang, Y.-A.; DeVilbiss, A.W.; O’Driscoll, C.A.; Ouellette, J.N.; Pope, N.J.; Camprecios, G.; Chang, C.-J.; Yang, D.; Bouhassira, E.E.; et al. The exosome complex establishes a barricade to erythroid maturation. Blood 2014, 124, 2285–2297.
- Aoyama, K.; Delaney, C.; Varnum-Finney, B.; Kohn, A.D.; Moon, R.T.; Bernstein, I.D. The Interaction of the Wnt and Notch Pathways Modulates Natural Killer Versus T Cell Differentiation. Stem Cells 2007, 25, 2488–2497.
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53.
- Lin, C.-H.; Lee, H.-T.; Lee, S.-D.; Lee, W.; Cho, C.-W.C.; Lin, S.-Z.; Wang, H.-J.; Okano, H.; Su, C.-Y.; Yu, Y.-L.; et al. Role of HIF-1α-activated Epac1 on HSC-mediated neuroplasticity in stroke model. Neurobiol. Dis. 2013, 58, 76–91.
- Wang, J.; Yu, L.; Jiang, C.; Chen, M.; Ou, C.; Wang, J. Bone marrow mononuclear cells exert long-term neuroprotection in a rat model of ischemic stroke by promoting arteriogenesis and angiogenesis. Brain Behav. Immun. 2013, 34, 56–66.
- Kennea, N.L.; Mehmet, H. Neural stem cells. J. Pathol. 2002, 197, 536–550.
- Ludwig, P.E.; Reddy, V.; Varacallo, M. Neuroanatomy, Neurons. . In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441977/ (accessed on 22 October 2023).
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; Lamantia, A.; McNamara, J.O.; Williams, S.M. (Eds.) Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10869/ (accessed on 22 October 2023).
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104.
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059.
- Ferrari, D.; Binda, E.; Filippis, L.D.; Vescovi, A.L. Isolation of Neural Stem Cells from Neural Tissues Using the Neurosphere Technique. Curr. Protoc. Stem Cell Biol. 2010, 15, 2D.6.1–2D.6.18.
- Reynolds, B.A.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710.
- Louis, S.A.; Mak, C.K.H. Enumerating Stem Cell Frequency: Neural Colony Forming Cell Assay. In Neural Progenitor Cells: Methods and Protocols; Reynolds, B.A., Deleyrolle, L.P., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 117–132.
- Zhou, Z.-D.; Kumari, U.; Xiao, Z.-C.; Tan, E.-K. Notch as a molecular switch in neural stem cells. IUBMB Life 2010, 62, 618–623.
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 2435–2448.
- Vieira, M.S.; Santos, A.K.; Vasconcellos, R.; Goulart, V.A.M.; Parreira, R.C.; Kihara, A.H.; Ulrich, H.; Resende, R.R. Neural stem cell differentiation into mature neurons: Mechanisms of regulation and biotechnological applications. Biotechnol. Adv. 2018, 36, 1946–1970.
- Yuan, T.; Liao, W.; Feng, N.-H.; Lou, Y.-L.; Niu, X.; Zhang, A.-J.; Wang, Y.; Deng, Z.-F. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res. Ther. 2013, 4, 73.
- Eckert, A.; Huang, L.; Gonzalez, R.; Kim, H.-S.; Hamblin, M.H.; Lee, J.-P. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits after Stroke. Stem Cells Transl. Med. 2015, 4, 841–851.
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 129.
- Watanabe, T.; Nagai, A.; Sheikh, A.M.; Mitaki, S.; Wakabayashi, K.; Kim, S.U.; Kobayashi, S.; Yamaguchi, S. A human neural stem cell line provides neuroprotection and improves neurological performance by early intervention of neuroinflammatory system. Brain Res. 2016, 1631, 194–203.
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796.
- Wechsler, L.R.; Bates, D.; Stroemer, P.; Andrews-Zwilling, Y.S.; Aizman, I. Cell Therapy for Chronic Stroke. Stroke 2018, 49, 1066–1074.
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019, 9, e01214.
- Zhang, H.-L.; Xie, X.-F.; Xiong, Y.-Q.; Liu, S.-M.; Hu, G.-Z.; Cao, W.-F.; Wu, X.-M. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 2018, 1680, 143–154.
- Vasconcelos-dos-Santos, A.; Rosado-de-Castro, P.H.; Lopes de Souza, S.A.; da Costa Silva, J.; Ramos, A.B.; Rodriguez de Freitas, G.; Barbosa da Fonseca, L.M.; Gutfilen, B.; Mendez-Otero, R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012, 9, 1–8.
- Rosado-de-Castro, P.H.; Schmidt Fda, R.; Battistella, V.; Lopes de Souza, S.A.; Gutfilen, B.; Goldenberg, R.C.; Kasai-Brunswick, T.H.; Vairo, L.; Silva, R.M.; Wajnberg, E.; et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen. Med. 2013, 8, 145–155.
- Yang, B.; Migliati, E.; Parsha, K.; Schaar, K.; Xi, X.; Aronowski, J.; Savitz, S.I. Intra-Arterial Delivery Is Not Superior to Intravenous Delivery of Autologous Bone Marrow Mononuclear Cells in Acute Ischemic Stroke. Stroke 2013, 44, 3463–3472.
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 2019, 50, 2835–2841.
- Chen, L.; Xi, H.; Huang, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. Multiple Cell Transplantation Based on an Intraparenchymal Approach for Patients with Chronic Phase Stroke. Cell Transplant. 2013, 22, 83–91.
- Boy, S.; Sauerbruch, S.; Kraemer, M.; Schormann, T.; Schlachetzki, F.; Schuierer, G.; Luerding, R.; Hennemann, B.; Orso, E.; Dabringhaus, A.; et al. Mobilisation of Hematopoietic CD34+ Precursor Cells in Patients with Acute Stroke Is Safe—Results of an Open-Labeled Non Randomized Phase I/II Trial. PLoS ONE 2011, 6, e23099.
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69.




