Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kirill Korneev | -- | 2153 | 2024-01-15 14:58:49 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2152 | 2024-01-16 01:02:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zheremyan, E.A.; Ustiugova, A.S.; Karamushka, N.M.; Uvarova, A.N.; Stasevich, E.M.; Bogolyubova, A.V.; Kuprash, D.V.; Korneev, K.V. Breg-Mediated Immunoregulation in the Skin. Encyclopedia. Available online: https://encyclopedia.pub/entry/53841 (accessed on 07 February 2026).
Zheremyan EA, Ustiugova AS, Karamushka NM, Uvarova AN, Stasevich EM, Bogolyubova AV, et al. Breg-Mediated Immunoregulation in the Skin. Encyclopedia. Available at: https://encyclopedia.pub/entry/53841. Accessed February 07, 2026.
Zheremyan, Elina A., Alina S. Ustiugova, Nina M. Karamushka, Aksinya N. Uvarova, Ekaterina M. Stasevich, Apollinariya V. Bogolyubova, Dmitry V. Kuprash, Kirill V. Korneev. "Breg-Mediated Immunoregulation in the Skin" Encyclopedia, https://encyclopedia.pub/entry/53841 (accessed February 07, 2026).
Zheremyan, E.A., Ustiugova, A.S., Karamushka, N.M., Uvarova, A.N., Stasevich, E.M., Bogolyubova, A.V., Kuprash, D.V., & Korneev, K.V. (2024, January 15). Breg-Mediated Immunoregulation in the Skin. In Encyclopedia. https://encyclopedia.pub/entry/53841
Zheremyan, Elina A., et al. "Breg-Mediated Immunoregulation in the Skin." Encyclopedia. Web. 15 January, 2024.
Copy Citation
Wound healing is a complex process involving a coordinated series of events aimed at restoring tissue integrity and function. Regulatory B cells (Bregs) are a subset of B lymphocytes that play an essential role in fine-tuning immune responses and maintaining immune homeostasis. Studies have suggested that Bregs are important players in cutaneous immunity.
regulatory B cells
skin homeostasis
wound healing
inflammatory skin pathology
tissue regeneration
immune regulation
1. Introduction
Inflammatory diseases are often accompanied by cutaneous abnormalities, ranging from minor skin irritation to severe chronic conditions [1]. In such diseases, the inflammatory process of the skin is usually driven by the activation of the immune system and the production of inflammatory mediators [2]. These inflammatory mediators cause vasodilation and increase vascular permeability, allowing immune cells to migrate to the site of inflammation. The activated immune cells then release additional pro-inflammatory cytokines and chemokines, amplifying the immune response and leading to further tissue damage and inflammation. Cutaneous wound healing is a complex process aimed at restoring skin tissue homeostasis and preventing further damage and infection. This process unfolds in an intricate sequence of phases, often occurring simultaneously rather than in a strictly linear fashion [3]. The initial inflammatory phase, encompassing vascular and cellular responses, commences with hemostasis marked by platelet aggregation and fibrin clot formation [4]. Subsequently, immune cells such as neutrophils, monocytes and lymphocytes infiltrate the wound bed, undertaking crucial roles in tissue debridement and cytokine release [5]. Transitioning to the repair phase involves the creation of granulation tissue, re-epithelialization and wound contraction, with fibroblasts, endothelial cells and keratinocytes as key players. Fibroblasts contribute to matrix formation, while endothelial cells drive angiogenesis. Keratinocytes migrate to cover the wound and myofibroblasts aid in contraction [6]. The maturation phase focuses on tissue remodeling, apoptosis and collagen cross-linking, leading to the formation of an acellular scar [7]. Thus, the complex process of wound healing is orchestrated by multiple immune cell types [8][9][10][11][12]. However, the role of B cells, critical players in the systemic immune response, still needs to be fully understood in the context of the local immune response in the skin. One type of B cell that deserves attention in the study of the wound healing processes is regulatory B cells (Bregs). The count of diverse subpopulations of regulatory B cells currently exceed ten [13] and unfortunately, there is no universally accepted specific marker that sets them apart from other B cell subsets.
2. Breg Homing and Induction in the Skin
Traditionally, B cells were thought to accumulate in the skin during inflammation but not under homeostatic conditions. However, B cells were later identified and characterized in the skin of humans, mice, and sheep, even in the absence of inflammation [14][15]. Within mammalian skin, B cells primarily reside in the dermis, exhibiting sparse dispersion as individual cells in homeostatic conditions and forming cell clusters or organized lymphoid structures during inflammation [16]. Based on the current limited knowledge, the homing and migration mechanisms of Bregs do not appear to be dramatically different from those of other B cells. In the classical paradigm of lymphocyte trafficking, orchestrated migration is governed by interactions involving distinct combinations of tissue-specific adhesion molecules (such as selectins and integrins) and their interplay with chemokines and corresponding receptors. This complex interplay ultimately facilitates the migration of cells through vascular endothelia and their infiltration into tissue sites [17].
3. Bregs Promote Cutaneous Wound Healing via Several Mechanisms in a Variety of Pathological and Healthy States
3.1. Maintaining Tissue Homeostasis
Anti-inflammatory cytokines, such as IL10 and TGFβ, are known to influence both immune cells and epithelial cells during wound healing [18][19]. Interestingly, the isoforms of TGFβ have different roles in wound healing machinery. In comparison, TGFβ1 and TGFβ2 are key players in the proliferative phase of wound healing, where they serve to promote signaling via SMAD and Wnt-dependent pathways to enhance scarring, whereas TGFβ3 reduces scarring [20]. Geherin and colleagues showed that Bregs reside in murine cutaneous tissue, where they accumulate during inflammation and secrete IL10.
3.2. Promotion of Angiogenesis
Angiogenesis is an essential physiological process occurring during repair after cutaneous injury [21]. Pathological angiogenesis has also been observed in carcinogenesis and chronic inflammation [22]. Van de Veen and colleagues identified a subpopulation of CD49b+CD73+ Bregs with high angiogenic potential that produces various angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), hepatocyte growth factor, axon guidance factor, and angiopoietins, orchestrating the formation of endothelial cell tubes [23].
3.3. Interaction with Other Types of Suppressor Cells
3.3.1. Regulatory T Cells
Regulatory T cells (Tregs) help to suppress excessive immune responses and promote immune tolerance. Tregs are important for regulating the inflammatory response in wound healing to prevent excessive tissue damage and promote tissue repair [24]. The skin regulatory T cell population comprises both resident and circulating Tregs, which can accumulate in the injured tissue [4]. Haertel and colleagues demonstrated that Treg depletion compromised normal acute wound healing: impaired re-epithelization, reduced wound closure, and delayed angiogenesis [25]. B cells play a crucial role in promoting the differentiation and activation of Tregs, as demonstrated by the reduced frequency of Foxp3+ Tregs in B cell-deficient mice [26].
3.3.2. Alternatively Activated (M2) Macrophages
Macrophages have critical functions at each stage of wound healing, namely inflammation, proliferation, and remodeling. Throughout the healing process, macrophages shift from primarily exhibiting pro-inflammatory traits (M1-like phenotypes) to displaying anti-inflammatory features (M2) [27]. However, chronic wounds that do not heal, such as diabetic ulcers, remain in the inflammatory phase of wound healing, and as a result, macrophages in the wound area retain pro-inflammatory properties [28]. Bregs can shift macrophage polarization from M1 to M2 by producing anti-inflammatory cytokines (IL10, TGFβ, etc.).
3.3.3. Other Immune Suppressor Cells
Invariant natural killer T cells (iNKT) promote skin wound healing by preventing a prolonged neutrophilic inflammatory response [29]. Bregs support iNKT proliferation, activation, and cytokine production in a CD1d-dependent manner [30]. Bregs can also modulate dendritic cell (DC) function in inflammation by reducing antigen presentation and pro-inflammatory cytokine productions by DCs or by modulating monocytes to differentiate into tolerogenic DCs [30].
3.4. Suppression of Effector Cells
3.4.1. Cytotoxic T Cells
Although the main immunosuppressive effect of Bregs on T cells is believed to be Treg induction, as discussed above, there are other mechanisms worth mentioning. Cytotoxic T cells are active participants in the early stages of acute wound healing [5]. Bregs suppressed the production of CCL5—a potent skin chemoattractant—by the skin epidermis preventing the infiltration of effector CD8+ T cells into the skin [31]. IL10-producing B cells have been described to suppress IFNγ and GrB expression by T killers [32][33].
3.4.2. Helper T Cells
Regarding helper T cells (Th), it has been reported that Bregs can suppress both Th1 and Th17 responses [34]. This process may be very beneficial in the case of diabetic ulcers, since the level of these inflammatory T helper subsets is increased in patients with diabetes mellitus [35]. B cell-derived TGFβ was found to inhibit Th1 and Th17 immune responses by decreasing the antigen-presenting capacity of dendritic cells [36]. B10 cells also inhibit Th1 and Th17 cells through IL10 production, thus ameliorating experimental arthritis [37]. Bregs have been found to induce CD4+ T cell death in general: experiments in diabetes models revealed that LPS-stimulated B cells express FasL and TGFβ and can induce apoptosis of both effector B and T lymphocytes [38].
3.4.3. Natural Killer Cells
During the late phases of wound healing, natural killer (NK) cells have a predominantly negative impact on tissue repair. NK-produced IFNγ polarizes macrophages toward the pro-inflammatory M1 phenotype, thereby enhancing immune cell infiltration at the wound site via macrophages producing cytokines such as IL1β, IL6, IL12, IL23, and TNF [5][39]. Therefore, NK-mediated inflammatory processes may hinder wound healing, especially in chronic wounds.
3.4.4. Dendritic Cells
The presence of plasmacytoid dendritic cells (pDCs) has been detected in cutaneous healing processes [40]. A regulatory feedback loop between pDCs and B cells has been demonstrated in both humans and mice. In response to inflammation, pDCs secrete IFNα, which induces B cells to produce IL10, subsequently suppressing IFNα production by pDCs. This regulatory feedback can be disrupted in autoimmune conditions such as systemic lupus erythematosus [41].
3.4.5. Neutrophils
Neutrophils are among the first circulating immune cells recruited to a wound that contribute to tissue injury by amplifying the inflammatory response [42]. The ability of Bregs to suppress neutrophils plays an important role in the proper resolution of an inflammatory response as shown in murine models of colitis and salmonella infection [43][44]. Peripheral IgM+ transitional B cells with regulatory properties were found to dampen excessive neutrophil activity in the lung [45].
3.4.6. Effector B Cells
Effector B cells can also be suppressed by their regulatory counterparts. Rosser and colleagues proposed three possible mechanisms for Breg-mediated suppression of antibody responses: (1) direct suppression of antibody-producing B cells; (2) suppression of T helper responses, which will inevitably lead to a decrease in plasma cell generation; (3) induction of Tregs, which could contribute to global suppression of inflammatory responses, including suppression of antibody production [34].
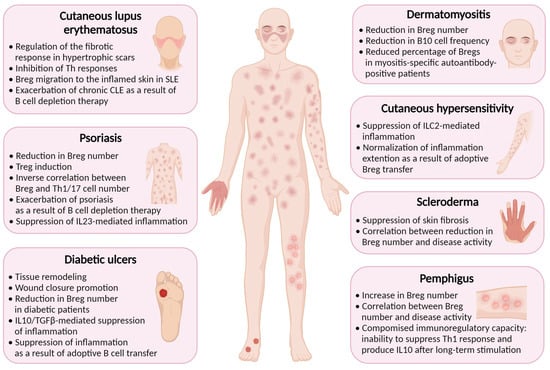
4. Breg-Mediated Wound Healing in Inflammatory Skin Diseases
Inflammation is a natural response of the immune system to injury or infection, and it plays a vital role in the healing process. Bregs have been found to play a role in wound healing and suppression of inflammation in several cutaneous pathologies associated with skin damage. Some inflammatory diseases, such as psoriasis, systemic sclerosis (scleroderma), cutaneous lupus erythematosus, cutaneous hypersensitivity, pemphigus, dermatomyositis, and diabetes, have been found to be associated with reduced Breg function (Figure 1) [16].

Figure 1. Regulatory B cells are implicated in the pathogenesis of inflammatory skin diseases. Created with BioRender.com, accessed on 24 November 2023.
4.1. Diabetes Mellitus
Diabetes is a chronic metabolic disorder that impairs the body’s ability to regulate blood glucose levels. In diabetes, chronic hyperglycemia, and impaired immune function, among other complications, can lead to a persistent state of low-grade inflammation with excessive production of cytokines and chemokines exacerbating skin ulceration and impairing the healing process [46][47]. Bregs are decreased in patients with diabetes [48], possibly contributing to delayed wound healing.
4.2. Psoriasis
Psoriasis is a chronic autoimmune disease affecting skin, nails, and joints [49]. In individuals with psoriasis, the immune system responds abnormally to skin cells, causing them to grow rapidly and resulting in a buildup of cells on the surface of the skin [50]. Patients with psoriasis have lower levels of IL10+ B cells in their bloodstream, which is consistent with studies in mice showing the crucial role of these cells in suppressing psoriasiform inflammation [51][52]. A number of IL10+ Breg cells was found to be inversely correlated with psoriasis severity and the number of pathogenic IL17A+CD3+ and IFNγ+CD3+ T cells [53].
4.3. Contact Hypersensitivity
Contact hypersensitivity (CHS) is an immune-mediated skin reaction that clinically manifests as allergic contact dermatitis [54]. Both CD4+ and CD8+ T cells have been shown to play a pivotal role in the progression of CHS [55]. The immune response is accompanied by the release of cytokines and other immune mediators that cause inflammation and the characteristic symptoms of CHS, such as redness, itching, and blistering.
4.4. Systemic Sclerosis (Scleroderma)
Systemic sclerosis is a chronic autoimmune disease that affects the skin and internal organs. It is characterized by the excessive production and deposition of collagen in various body tissues, leading to the hardening and thickening of the skin and other tissues [56]. Systemic sclerosis can vary widely in its severity and manifestations, and conservative treatment options rely upon immunosuppressive medications to reduce the immune response and alleviate symptoms [57].
4.5. Cutaneous Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) is a cutaneous manifestation of systemic lupus erythematosus (SLE) that affects the skin, occurring in 80% of cases [58]. In a mouse model of SLE, induction of Bregs has been found to suppress both skin and systemic disease [59]. In SLE patients, IL10+ B cells localize in the inflamed skin [60]. Patients with hypertrophic scars demonstrate a higher percentage of Breg cells compared to healthy donors, suggesting that Bregs may regulate the fibrotic response in CLE, presenting a potential therapeutic target [61].
4.6. Pemphigus
Pemphigus is a rare autoimmune disease that is characterized by intraepidermal blisters caused by autoantibodies against desmoglein 1 and 3 [62]. One study has revealed that the frequency of CD19+CD24hiCD38hi Bregs in PBMCs of pemphigus patients was elevated compared with healthy controls, but they were functionally impaired, unable to suppress Th1 immune response [62]. The increase in Breg number can be linked with elevated IL21 level, which is characteristic of pemphigus patients, and is known to promote Bregs [63].
4.7. Dermatomyositis
Dermatomyositis (DM) is a systemic autoimmune disease primarily affecting skin and muscles and which is characterized by dysregulation of hyperactivated B and T cells, leading to autoantibody production and aberrant cytokine production [64]. In DM patients, aggregates of lymphocytes are found in skin lesions [65]. A very comprehensive study conducted by Li et al. revealed that Breg deficiency is an important immunopathogenic feature of DM [66]. CD19+CD24hiCD38hi Breg level was found to be significantly decreased in the peripheral blood of DM patients, and IL10+ B cell levels were also lower compared to those of healthy controls. Myositis-specific autoantibody (MSA)-positive patients were shown to have lower percentage of CD19+CD24hiCD38hi Bregs than in MSA-negative individuals, thus supporting possible role of Bregs in the control of autoantibody production [66].
References
- Chiricozzi, A.; Zhang, S.; Dattola, A.; Cannizzaro, M.V.; Gabellini, M.; Chimenti, S.; Nistico, S.P. New Insights into the Pathogenesis of Cutaneous Autoimmune Disorders. J. Biol. Regul. Homeost. Agents 2012, 26, 165–170.
- Vesely, M.D. Getting Under the Skin: Targeting Cutaneous Autoimmune Disease. Yale J. Biol. Med. 2020, 93, 197–206.
- Lux, C.N. Wound Healing in Animals: A Review of Physiology and Clinical Evaluation. Vet. Dermatol. 2022, 33, 91-e27.
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315.
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700.
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound Bed Preparation: A Systematic Approach to Wound Management. Wound Repair Regen. 2003, 11 (Suppl. S1), S1–S28.
- Visha, M.G.; Karunagaran, M. A Review on Wound Healing. Int. J. Clin. Correl. 2019, 3, 50–59.
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014.
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and Wound Healing: An Update. Regen. Med. 2018, 13, 491–495.
- Wilgus, T.A. Immune Cells in the Healing Skin Wound: Influential Players at Each Stage of Repair. Pharmacol. Res. 2008, 58, 112–116.
- Sun, X.; Joost, S.; Kasper, M. Plasticity of Epithelial Cells during Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041232.
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231.
- Glass, D.R.; Tsai, A.G.; Oliveria, J.P.; Hartmann, F.J.; Kimmey, S.C.; Calderon, A.A.; Borges, L.; Glass, M.C.; Wagar, L.E.; Davis, M.M.; et al. An Integrated Multi-Omic Single-Cell Atlas of Human B Cell Identity. Immunity 2020, 53, 217–232.
- Geherin, S.A.; Gómez, D.; Glabman, R.A.; Ruthel, G.; Hamann, A.; Debes, G.F. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require α4β1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J. Immunol. 2016, 196, 2514–2525.
- Geherin, S.A.; Fintushel, S.R.; Lee, M.H.; Wilson, R.P.; Patel, R.T.; Alt, C.; Young, A.J.; Hay, J.B.; Debes, G.F. The Skin, a Novel Niche for Recirculating B Cells. J. Immunol. 2012, 188, 6027–6035.
- Debes, G.F.; McGettigan, S.E. Skin-Associated B Cells in Health and Inflammation. J. Immunol. 2019, 202, 1659–1666.
- Shimizu, Y.; Newman, W.; Tanaka, Y.; Shaw, S. Lymphocyte Interactions with Endothelial Cells. Immunol. Today 1992, 13, 106–112.
- Quiros, M.; Nishio, H.; Neumann, P.A.; Siuda, D.; Brazil, J.C.; Azcutia, V.; Hilgarth, R.; O’Leary, M.N.; Garcia-Hernandez, V.; Leoni, G.; et al. Macrophage-Derived IL-10 Mediates Mucosal Repair by Epithelial WISP-1 Signaling. J. Clin. Investig. 2017, 127, 3510–3520.
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306.
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous Scarring: Pathophysiology, Molecular Mechanisms, and Scar Reduction Therapeutics Part I. The Molecular Basis of Scar Formation. J. Am. Acad. Dermatol. 2012, 66, 1–10.
- Varricchi, G.; Granata, F.; Loffredo, S.; Genovese, A.; Marone, G. Angiogenesis and Lymphangiogenesis in Inflammatory Skin Disorders. J. Am. Acad. Dermatol. 2015, 73, 144–153.
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399.
- van de Veen, W.; Globinska, A.; Jansen, K.; Straumann, A.; Kubo, T.; Verschoor, D.; Wirz, O.F.; Castro-Giner, F.; Tan, G.; Rückert, B.; et al. A Novel Proangiogenic B Cell Subset Is Increased in Cancer and Chronic Inflammation. Sci. Adv. 2020, 6, eaaz3559.
- Kalekar, L.A.; Rosenblum, M.D. Regulatory T Cells in Inflammatory Skin Disease: From Mice to Humans. Int. Immunol. 2019, 31, 457–463.
- Haertel, E.; Joshi, N.; Hiebert, P.; Kopf, M.; Werner, S. Regulatory T Cells Are Required for Normal and Activin-Promoted Wound Repair in Mice. Eur. J. Immunol. 2018, 48, 1001–1013.
- Sun, J.-B.; Flach, C.-F.; Czerkinsky, C.; Holmgren, J. B Lymphocytes Promote Expansion of Regulatory T Cells in Oral Tolerance: Powerful Induction by Antigen Coupled to Cholera Toxin B Subunit. J. Immunol. 2008, 181, 8278–8287.
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364.
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419.
- Tanno, H.; Kawakami, K.; Kanno, E.; Suzuki, A.; Takagi, N.; Yamamoto, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Invariant NKT Cells Promote Skin Wound Healing by Preventing a Prolonged Neutrophilic Inflammatory Response. Wound Repair Regen. 2017, 25, 805–815.
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795.
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B Regulatory Cells and the Tumor-Promoting Actions of TNF-α during Squamous Carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667.
- Wei, X.; Jin, Y.; Tian, Y.; Zhang, H.; Wu, J.; Lu, W.; Lu, X. Regulatory B Cells Contribute to the Impaired Antitumor Immunity in Ovarian Cancer Patients. Tumour Biol. 2016, 37, 6581–6588.
- Chen, Z.; Zhu, Y.; Du, R.; Pang, N.; Zhang, F.; Dong, D.; Ding, J.; Ding, Y. Role of Regulatory B Cells in the Progression of Cervical Cancer. Mediat. Inflamm. 2019, 2019, 6519427.
- Rosser, E.C.; Blair, P.A.; Mauri, C. Cellular Targets of Regulatory B Cell-Mediated Suppression. Mol. Immunol. 2014, 62, 296–304.
- Strang, H.; Kaul, A.; Parikh, U.; Masri, L.; Saravanan, S.; Li, H.; Miao, Q.; Balaji, S. Chapter 11—Role of Cytokines and Chemokines in Wound Healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: New York, NY, USA, 2020; pp. 197–235. ISBN 9780128164136.
- Bjarnadóttir, K.; Benkhoucha, M.; Merkler, D.; Weber, M.S.; Payne, N.L.; Bernard, C.C.A.; Molnarfi, N.; Lalive, P.H. B Cell-Derived Transforming Growth Factor-β1 Expression Limits the Induction Phase of Autoimmune Neuroinflammation. Sci. Rep. 2016, 6, 34594.
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of Arthritis by Interleukin 10-Producing B Cells. J. Exp. Med. 2003, 197, 489–501.
- Tian, J.; Zekzer, D.; Hanssen, L.; Lu, Y.; Olcott, A.; Kaufman, D.L. Lipopolysaccharide-Activated B Cells down-Regulate Th1 Immunity and Prevent Autoimmune Diabetes in Nonobese Diabetic Mice. J. Immunol. 2001, 167, 1081–1089.
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928.
- Tomasini, D.; Mentzel, T.; Hantschke, M.; Cerri, A.; Paredes, B.; Rütten, A.; Schärer, L.; Kutzner, H. Plasmacytoid Dendritic Cells: An Overview of Their Presence and Distribution in Different Inflammatory Skin Diseases, with Special Emphasis on Jessner’s Lymphocytic Infiltrate of the Skin and Cutaneous Lupus Erythematosus. J. Cutan. Pathol. 2010, 37, 1132–1139.
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697.
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS Pathog. 2015, 11, e1004651.
- Maseda, D.; Candando, K.M.; Smith, S.H.; Kalampokis, I.; Weaver, C.T.; Plevy, S.E.; Poe, J.C.; Tedder, T.F. Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-γ+CD4+ T Cell Numbers during Colitis Development in Mice. J. Immunol. 2013, 191, 2780–2795.
- Neves, P.; Lampropoulou, V.; Calderon-Gomez, E.; Roch, T.; Stervbo, U.; Shen, P.; Kühl, A.A.; Loddenkemper, C.; Haury, M.; Nedospasov, S.A.; et al. Signaling via the MyD88 Adaptor Protein in B Cells Suppresses Protective Immunity during Salmonella Typhimurium Infection. Immunity 2010, 33, 777–790.
- Podstawka, J.; Sinha, S.; Hiroki, C.H.; Sarden, N.; Granton, E.; Labit, E.; Kim, J.H.; Andonegui, G.; Lou, Y.; Snarr, B.D.; et al. Marginating Transitional B Cells Modulate Neutrophils in the Lung during Inflammation and Pneumonia. J. Exp. Med. 2021, 218, e20210409.
- Rodríguez-Rodríguez, N.; Martínez-Jiménez, I.; García-Ojalvo, A.; Mendoza-Mari, Y.; Guillén-Nieto, G.; Armstrong, D.G.; Berlanga-Acosta, J. Wound Chronicity, Impaired Immunity and Infection in Diabetic Patients. MEDICC Rev. 2022, 24, 44–58.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615.
- Mendez-Frausto, G.; Romero-Aguilera, G.; Sanchez-Gutierrez, R.; García-Jacobo, R.E.; Lara-Ramírez, E.E.; Uresti-Rivera, E.E.; Gonzalez-Amaro, R.; Enciso-Moreno, J.A.; García-Hernández, M.H. B Regulatory Cells Associated with Changes in Biochemical and Inflammatory Parameters in Normal-Glycemic Individuals, Pre-Diabetes and T2DM Patients. Diabetes Res. Clin. Pract. 2021, 173, 108692.
- Goldman, M.P. Treatment of Varicose and Telangiectatic Leg Veins: Double-Blind Prospective Comparative Trial between Aethoxyskerol and Sotradecol. Dermatol. Surg. 2002, 28, 52–55.
- Grigorieva, E.; Khailov, E.; Deignan, P. Optimal Treatment Strategies for Control Model of Psoriasis. In Proceedings of the 2017 Proceedings of the Conference on Control and its Applications (CT), Philadelphia, PA, USA, 10–12 July 2017; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2017; pp. 86–93.
- Yanaba, K.; Kamata, M.; Ishiura, N.; Shibata, S.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Tedder, T.F.; Sato, S. Regulatory B Cells Suppress Imiquimod-Induced, Psoriasis-like Skin Inflammation. J. Leukoc. Biol. 2013, 94, 563–573.
- Hayashi, M.; Yanaba, K.; Umezawa, Y.; Yoshihara, Y.; Kikuchi, S.; Ishiuji, Y.; Saeki, H.; Nakagawa, H. IL-10-Producing Regulatory B Cells Are Decreased in Patients with Psoriasis. J. Dermatol. Sci. 2016, 81, 93–100.
- Mavropoulos, A.; Varna, A.; Zafiriou, E.; Liaskos, C.; Alexiou, I.; Roussaki-Schulze, A.; Vlychou, M.; Katsiari, C.; Bogdanos, D.P.; Sakkas, L.I. IL-10 Producing Bregs Are Impaired in Psoriatic Arthritis and Psoriasis and Inversely Correlate with IL-17- and IFNγ-Producing T Cells. Clin. Immunol. 2017, 184, 33–41.
- Grabbe, S.; Schwarz, T. Immunoregulatory Mechanisms Involved in Elicitation of Allergic Contact Hypersensitivity. Immunol. Today 1998, 19, 37–44.
- Saint-Mezard, P.; Berard, F.; Dubois, B.; Kaiserlian, D.; Nicolas, J.-F. The Role of CD4+ and CD8+ T Cells in Contact Hypersensitivity and Allergic Contact Dermatitis. Eur. J. Dermatol. 2004, 14, 131–138.
- Rosendahl, A.-H.; Schönborn, K.; Krieg, T. Pathophysiology of Systemic Sclerosis (scleroderma). Kaohsiung J. Med. Sci. 2022, 38, 187–195.
- Zhao, M.; Wu, J.; Wu, H.; Sawalha, A.H.; Lu, Q. Clinical Treatment Options in Scleroderma: Recommendations and Comprehensive Review. Clin. Rev. Allergy Immunol. 2022, 62, 273–291.
- Rothfield, N.; Sontheimer, R.D.; Bernstein, M. Lupus Erythematosus: Systemic and Cutaneous Manifestations. Clin. Dermatol. 2006, 24, 348–362.
- Maz, M.P.; Martens, J.W.S.; Hannoudi, A.; Reddy, A.L.; Hile, G.A.; Kahlenberg, J.M. Recent Advances in Cutaneous Lupus. J. Autoimmun. 2022, 132, 102865.
- Yang, X.; Yang, J.; Chu, Y.; Xue, Y.; Xuan, D.; Zheng, S.; Zou, H. T Follicular Helper Cells and Regulatory B Cells Dynamics in Systemic Lupus Erythematosus. PLoS ONE 2014, 9, e88441.
- Méndez-Flores, S.; Hernández-Molina, G.; Enríquez, A.B.; Faz-Muñoz, D.; Esquivel, Y.; Pacheco-Molina, C.; Furuzawa-Carballeda, J. Cytokines and Effector/Regulatory Cells Characterization in the Physiopathology of Cutaneous Lupus Erythematous: A Cross-Sectional Study. Mediat. Inflamm. 2016, 2016, 7074829.
- Hébert, V.; Petit, M.; Maho-Vaillant, M.; Golinski, M.-L.; Riou, G.; Derambure, C.; Boyer, O.; Joly, P.; Calbo, S. Modifications of the Transcriptomic Profile of Autoreactive B Cells From Pemphigus Patients After Treatment With Rituximab or a Standard Corticosteroid Regimen. Front. Immunol. 2019, 10, 1794.
- Zhu, H.-Q.; Xu, R.-C.; Chen, Y.-Y.; Yuan, H.-J.; Cao, H.; Zhao, X.-Q.; Zheng, J.; Wang, Y.; Pan, M. Impaired Function of CD19+CD24hiCD38hi Regulatory B Cells in Patients with Pemphigus. Br. J. Dermatol. 2015, 172, 101–110.
- Zong, M.; Lundberg, I.E. Pathogenesis, Classification and Treatment of Inflammatory Myopathies. Nat. Rev. Rheumatol. 2011, 7, 297–306.
- Magro, C.M.; Segal, J.P.; Crowson, A.N.; Chadwick, P. The Phenotypic Profile of Dermatomyositis and Lupus Erythematosus: A Comparative Analysis. J. Cutan. Pathol. 2010, 37, 659–671.
- Li, W.; Tian, X.; Lu, X.; Peng, Q.; Shu, X.; Yang, H.; Li, Y.; Wang, Y.; Zhang, X.; Liu, Q.; et al. Significant Decrease in Peripheral Regulatory B Cells Is an Immunopathogenic Feature of Dermatomyositis. Sci. Rep. 2016, 6, 27479.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
430
Revisions:
2 times
(View History)
Update Date:
16 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




